 Found 1019 hits with Last Name = 'brookfield' and Initial = 'fa'
Found 1019 hits with Last Name = 'brookfield' and Initial = 'fa' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037076
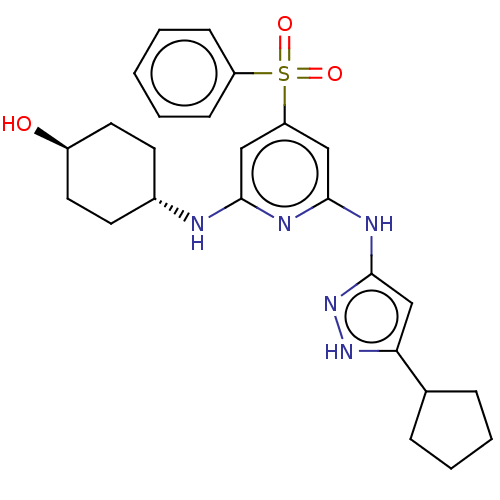
(CHEMBL3355737)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)C2CCCC2)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(11.82,-31.11,;11.83,-29.57,;13.16,-28.8,;13.17,-27.26,;11.84,-26.5,;10.5,-27.26,;10.5,-28.8,;11.83,-24.96,;10.5,-24.19,;9.16,-24.96,;7.83,-24.19,;7.83,-22.65,;9.16,-21.88,;9.15,-20.34,;10.49,-19.56,;11.9,-20.19,;12.93,-19.05,;12.15,-17.72,;10.65,-18.04,;14.46,-19.19,;15.24,-20.52,;16.74,-20.2,;16.9,-18.67,;15.49,-18.04,;10.49,-22.64,;6.49,-24.96,;7.25,-26.29,;5.72,-26.28,;5.16,-24.19,;5.17,-22.65,;3.84,-21.88,;2.5,-22.65,;2.51,-24.2,;3.84,-24.96,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037066
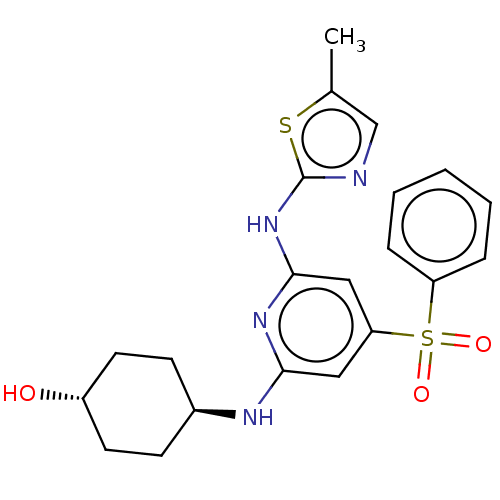
(CHEMBL3355728)Show SMILES Cc1cnc(Nc2cc(cc(N[C@H]3CC[C@H](O)CC3)n2)S(=O)(=O)c2ccccc2)s1 |r,wU:12.11,wD:15.15,(26,-11.56,;24.47,-11.4,;23.69,-10.07,;22.19,-10.39,;22.03,-11.92,;20.69,-12.69,;20.7,-14.23,;19.37,-15,;19.37,-16.55,;20.7,-17.32,;22.04,-16.55,;23.37,-17.31,;23.38,-18.85,;24.71,-19.61,;24.7,-21.15,;23.37,-21.92,;23.37,-23.46,;22.04,-21.15,;22.04,-19.62,;22.03,-15,;18.03,-17.31,;18.79,-18.64,;17.26,-18.64,;16.7,-16.54,;16.71,-15,;15.38,-14.23,;14.04,-15,;14.05,-16.55,;15.38,-17.31,;23.44,-12.55,)| Show InChI InChI=1S/C21H24N4O3S2/c1-14-13-22-21(29-14)25-20-12-18(30(27,28)17-5-3-2-4-6-17)11-19(24-20)23-15-7-9-16(26)10-8-15/h2-6,11-13,15-16,26H,7-10H2,1H3,(H2,22,23,24,25)/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037077
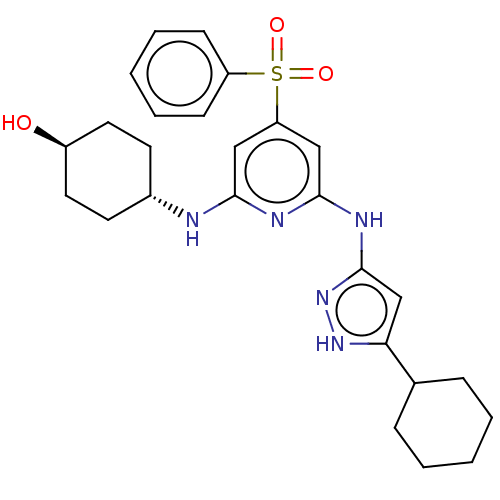
(CHEMBL3355738)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)C2CCCCC2)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(26.39,-31.95,;26.39,-30.41,;27.73,-29.64,;27.73,-28.09,;26.4,-27.34,;25.06,-28.1,;25.06,-29.63,;26.4,-25.8,;25.06,-25.03,;23.73,-25.8,;22.39,-25.03,;22.39,-23.48,;23.72,-22.71,;23.72,-21.17,;25.05,-20.4,;26.46,-21.03,;27.49,-19.88,;26.71,-18.55,;25.21,-18.88,;29.01,-20.04,;29.64,-21.44,;31.16,-21.6,;32.07,-20.36,;31.44,-18.95,;29.91,-18.79,;25.06,-23.48,;21.05,-25.79,;21.81,-27.12,;20.28,-27.12,;19.72,-25.03,;19.73,-23.49,;18.4,-22.72,;17.07,-23.49,;17.07,-25.03,;18.4,-25.8,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50174361
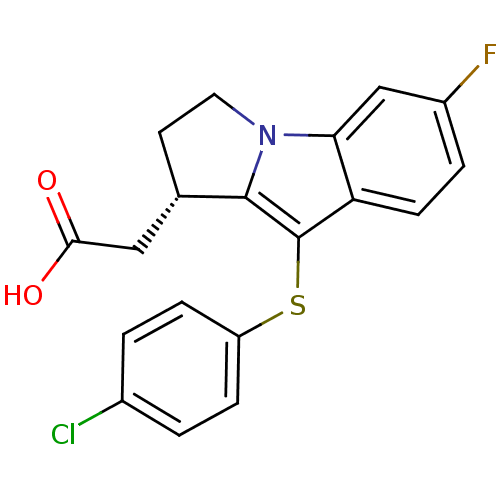
(CHEMBL370606 | L-888607 | [(S)-9-(4-Chloro-phenyls...)Show SMILES OC(=O)C[C@@H]1CCn2c1c(Sc1ccc(Cl)cc1)c1ccc(F)cc21 Show InChI InChI=1S/C19H15ClFNO2S/c20-12-1-4-14(5-2-12)25-19-15-6-3-13(21)10-16(15)22-8-7-11(18(19)22)9-17(23)24/h1-6,10-11H,7-9H2,(H,23,24)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037070

