 Found 914 hits with Last Name = 'brown' and Initial = 'ad'
Found 914 hits with Last Name = 'brown' and Initial = 'ad' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50066789
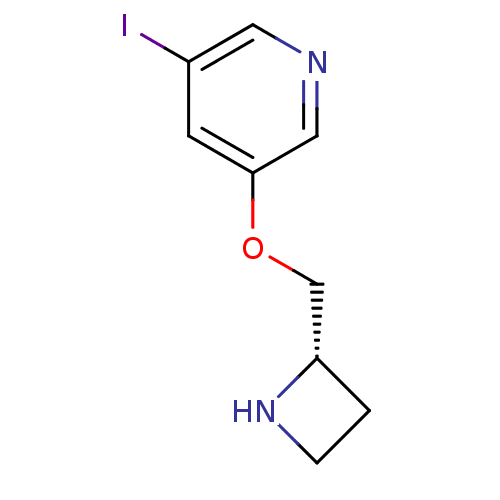
(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from alpha4beta2 nAChR in rat brain membrane |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to alpha4beta2 nAChR in rat cortex |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50354983
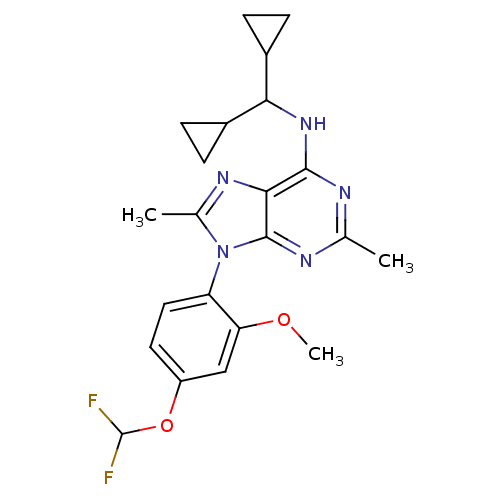
(CHEMBL1836947)Show SMILES COc1cc(OC(F)F)ccc1-n1c(C)nc2c(NC(C3CC3)C3CC3)nc(C)nc12 |(44.5,-44.78,;42.97,-44.81,;42.22,-46.16,;43.02,-47.47,;42.27,-48.83,;43.06,-50.15,;42.32,-51.49,;43.11,-52.81,;40.78,-51.52,;40.73,-48.85,;39.94,-47.53,;40.69,-46.19,;39.9,-44.87,;40.81,-43.62,;42.35,-43.62,;39.9,-42.36,;38.42,-42.84,;37.09,-42.08,;37.08,-40.54,;35.75,-39.77,;35.74,-38.23,;36.51,-36.91,;34.97,-36.91,;34.42,-40.55,;32.88,-40.55,;33.65,-41.88,;35.76,-42.85,;35.76,-44.39,;34.42,-45.16,;37.09,-45.16,;38.42,-44.39,)| Show InChI InChI=1S/C22H25F2N5O2/c1-11-25-20(28-18(13-4-5-13)14-6-7-14)19-21(26-11)29(12(2)27-19)16-9-8-15(31-22(23)24)10-17(16)30-3/h8-10,13-14,18,22H,4-7H2,1-3H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.291 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CRF1 receptor expressed in CHO cells assessed as inhibition of CRF-induced cAMP formation |
Bioorg Med Chem Lett 21: 6108-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.040
BindingDB Entry DOI: 10.7270/Q2F19046 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113787