(CHEMBL3355732)Show SMILES COc1ccc(cc1)-c1cc(Nc2cc(cc(N[C@H]3CC[C@H](O)CC3)n2)S(=O)(=O)c2ccccc2)n[nH]1 |r,wU:18.18,wD:21.22,(12.08,-20.69,;11.44,-19.28,;9.91,-19.13,;9.01,-20.38,;7.48,-20.22,;6.85,-18.81,;7.74,-17.57,;9.27,-17.72,;5.32,-18.66,;4.29,-19.8,;2.88,-19.17,;1.55,-19.95,;1.55,-21.49,;.22,-22.26,;.22,-23.8,;1.55,-24.57,;2.89,-23.8,;4.23,-24.57,;4.23,-26.11,;5.56,-26.87,;5.56,-28.41,;4.22,-29.18,;4.22,-30.72,;2.89,-28.41,;2.89,-26.87,;2.89,-22.25,;-1.12,-24.57,;-.36,-25.9,;-1.9,-25.89,;-2.45,-23.8,;-2.45,-22.26,;-3.78,-21.49,;-5.12,-22.26,;-5.1,-23.81,;-3.78,-24.57,;3.04,-17.65,;4.54,-17.32,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037074

(CHEMBL3355736)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)C2CCC2)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(26.41,-16.6,;26.41,-15.06,;27.75,-14.29,;27.75,-12.74,;26.42,-11.99,;25.08,-12.75,;25.08,-14.28,;26.42,-10.45,;25.08,-9.68,;23.75,-10.45,;22.41,-9.68,;22.41,-8.14,;23.74,-7.36,;23.74,-5.82,;25.07,-5.05,;26.48,-5.68,;27.51,-4.53,;26.74,-3.2,;25.23,-3.53,;29.04,-4.69,;30.01,-5.89,;31.2,-4.92,;30.23,-3.72,;25.08,-8.13,;21.07,-10.45,;21.83,-11.78,;20.3,-11.77,;19.74,-9.68,;19.75,-8.14,;18.42,-7.37,;17.09,-8.14,;17.09,-9.68,;18.42,-10.45,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037071
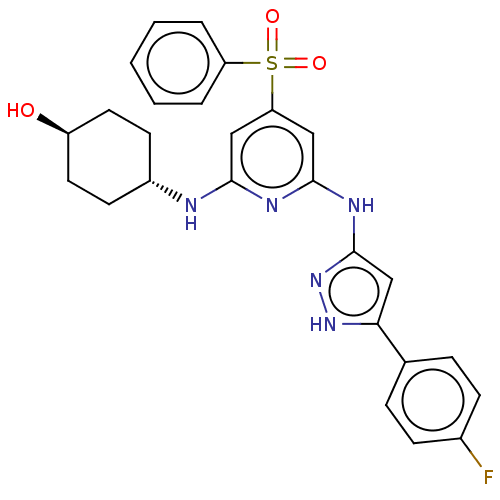
(CHEMBL3355733)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)-c2ccc(F)cc2)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(22.41,-30.72,;22.41,-29.18,;23.75,-28.41,;23.75,-26.87,;22.42,-26.11,;21.08,-26.87,;21.08,-28.41,;22.42,-24.57,;21.08,-23.8,;19.75,-24.57,;18.41,-23.8,;18.41,-22.26,;19.74,-21.49,;19.74,-19.95,;21.07,-19.17,;22.48,-19.8,;23.51,-18.66,;22.74,-17.32,;21.23,-17.65,;25.04,-18.81,;25.67,-20.22,;27.2,-20.38,;28.1,-19.13,;29.63,-19.28,;27.46,-17.72,;25.94,-17.57,;21.08,-22.25,;17.07,-24.57,;17.83,-25.9,;16.3,-25.89,;15.74,-23.8,;15.75,-22.26,;14.42,-21.49,;13.09,-22.26,;13.09,-23.81,;14.42,-24.57,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037068
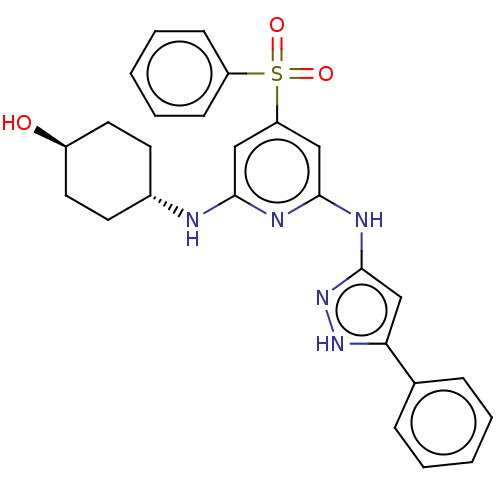
(CHEMBL3355730)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)-c2ccccc2)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(6.57,-15.01,;6.58,-13.47,;7.91,-12.7,;7.92,-11.16,;6.59,-10.4,;5.25,-11.16,;5.25,-12.7,;6.58,-8.86,;5.25,-8.09,;3.91,-8.86,;2.58,-8.09,;2.58,-6.55,;3.91,-5.78,;3.9,-4.24,;5.23,-3.46,;6.65,-4.09,;7.68,-2.95,;6.9,-1.62,;5.4,-1.94,;9.21,-3.1,;9.83,-4.51,;11.37,-4.67,;12.27,-3.42,;11.63,-2.01,;10.1,-1.86,;5.24,-6.54,;1.24,-8.86,;2,-10.19,;.47,-10.18,;-.1,-8.09,;-.09,-6.55,;-1.42,-5.78,;-2.76,-6.55,;-2.75,-8.1,;-1.42,-8.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442147
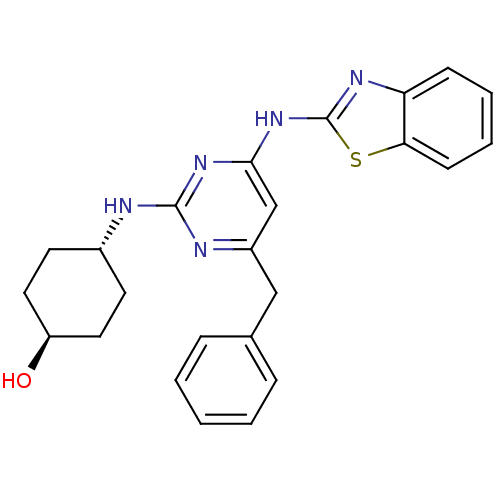
(CHEMBL2441269)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccccc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C24H25N5OS/c30-19-12-10-17(11-13-19)25-23-26-18(14-16-6-2-1-3-7-16)15-22(28-23)29-24-27-20-8-4-5-9-21(20)31-24/h1-9,15,17,19,30H,10-14H2,(H2,25,26,27,28,29)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037063
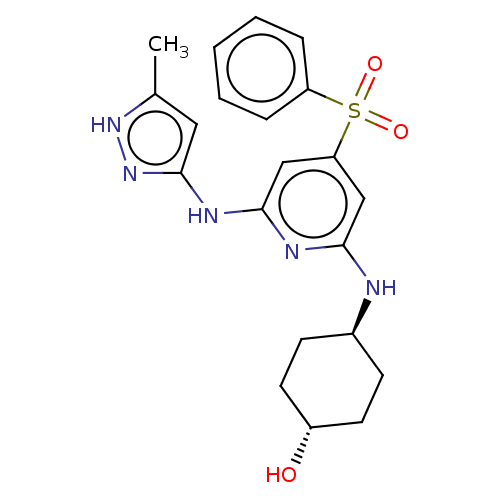
(CHEMBL3355726)Show SMILES Cc1cc(Nc2cc(cc(N[C@H]3CC[C@H](O)CC3)n2)S(=O)(=O)c2ccccc2)n[nH]1 |r,wU:11.10,wD:14.14,(15.1,-16.36,;13.56,-16.21,;12.54,-17.35,;11.12,-16.72,;9.79,-17.5,;9.8,-19.04,;8.47,-19.81,;8.46,-21.35,;9.8,-22.12,;11.14,-21.35,;12.47,-22.12,;12.47,-23.66,;13.81,-24.42,;13.8,-25.96,;12.47,-26.73,;12.46,-28.27,;11.13,-25.96,;11.14,-24.42,;11.13,-19.8,;7.12,-22.12,;7.89,-23.45,;6.35,-23.44,;5.8,-21.35,;5.81,-19.81,;4.48,-19.04,;3.14,-19.81,;3.14,-21.36,;4.48,-22.12,;11.28,-15.2,;12.79,-14.88,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037069
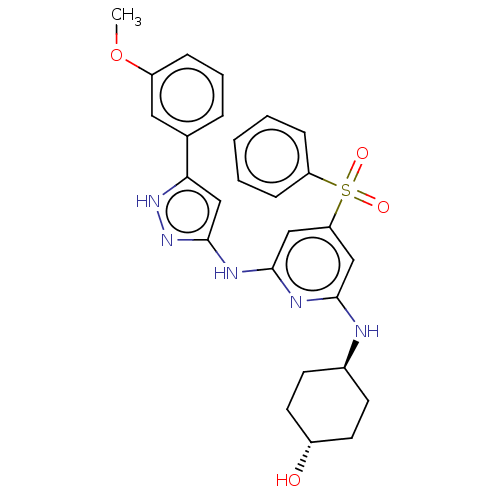
(CHEMBL3355731)Show SMILES COc1cccc(c1)-c1cc(Nc2cc(cc(N[C@H]3CC[C@H](O)CC3)n2)S(=O)(=O)c2ccccc2)n[nH]1 |r,wU:18.18,wD:21.22,(24.8,-7.73,;25.7,-6.49,;25.07,-5.08,;25.97,-3.83,;25.33,-2.42,;23.81,-2.27,;22.91,-3.52,;23.54,-4.92,;21.38,-3.36,;20.35,-4.51,;18.94,-3.88,;17.61,-4.65,;17.61,-6.19,;16.28,-6.96,;16.28,-8.51,;17.62,-9.28,;18.95,-8.5,;20.29,-9.27,;20.29,-10.81,;21.62,-11.57,;21.62,-13.11,;20.28,-13.88,;20.28,-15.42,;18.95,-13.11,;18.95,-11.58,;18.95,-6.95,;14.94,-9.27,;15.7,-10.6,;14.17,-10.6,;13.61,-8.5,;13.62,-6.96,;12.29,-6.19,;10.96,-6.96,;10.96,-8.51,;12.29,-9.27,;19.1,-2.35,;20.61,-2.03,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037067
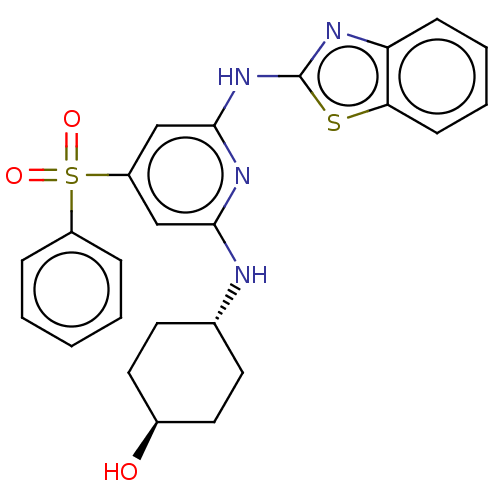
(CHEMBL3355729)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2nc3ccccc3s2)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(11.37,-36.31,;11.37,-34.77,;12.71,-34,;12.71,-32.45,;11.38,-31.69,;10.04,-32.46,;10.04,-33.99,;11.38,-30.15,;10.04,-29.39,;8.7,-30.16,;7.37,-29.39,;7.37,-27.84,;8.7,-27.07,;8.7,-25.53,;10.03,-24.76,;10.19,-23.23,;11.7,-22.91,;12.46,-21.58,;13.99,-21.57,;14.77,-22.9,;14,-24.24,;12.47,-24.24,;11.44,-25.39,;10.04,-27.84,;6.03,-30.15,;6.79,-31.48,;5.26,-31.48,;4.7,-29.39,;4.71,-27.85,;3.38,-27.07,;2.04,-27.84,;2.05,-29.39,;3.38,-30.16,)| Show InChI InChI=1S/C24H24N4O3S2/c29-17-12-10-16(11-13-17)25-22-14-19(33(30,31)18-6-2-1-3-7-18)15-23(27-22)28-24-26-20-8-4-5-9-21(20)32-24/h1-9,14-17,29H,10-13H2,(H2,25,26,27,28)/t16-,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037065
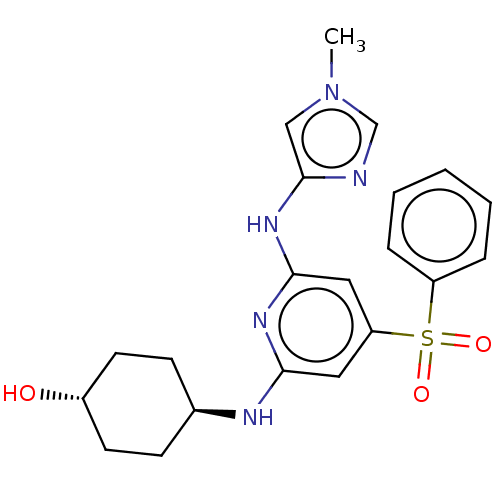
(CHEMBL3355727)Show SMILES Cn1cnc(Nc2cc(cc(N[C@H]3CC[C@H](O)CC3)n2)S(=O)(=O)c2ccccc2)c1 |r,wU:12.11,wD:15.15,(11.72,-7.94,;10.19,-7.78,;9.41,-6.45,;7.91,-6.78,;7.75,-8.3,;6.42,-9.08,;6.42,-10.61,;5.09,-11.39,;5.09,-12.93,;6.42,-13.7,;7.76,-12.93,;9.1,-13.7,;9.1,-15.24,;10.43,-15.99,;10.43,-17.54,;9.09,-18.31,;9.09,-19.85,;7.76,-17.53,;7.76,-16,;7.75,-11.38,;3.75,-13.69,;4.51,-15.02,;2.98,-15.02,;2.42,-12.93,;2.43,-11.39,;1.1,-10.62,;-.25,-11.39,;-.23,-12.93,;1.1,-13.7,;9.16,-8.93,)| Show InChI InChI=1S/C21H25N5O3S/c1-26-13-21(22-14-26)25-20-12-18(30(28,29)17-5-3-2-4-6-17)11-19(24-20)23-15-7-9-16(27)10-8-15/h2-6,11-16,27H,7-10H2,1H3,(H2,23,24,25)/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50174354
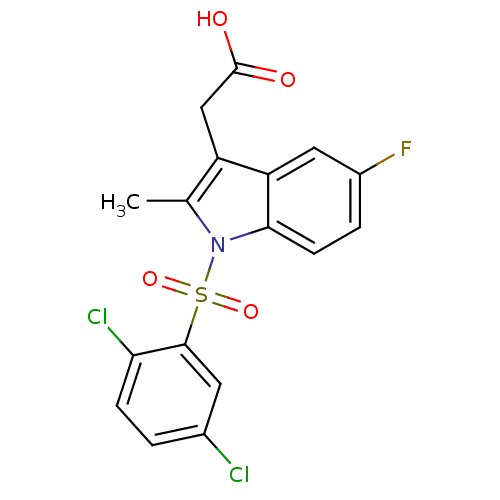
(CHEMBL373294 | [1-(2,5-Dichloro-benzenesulfonyl)-5...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1cc(Cl)ccc1Cl Show InChI InChI=1S/C17H12Cl2FNO4S/c1-9-12(8-17(22)23)13-7-11(20)3-5-15(13)21(9)26(24,25)16-6-10(18)2-4-14(16)19/h2-7H,8H2,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037072
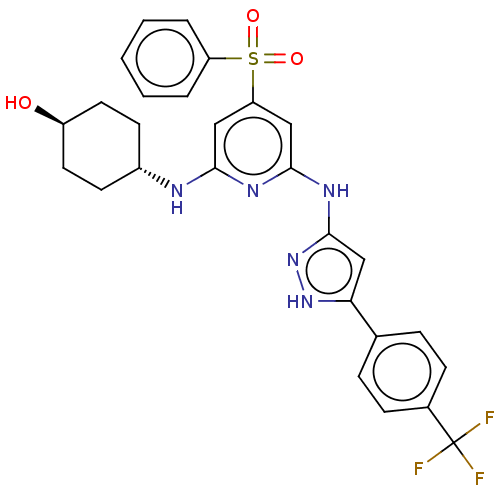
(CHEMBL3355734)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)-c2ccc(cc2)C(F)(F)F)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(7.54,-17.72,;7.54,-16.18,;8.88,-15.41,;8.88,-13.86,;7.55,-13.11,;6.21,-13.87,;6.21,-15.4,;7.55,-11.57,;6.21,-10.8,;4.88,-11.57,;3.54,-10.8,;3.54,-9.26,;4.87,-8.48,;4.87,-6.94,;6.2,-6.17,;7.61,-6.8,;8.64,-5.65,;7.87,-4.32,;6.36,-4.65,;10.17,-5.81,;10.8,-7.22,;12.33,-7.38,;13.23,-6.13,;12.6,-4.72,;11.07,-4.57,;14.77,-6.28,;15.4,-7.68,;15.67,-5.03,;16.3,-6.28,;6.21,-9.25,;2.2,-11.57,;2.96,-12.9,;1.43,-12.89,;.87,-10.8,;.88,-9.26,;-.45,-8.49,;-1.79,-9.26,;-1.78,-10.8,;-.46,-11.57,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50174357