(CHEMBL81056 | S-(2-{2-[2-(4-Carbamimidoyl-phenoxy)...)Show SMILES NC(=N)c1ccc(OCC[C@@H]2CCCCN2C(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 Show InChI InChI=1S/C25H38N4O4/c26-24(27)19-9-11-21(12-10-19)33-15-13-20-8-4-5-14-29(20)25(32)22(28-17-23(30)31)16-18-6-2-1-3-7-18/h9-12,18,20,22,28H,1-8,13-17H2,(H3,26,27)(H,30,31)/t20-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50354981
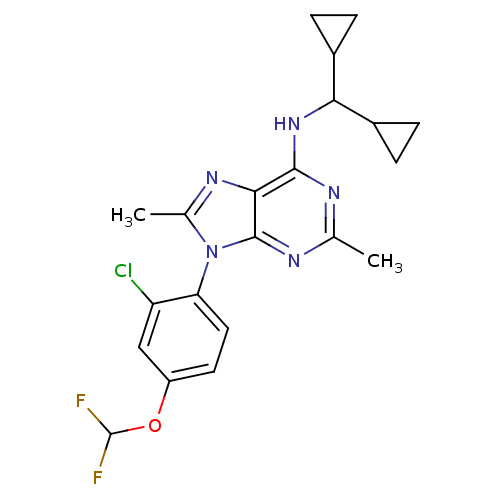
(CHEMBL1836945)Show SMILES Cc1nc2c(NC(C3CC3)C3CC3)nc(C)nc2n1-c1ccc(OC(F)F)cc1Cl |(10.94,-43.27,;9.4,-43.27,;8.49,-42.02,;7.02,-42.5,;5.68,-41.73,;5.68,-40.19,;4.34,-39.43,;4.34,-37.89,;5.1,-36.57,;3.56,-36.56,;3.01,-40.2,;1.47,-40.21,;2.24,-41.54,;4.35,-42.51,;4.35,-44.05,;3.02,-44.82,;5.69,-44.82,;7.02,-44.05,;8.49,-44.53,;9.28,-45.85,;8.53,-47.19,;9.32,-48.51,;10.86,-48.49,;11.66,-49.8,;10.91,-51.15,;11.7,-52.47,;9.37,-51.18,;11.61,-47.13,;10.82,-45.82,;11.56,-44.47,)| Show InChI InChI=1S/C21H22ClF2N5O/c1-10-25-19(28-17(12-3-4-12)13-5-6-13)18-20(26-10)29(11(2)27-18)16-8-7-14(9-15(16)22)30-21(23)24/h7-9,12-13,17,21H,3-6H2,1-2H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.373 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CRF1 receptor expressed in CHO cells assessed as inhibition of CRF-induced cAMP formation |
Bioorg Med Chem Lett 21: 6108-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.040
BindingDB Entry DOI: 10.7270/Q2F19046 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50067424
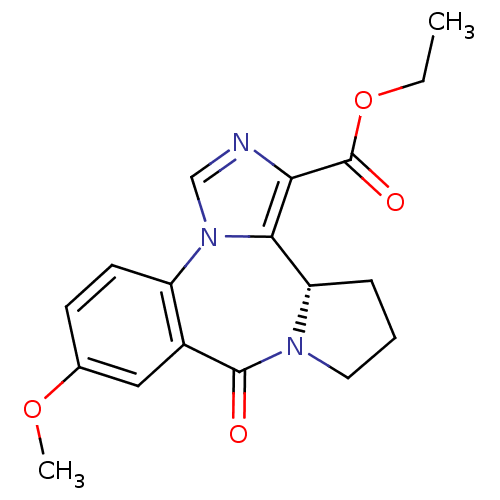
((S)-9-Methoxy-7-oxo-3b,4,5,6-tetrahydro-7H-2,6a,11...)Show SMILES CCOC(=O)c1ncn-2c1[C@@H]1CCCN1C(=O)c1cc(OC)ccc-21 Show InChI InChI=1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50142570
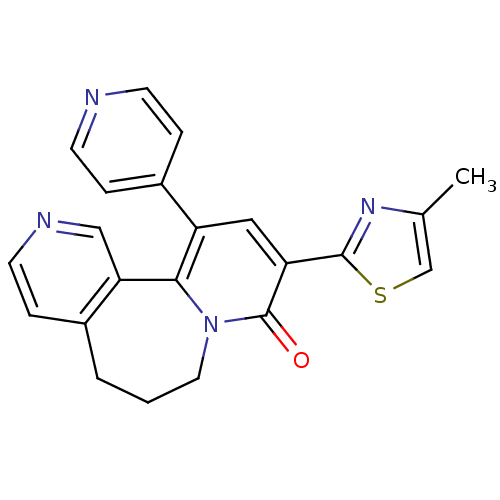
(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113790
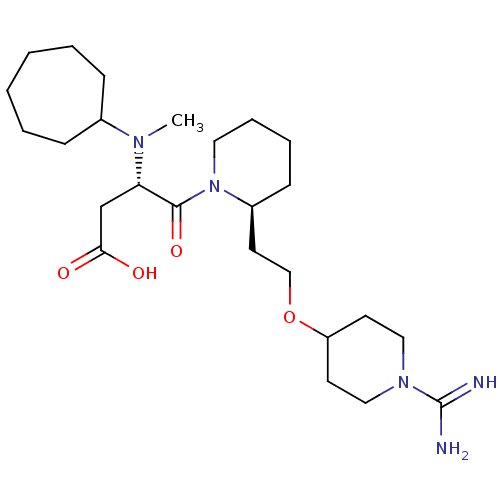
(CHEMBL84389 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...)Show SMILES CN([C@@H](CC(O)=O)C(=O)N1CCCC[C@H]1CCOC1CCN(CC1)C(N)=N)C1CCCCCC1 Show InChI InChI=1S/C25H45N5O4/c1-28(19-8-4-2-3-5-9-19)22(18-23(31)32)24(33)30-14-7-6-10-20(30)13-17-34-21-11-15-29(16-12-21)25(26)27/h19-22H,2-18H2,1H3,(H3,26,27)(H,31,32)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50142570

(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50179998
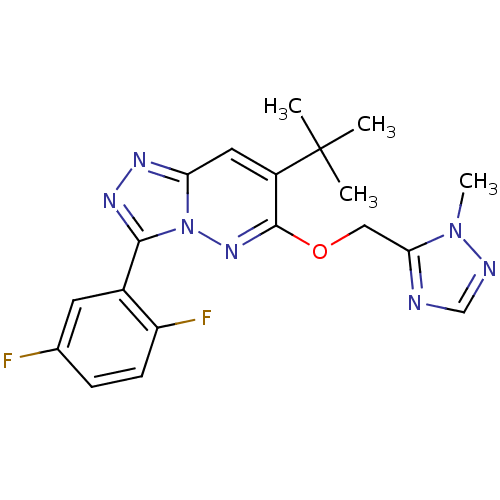
(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50179998

(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha2 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113791

(CHEMBL84229 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...)Show SMILES CN([C@@H](CC(O)=O)C(=O)N1CCCC[C@H]1CCOC1CCN(CC1)C(N)=N)C1CCC=CCC1 |c:32| Show InChI InChI=1S/C25H43N5O4/c1-28(19-8-4-2-3-5-9-19)22(18-23(31)32)24(33)30-14-7-6-10-20(30)13-17-34-21-11-15-29(16-12-21)25(26)27/h2-3,19-22H,4-18H2,1H3,(H3,26,27)(H,31,32)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha3 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113785
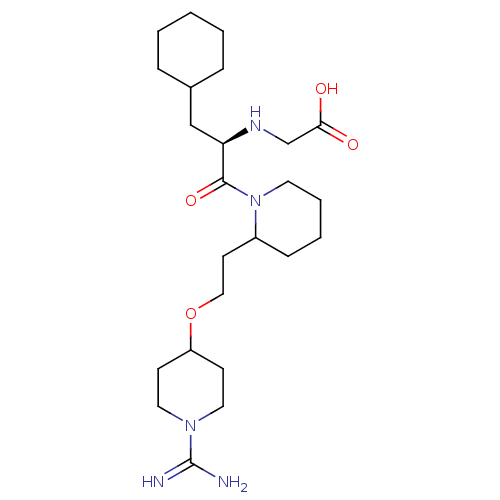
(CHEMBL309670 | RS-(2-{2-[2-(1-Carbamimidoyl-piperi...)Show SMILES NC(=N)N1CCC(CC1)OCCC1CCCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O Show InChI InChI=1S/C24H43N5O4/c25-24(26)28-13-9-20(10-14-28)33-15-11-19-8-4-5-12-29(19)23(32)21(27-17-22(30)31)16-18-6-2-1-3-7-18/h18-21,27H,1-17H2,(H3,25,26)(H,30,31)/t19?,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113799
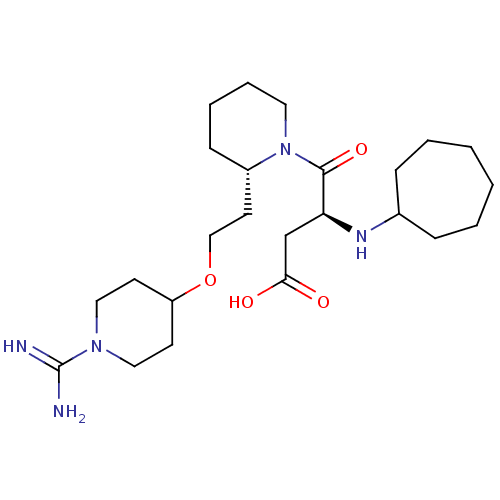
(CHEMBL309403 | S-4-{2-[2-(1-Carbamimidoyl-piperidi...)Show SMILES NC(=N)N1CCC(CC1)OCC[C@@H]1CCCCN1C(=O)[C@H](CC(O)=O)NC1CCCCCC1 Show InChI InChI=1S/C24H43N5O4/c25-24(26)28-14-10-20(11-15-28)33-16-12-19-9-5-6-13-29(19)23(32)21(17-22(30)31)27-18-7-3-1-2-4-8-18/h18-21,27H,1-17H2,(H3,25,26)(H,30,31)/t19-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM50142570