(CHEMBL199040 | [1-(4-Chloro-benzenesulfonyl)-5-flu...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C17H13ClFNO4S/c1-10-14(9-17(21)22)15-8-12(19)4-7-16(15)20(10)25(23,24)13-5-2-11(18)3-6-13/h2-8H,9H2,1H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
In vitro binding affinity (agonistic) towards human CRTH2 receptor expressed in CHO cells; range 15 to 25 nM |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037060
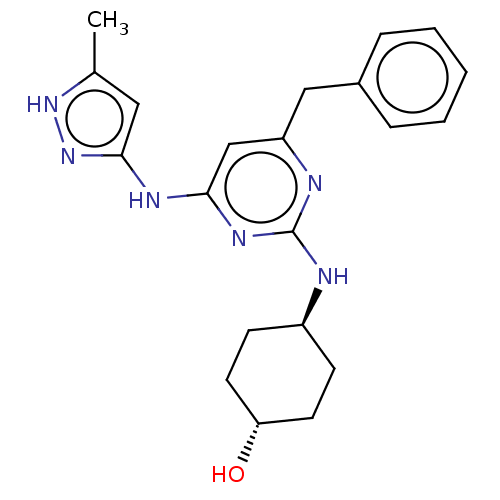
(CHEMBL3355311)Show SMILES Cc1cc(Nc2cc(Cc3ccccc3)nc(N[C@H]3CC[C@H](O)CC3)n2)n[nH]1 |r,wU:18.18,wD:21.22,(15.15,-15.42,;13.62,-15.27,;12.59,-16.41,;11.18,-15.78,;9.85,-16.56,;9.85,-18.1,;8.52,-18.87,;8.52,-20.41,;7.19,-21.18,;5.85,-20.41,;5.86,-18.87,;4.53,-18.1,;3.19,-18.87,;3.2,-20.42,;4.53,-21.18,;9.85,-21.18,;11.19,-20.41,;12.53,-21.18,;12.53,-22.72,;13.86,-23.48,;13.86,-25.02,;12.52,-25.79,;12.52,-27.33,;11.19,-25.02,;11.19,-23.48,;11.19,-18.86,;11.34,-14.26,;12.84,-13.93,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037073

(CHEMBL3355735)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)-c2ccc(OC(F)(F)F)cc2)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(5.56,-17.07,;5.57,-15.53,;6.9,-14.76,;6.91,-13.22,;5.58,-12.46,;4.24,-13.22,;4.24,-14.76,;5.57,-10.92,;4.24,-10.15,;2.9,-10.92,;1.57,-10.15,;1.57,-8.61,;2.9,-7.84,;2.89,-6.3,;4.22,-5.53,;5.64,-6.16,;6.66,-5.01,;5.89,-3.68,;4.39,-4,;8.2,-5.16,;8.82,-6.57,;10.35,-6.73,;11.26,-5.48,;12.79,-5.63,;13.42,-7.04,;14.95,-7.19,;12.52,-8.29,;14.18,-8.37,;10.62,-4.07,;9.09,-3.92,;4.23,-8.6,;.23,-10.92,;.99,-12.25,;-.56,-12.24,;-1.11,-10.15,;-1.1,-8.61,;-2.43,-7.84,;-3.77,-8.61,;-3.76,-10.16,;-2.43,-10.92,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50174351
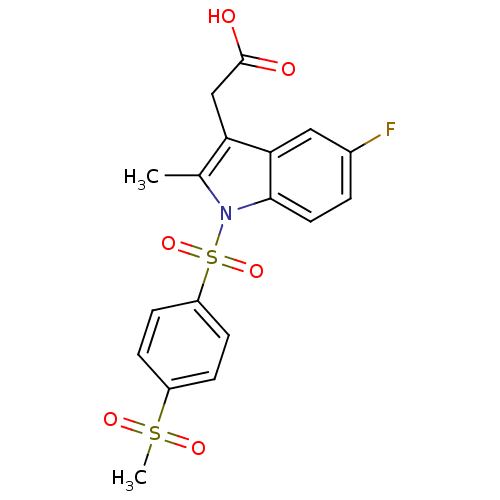
(CHEMBL196707 | [5-Fluoro-1-(4-methanesulfonyl-benz...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H16FNO6S2/c1-11-15(10-18(21)22)16-9-12(19)3-8-17(16)20(11)28(25,26)14-6-4-13(5-7-14)27(2,23)24/h3-9H,10H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50174352
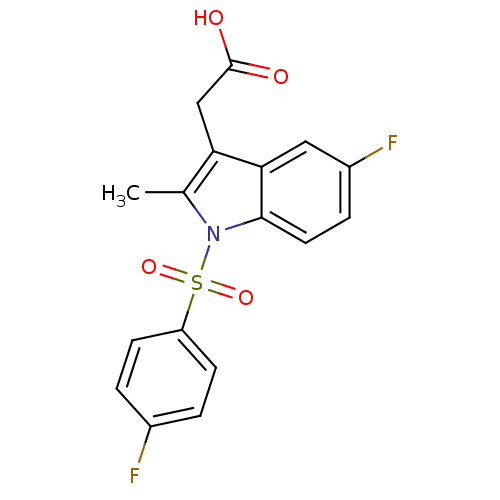
(CHEMBL370257 | [5-Fluoro-1-(4-fluoro-benzenesulfon...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C17H13F2NO4S/c1-10-14(9-17(21)22)15-8-12(19)4-7-16(15)20(10)25(23,24)13-5-2-11(18)3-6-13/h2-8H,9H2,1H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037062
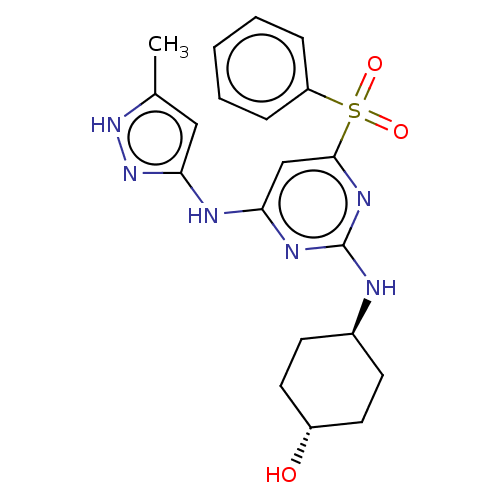
(CHEMBL3355312)Show SMILES Cc1cc(Nc2cc(nc(N[C@H]3CC[C@H](O)CC3)n2)S(=O)(=O)c2ccccc2)n[nH]1 |r,wU:11.10,wD:14.14,(15.1,-16.36,;13.56,-16.21,;12.54,-17.36,;11.12,-16.73,;9.79,-17.5,;9.8,-19.04,;8.47,-19.81,;8.47,-21.35,;9.8,-22.12,;11.14,-21.35,;12.47,-22.12,;12.47,-23.66,;13.81,-24.42,;13.8,-25.96,;12.47,-26.73,;12.46,-28.27,;11.13,-25.96,;11.14,-24.42,;11.13,-19.8,;7.12,-22.12,;7.89,-23.45,;6.35,-23.44,;5.8,-21.35,;5.81,-19.81,;4.48,-19.04,;3.14,-19.81,;3.14,-21.36,;4.48,-22.12,;11.28,-15.2,;12.79,-14.88,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50174353
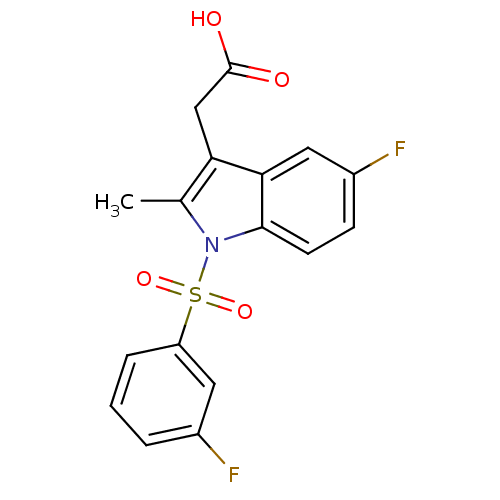
(CHEMBL196617 | [5-Fluoro-1-(3-fluoro-benzenesulfon...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C17H13F2NO4S/c1-10-14(9-17(21)22)15-8-12(19)5-6-16(15)20(10)25(23,24)13-4-2-3-11(18)7-13/h2-8H,9H2,1H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50174358