(9-(4-Methyl-thiazol-2-yl)-11-pyridin-4-yl-6,7-dihy...)Show SMILES Cc1csc(n1)-c1cc(-c2ccncc2)c2-c3cnccc3CCCn2c1=O Show InChI InChI=1S/C22H18N4OS/c1-14-13-28-21(25-14)18-11-17(16-4-7-23-8-5-16)20-19-12-24-9-6-15(19)3-2-10-26(20)22(18)27/h4-9,11-13H,2-3,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha1 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113794
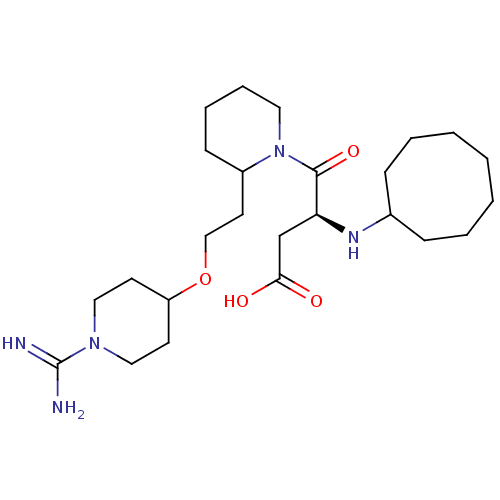
(CHEMBL82658 | RS-4-{2-[2-(1-Carbamimidoyl-piperidi...)Show SMILES NC(=N)N1CCC(CC1)OCCC1CCCCN1C(=O)[C@H](CC(O)=O)NC1CCCCCCC1 Show InChI InChI=1S/C25H45N5O4/c26-25(27)29-15-11-21(12-16-29)34-17-13-20-10-6-7-14-30(20)24(33)22(18-23(31)32)28-19-8-4-2-1-3-5-9-19/h19-22,28H,1-18H2,(H3,26,27)(H,31,32)/t20?,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50156083

(3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...)Show SMILES Cc1cc(no1)-c1nncc2c(c(OCc3ncnn3C)nn12)C(C)(C)C Show InChI InChI=1S/C17H20N8O2/c1-10-6-11(23-27-10)15-21-19-7-12-14(17(2,3)4)16(22-25(12)15)26-8-13-18-9-20-24(13)5/h6-7,9H,8H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50087713
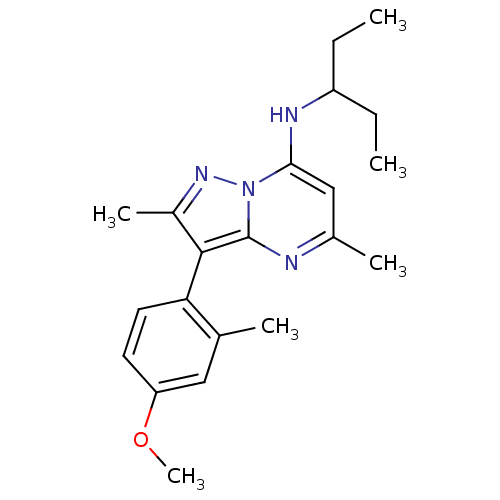
((1-Ethyl-propyl)-[3-(4-methoxy-2-methyl-phenyl)-2,...)Show SMILES CCC(CC)Nc1cc(C)nc2c(c(C)nn12)-c1ccc(OC)cc1C |(-4.03,1.25,;-2.68,.48,;-1.35,1.27,;-1.37,2.81,;-2.86,3.21,;-.02,.5,;-.02,-1.04,;-1.34,-1.81,;-1.34,-3.35,;-2.66,-4.12,;,-4.12,;1.33,-3.35,;2.8,-3.8,;3.7,-2.55,;5.24,-2.53,;2.77,-1.32,;1.33,-1.81,;3.3,-5.26,;2.28,-6.39,;2.77,-7.87,;4.28,-8.16,;4.78,-9.63,;3.76,-10.79,;5.3,-7,;4.79,-5.55,;5.82,-4.4,)| Show InChI InChI=1S/C21H28N4O/c1-7-16(8-2)23-19-12-14(4)22-21-20(15(5)24-25(19)21)18-10-9-17(26-6)11-13(18)3/h9-12,16,23H,7-8H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CRF1 receptor expressed in CHO cells assessed as inhibition of CRF-induced cAMP formation |