((1-Benzenesulfonyl-5-fluoro-2-methyl-1H-indol-3-yl...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C17H14FNO4S/c1-11-14(10-17(20)21)15-9-12(18)7-8-16(15)19(11)24(22,23)13-5-3-2-4-6-13/h2-9H,10H2,1H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50174360

(CHEMBL364299 | [5-Fluoro-1-(4-methoxy-benzenesulfo...)Show SMILES COc1ccc(cc1)S(=O)(=O)n1c(C)c(CC(O)=O)c2cc(F)ccc12 Show InChI InChI=1S/C18H16FNO5S/c1-11-15(10-18(21)22)16-9-12(19)3-8-17(16)20(11)26(23,24)14-6-4-13(25-2)5-7-14/h3-9H,10H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50174356
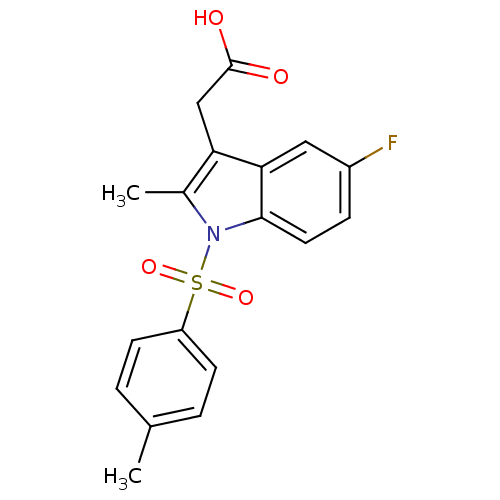
(CHEMBL193753 | [5-Fluoro-2-methyl-1-(toluene-4-sul...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C18H16FNO4S/c1-11-3-6-14(7-4-11)25(23,24)20-12(2)15(10-18(21)22)16-9-13(19)5-8-17(16)20/h3-9H,10H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50174359
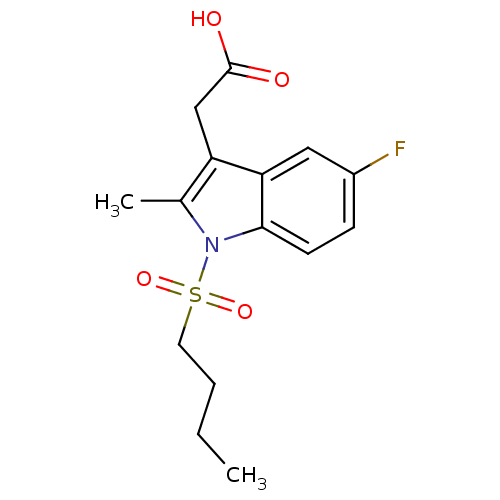
(CHEMBL194918 | [1-(Butane-1-sulfonyl)-5-fluoro-2-m...)Show InChI InChI=1S/C15H18FNO4S/c1-3-4-7-22(20,21)17-10(2)12(9-15(18)19)13-8-11(16)5-6-14(13)17/h5-6,8H,3-4,7,9H2,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 634 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50174354

(CHEMBL373294 | [1-(2,5-Dichloro-benzenesulfonyl)-5...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1cc(Cl)ccc1Cl Show InChI InChI=1S/C17H12Cl2FNO4S/c1-9-12(8-17(22)23)13-7-11(20)3-5-15(13)21(9)26(24,25)16-6-10(18)2-4-14(16)19/h2-7H,8H2,1H3,(H,22,23) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human DP receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50174355

(CHEMBL194019 | [1-(1,2-Dimethyl-1H-imidazole-4-sul...)Show SMILES Cc1nc(cn1C)S(=O)(=O)n1c(C)c(CC(O)=O)c2cc(F)ccc12 Show InChI InChI=1S/C16H16FN3O4S/c1-9-12(7-16(21)22)13-6-11(17)4-5-14(13)20(9)25(23,24)15-8-19(3)10(2)18-15/h4-6,8H,7H2,1-3H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037059
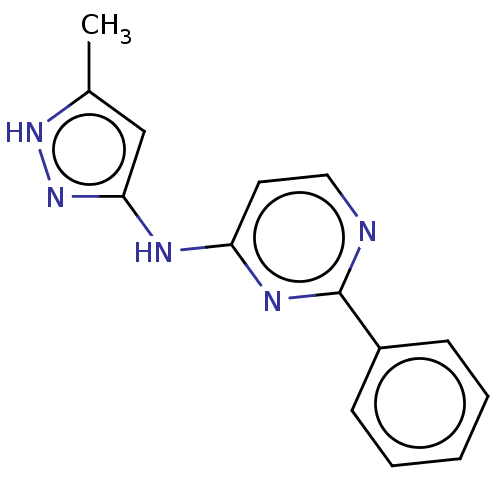
(CHEMBL3355310)Show InChI InChI=1S/C14H13N5/c1-10-9-13(19-18-10)16-12-7-8-15-14(17-12)11-5-3-2-4-6-11/h2-9H,1H3,(H2,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM50110164
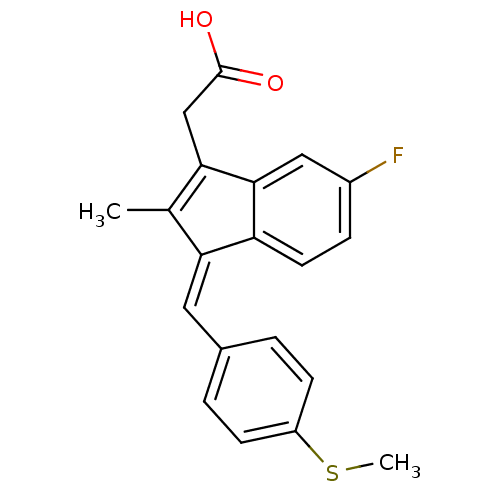
((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...)Show SMILES CSc1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:9| Show InChI InChI=1S/C20H17FO2S/c1-12-17(9-13-3-6-15(24-2)7-4-13)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50174358

((1-Benzenesulfonyl-5-fluoro-2-methyl-1H-indol-3-yl...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C17H14FNO4S/c1-11-14(10-17(20)21)15-9-12(18)7-8-16(15)19(11)24(22,23)13-5-3-2-4-6-13/h2-9H,10H2,1H3,(H,20,21) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human DP receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50174353

(CHEMBL196617 | [5-Fluoro-1-(3-fluoro-benzenesulfon...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C17H13F2NO4S/c1-10-14(9-17(21)22)15-8-12(19)5-6-16(15)20(10)25(23,24)13-4-2-3-11(18)7-13/h2-8H,9H2,1H3,(H,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human DP receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50174360

(CHEMBL364299 | [5-Fluoro-1-(4-methoxy-benzenesulfo...)Show SMILES COc1ccc(cc1)S(=O)(=O)n1c(C)c(CC(O)=O)c2cc(F)ccc12 Show InChI InChI=1S/C18H16FNO5S/c1-11-15(10-18(21)22)16-9-12(19)3-8-17(16)20(11)26(23,24)14-6-4-13(25-2)5-7-14/h3-9H,10H2,1-2H3,(H,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human DP receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human CRTH2 receptor expressed in CHO cells; range 25 nM to 8 uM |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50174357

(CHEMBL199040 | [1-(4-Chloro-benzenesulfonyl)-5-flu...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C17H13ClFNO4S/c1-10-14(9-17(21)22)15-8-12(19)4-7-16(15)20(10)25(23,24)13-5-2-11(18)3-6-13/h2-8H,9H2,1H3,(H,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human DP receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50174359

(CHEMBL194918 | [1-(Butane-1-sulfonyl)-5-fluoro-2-m...)Show InChI InChI=1S/C15H18FNO4S/c1-3-4-7-22(20,21)17-10(2)12(9-15(18)19)13-8-11(16)5-6-14(13)17/h5-6,8H,3-4,7,9H2,1-2H3,(H,18,19) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human DP receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50174351

(CHEMBL196707 | [5-Fluoro-1-(4-methanesulfonyl-benz...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H16FNO6S2/c1-11-15(10-18(21)22)16-9-12(19)3-8-17(16)20(11)28(25,26)14-6-4-13(5-7-14)27(2,23)24/h3-9H,10H2,1-2H3,(H,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human DP receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50174356

(CHEMBL193753 | [5-Fluoro-2-methyl-1-(toluene-4-sul...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C18H16FNO4S/c1-11-3-6-14(7-4-11)25(23,24)20-12(2)15(10-18(21)22)16-9-13(19)5-8-17(16)20/h3-9H,10H2,1-2H3,(H,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human DP receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50174352

(CHEMBL370257 | [5-Fluoro-1-(4-fluoro-benzenesulfon...)Show SMILES Cc1c(CC(O)=O)c2cc(F)ccc2n1S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C17H13F2NO4S/c1-10-14(9-17(21)22)15-8-12(19)4-7-16(15)20(10)25(23,24)13-5-2-11(18)3-6-13/h2-8H,9H2,1H3,(H,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human DP receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50174355