Bioorg Med Chem Lett 21: 6108-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.040
BindingDB Entry DOI: 10.7270/Q2F19046 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113802
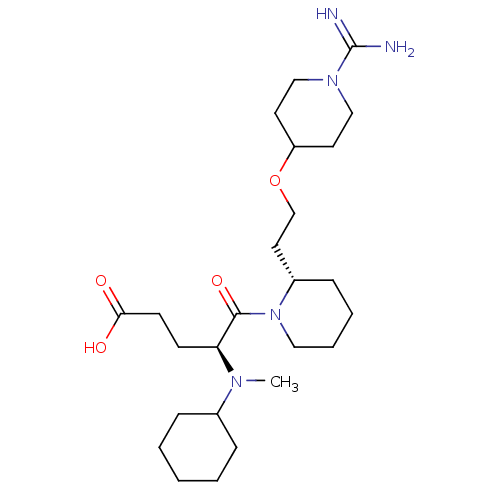
(5-{2-[2-(1-Carbamimidoyl-piperidin-4-yloxy)-ethyl]...)Show SMILES CN([C@@H](CCC(O)=O)C(=O)N1CCCC[C@H]1CCOC1CCN(CC1)C(N)=N)C1CCCCC1 Show InChI InChI=1S/C25H45N5O4/c1-28(19-7-3-2-4-8-19)22(10-11-23(31)32)24(33)30-15-6-5-9-20(30)14-18-34-21-12-16-29(17-13-21)25(26)27/h19-22H,2-18H2,1H3,(H3,26,27)(H,31,32)/t20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113792
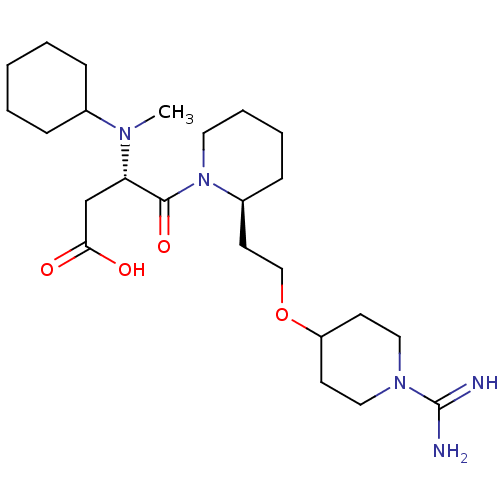
(CHEMBL81521 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...)Show SMILES CN([C@@H](CC(O)=O)C(=O)N1CCCC[C@H]1CCOC1CCN(CC1)C(N)=N)C1CCCCC1 Show InChI InChI=1S/C24H43N5O4/c1-27(18-7-3-2-4-8-18)21(17-22(30)31)23(32)29-13-6-5-9-19(29)12-16-33-20-10-14-28(15-11-20)24(25)26/h18-21H,2-17H2,1H3,(H3,25,26)(H,30,31)/t19-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(Homo sapiens (Human)) | BDBM50179998

(7-tert-butyl-3-(2,5-difluorophenyl)-6-((2-methyl-2...)Show SMILES Cn1ncnc1COc1nn2c(nnc2cc1C(C)(C)C)-c1cc(F)ccc1F Show InChI InChI=1S/C19H19F2N7O/c1-19(2,3)13-8-15-24-25-17(12-7-11(20)5-6-14(12)21)28(15)26-18(13)29-9-16-22-10-23-27(16)4/h5-8,10H,9H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of GABAA alpha5 (unknown origin) |
J Med Chem 56: 593-624 (2013)
Article DOI: 10.1021/jm3011433
BindingDB Entry DOI: 10.7270/Q23B61GZ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Bos taurus) | BDBM50113792