(CHEMBL194019 | [1-(1,2-Dimethyl-1H-imidazole-4-sul...)Show SMILES Cc1nc(cn1C)S(=O)(=O)n1c(C)c(CC(O)=O)c2cc(F)ccc12 Show InChI InChI=1S/C16H16FN3O4S/c1-9-12(7-16(21)22)13-6-11(17)4-5-14(13)20(9)25(23,24)15-8-19(3)10(2)18-15/h4-6,8H,7H2,1-3H3,(H,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oxagen Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human DP receptor expressed in CHO cells |
J Med Chem 48: 6174-7 (2005)
Article DOI: 10.1021/jm050519b
BindingDB Entry DOI: 10.7270/Q2KK9B9B |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072078
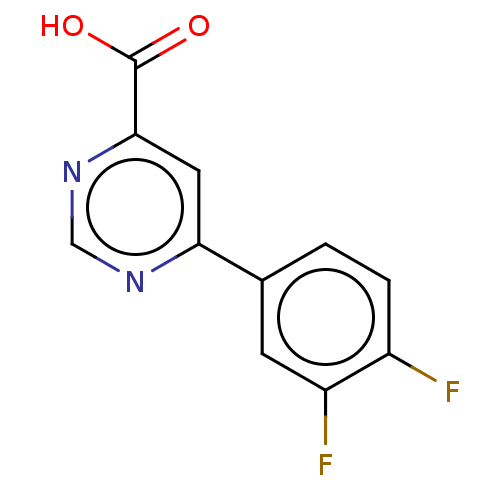
(CHEMBL3407905)Show InChI InChI=1S/C11H6F2N2O2/c12-7-2-1-6(3-8(7)13)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072123
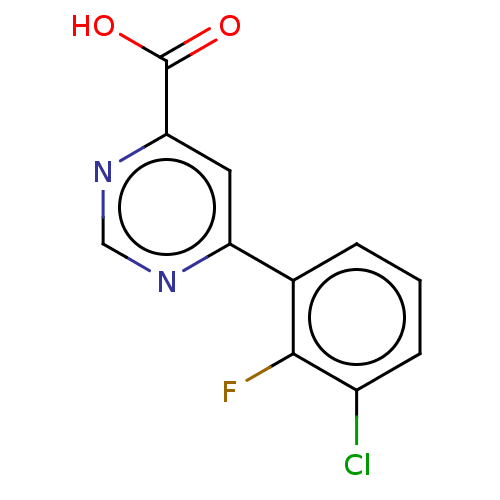
(CHEMBL3407904)Show InChI InChI=1S/C11H6ClFN2O2/c12-7-3-1-2-6(10(7)13)8-4-9(11(16)17)15-5-14-8/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072122
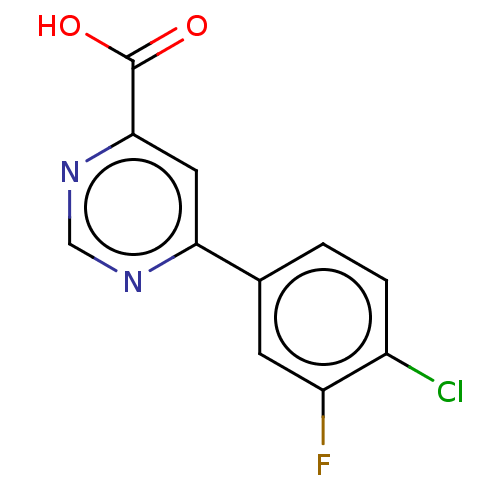
(CHEMBL3407903)Show InChI InChI=1S/C11H6ClFN2O2/c12-7-2-1-6(3-8(7)13)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072120
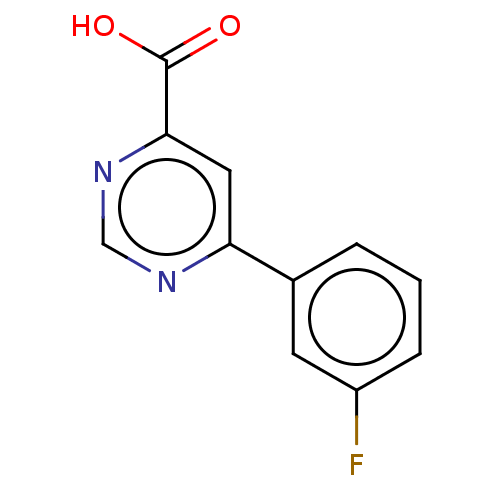
(CHEMBL3407901)Show InChI InChI=1S/C11H7FN2O2/c12-8-3-1-2-7(4-8)9-5-10(11(15)16)14-6-13-9/h1-6H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072082
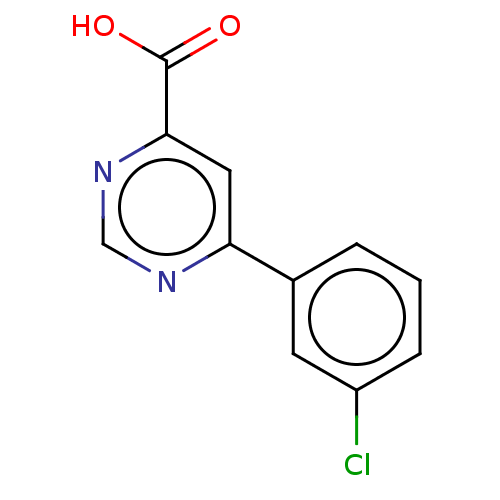
(CHEMBL3407865)Show InChI InChI=1S/C11H7ClN2O2/c12-8-3-1-2-7(4-8)9-5-10(11(15)16)14-6-13-9/h1-6H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072081
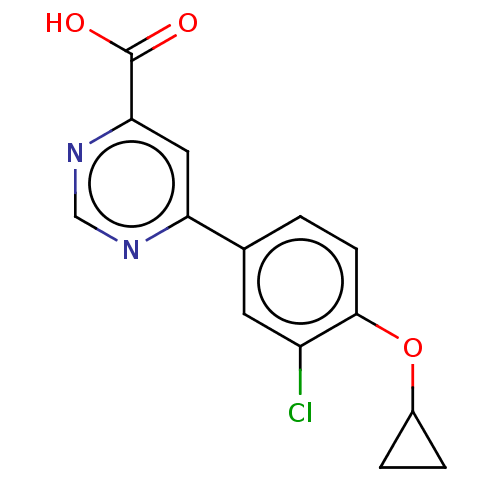
(CHEMBL3407922)Show InChI InChI=1S/C14H11ClN2O3/c15-10-5-8(1-4-13(10)20-9-2-3-9)11-6-12(14(18)19)17-7-16-11/h1,4-7,9H,2-3H2,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072077

(CHEMBL3407866)Show InChI InChI=1S/C11H6Cl2N2O2/c12-7-2-1-6(3-8(7)13)9-4-10(11(16)17)15-5-14-9/h1-5H,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072079
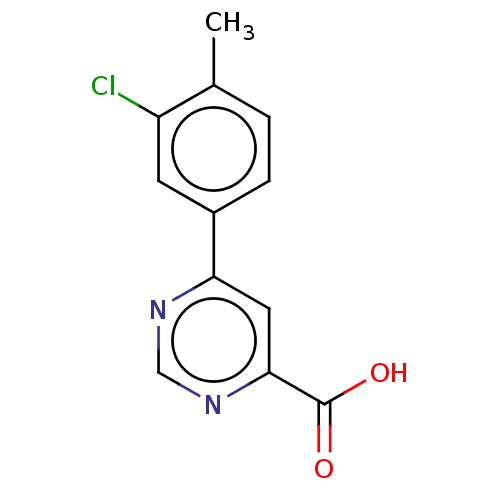
(CHEMBL3407913)Show InChI InChI=1S/C12H9ClN2O2/c1-7-2-3-8(4-9(7)13)10-5-11(12(16)17)15-6-14-10/h2-6H,1H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50072131
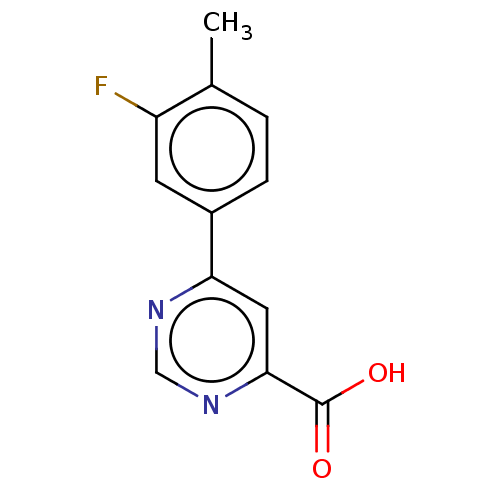
(CHEMBL3407914)Show InChI InChI=1S/C12H9FN2O2/c1-7-2-3-8(4-9(7)13)10-5-11(12(16)17)15-6-14-10/h2-6H,1H3,(H,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (U.K.) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysis |
J Med Chem 58: 1159-83 (2015)
Article DOI: 10.1021/jm501350y
BindingDB Entry DOI: 10.7270/Q2445P5N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data