(CHEMBL81521 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...)Show SMILES CN([C@@H](CC(O)=O)C(=O)N1CCCC[C@H]1CCOC1CCN(CC1)C(N)=N)C1CCCCC1 Show InChI InChI=1S/C24H43N5O4/c1-27(18-7-3-2-4-8-18)21(17-22(30)31)23(32)29-13-6-5-9-19(29)12-16-33-20-10-14-28(15-11-20)24(25)26/h18-21H,2-17H2,1H3,(H3,25,26)(H,30,31)/t19-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory effect on plasmin in bovine plasma |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315675
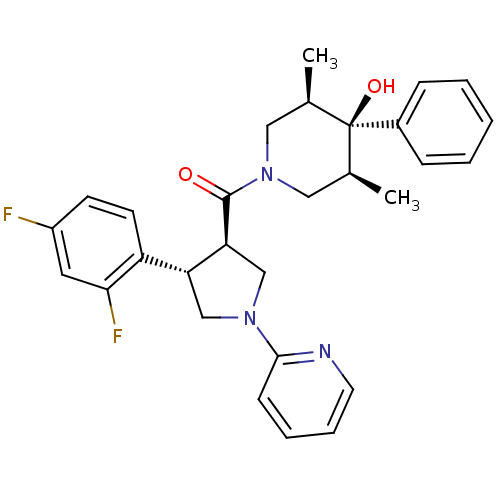
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridin-2-yl)py...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1ccccn1 |r| Show InChI InChI=1S/C29H31F2N3O2/c1-19-15-34(16-20(2)29(19,36)21-8-4-3-5-9-21)28(35)25-18-33(27-10-6-7-13-32-27)17-24(25)23-12-11-22(30)14-26(23)31/h3-14,19-20,24-25,36H,15-18H2,1-2H3/t19-,20+,24-,25+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Bos taurus) | BDBM50113792

(CHEMBL81521 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...)Show SMILES CN([C@@H](CC(O)=O)C(=O)N1CCCC[C@H]1CCOC1CCN(CC1)C(N)=N)C1CCCCC1 Show InChI InChI=1S/C24H43N5O4/c1-27(18-7-3-2-4-8-18)21(17-22(30)31)23(32)29-13-6-5-9-19(29)12-16-33-20-10-14-28(15-11-20)24(25)26/h18-21H,2-17H2,1H3,(H3,25,26)(H,30,31)/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory effect on Coagulation factor Xa (FXa) from bovine plasma |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315688
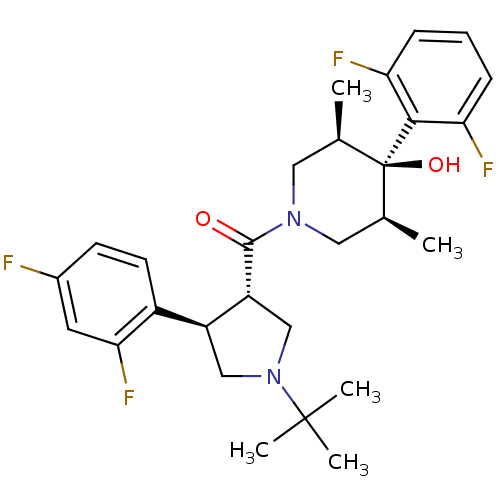
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1c(F)cccc1F)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H34F4N2O2/c1-16-12-33(13-17(2)28(16,36)25-22(30)7-6-8-23(25)31)26(35)21-15-34(27(3,4)5)14-20(21)19-10-9-18(29)11-24(19)32/h6-11,16-17,20-21,36H,12-15H2,1-5H3/t16-,17+,20-,21+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50354980
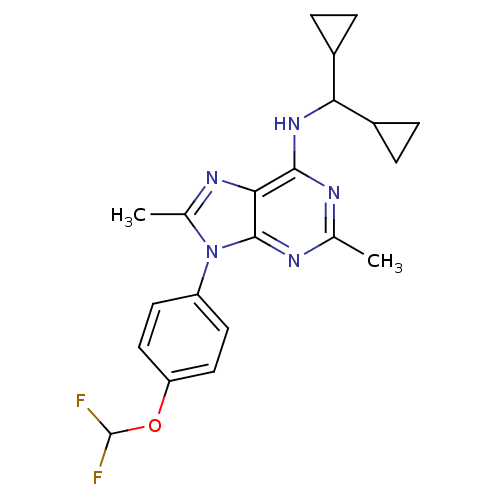
(CHEMBL1836944)Show SMILES Cc1nc2c(NC(C3CC3)C3CC3)nc(C)nc2n1-c1ccc(OC(F)F)cc1 Show InChI InChI=1S/C21H23F2N5O/c1-11-24-19(27-17(13-3-4-13)14-5-6-14)18-20(25-11)28(12(2)26-18)15-7-9-16(10-8-15)29-21(22)23/h7-10,13-14,17,21H,3-6H2,1-2H3,(H,24,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CRF1 receptor expressed in CHO cells assessed as inhibition of CRF-induced cAMP formation |
Bioorg Med Chem Lett 21: 6108-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.040
BindingDB Entry DOI: 10.7270/Q2F19046 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315674
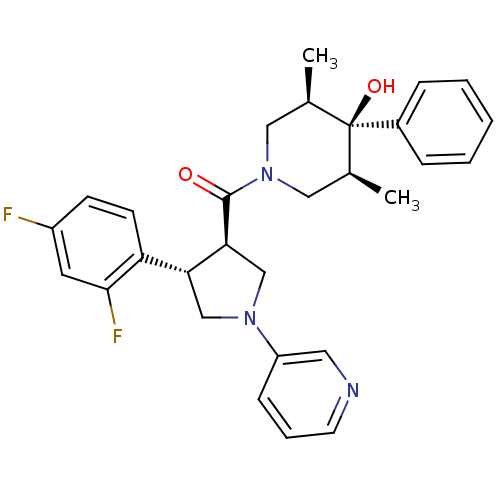
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridin-3-yl)py...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnc1 |r| Show InChI InChI=1S/C29H31F2N3O2/c1-19-15-34(16-20(2)29(19,36)21-7-4-3-5-8-21)28(35)26-18-33(23-9-6-12-32-14-23)17-25(26)24-11-10-22(30)13-27(24)31/h3-14,19-20,25-26,36H,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315676
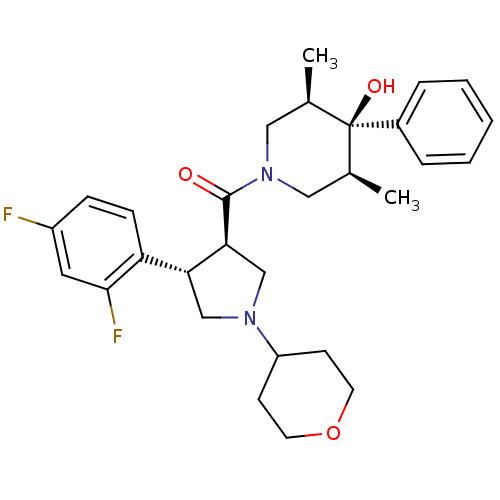
(((3R,4S)-4-(2,4-difluorophenyl)-1-(tetrahydro-2H-p...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCOCC1 |r| Show InChI InChI=1S/C29H36F2N2O3/c1-19-15-33(16-20(2)29(19,35)21-6-4-3-5-7-21)28(34)26-18-32(23-10-12-36-13-11-23)17-25(26)24-9-8-22(30)14-27(24)31/h3-9,14,19-20,23,25-26,35H,10-13,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315686
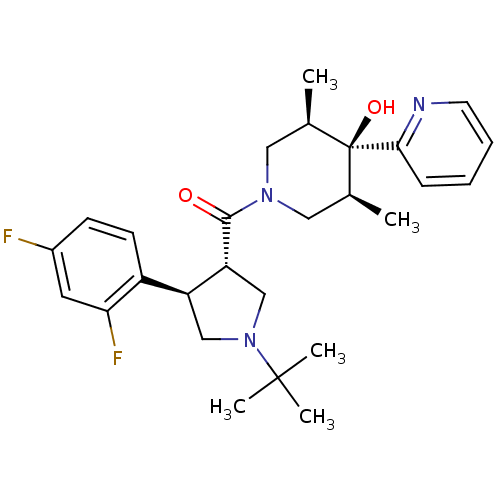
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccn1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C27H35F2N3O2/c1-17-13-31(14-18(2)27(17,34)24-8-6-7-11-30-24)25(33)22-16-32(26(3,4)5)15-21(22)20-10-9-19(28)12-23(20)29/h6-12,17-18,21-22,34H,13-16H2,1-5H3/t17-,18+,21-,22+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315677

(((3R,4S)-1-cyclobutyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCC1 |r| Show InChI InChI=1S/C28H34F2N2O2/c1-18-14-32(15-19(2)28(18,34)20-7-4-3-5-8-20)27(33)25-17-31(22-9-6-10-22)16-24(25)23-12-11-21(29)13-26(23)30/h3-5,7-8,11-13,18-19,22,24-25,34H,6,9-10,14-17H2,1-2H3/t18-,19+,24-,25+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50257624
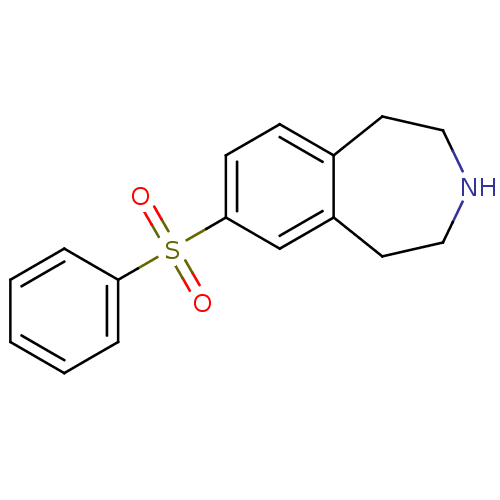
(7-(phenylsulfonyl)-2,3,4,5-tetrahydro-1H-benzo[d]a...)Show InChI InChI=1S/C16H17NO2S/c18-20(19,15-4-2-1-3-5-15)16-7-6-13-8-10-17-11-9-14(13)12-16/h1-7,12,17H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine form human recombinant 5HT2C receptor expressed in mouse Swiss 3T3 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1871-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.071
BindingDB Entry DOI: 10.7270/Q2GB23XH |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315680
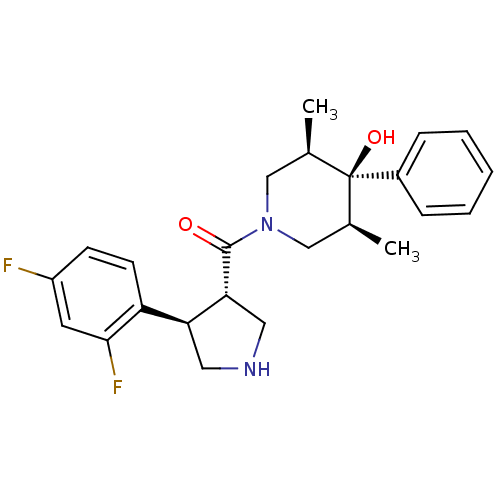
(((3S,4R)-4-(2,4-difluorophenyl)pyrrolidin-3-yl)((3...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@@H]1CNC[C@H]1c1ccc(F)cc1F |r| Show InChI InChI=1S/C24H28F2N2O2/c1-15-13-28(14-16(2)24(15,30)17-6-4-3-5-7-17)23(29)21-12-27-11-20(21)19-9-8-18(25)10-22(19)26/h3-10,15-16,20-21,27,30H,11-14H2,1-2H3/t15-,16+,20-,21+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50257613
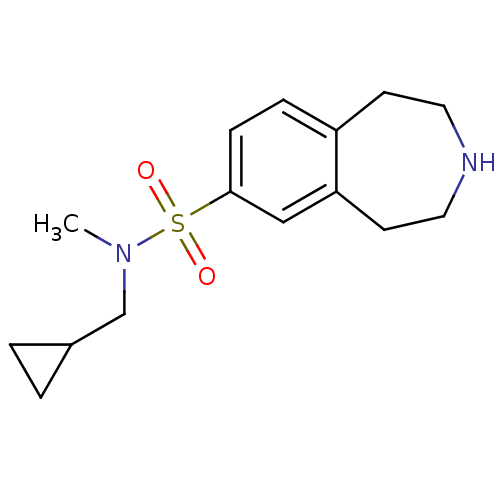
(CHEMBL493951 | N-(cyclopropylmethyl)-N-methyl-2,3,...)Show InChI InChI=1S/C15H22N2O2S/c1-17(11-12-2-3-12)20(18,19)15-5-4-13-6-8-16-9-7-14(13)10-15/h4-5,10,12,16H,2-3,6-9,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine form human recombinant 5HT2C receptor expressed in mouse Swiss 3T3 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1871-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.071
BindingDB Entry DOI: 10.7270/Q2GB23XH |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50354984
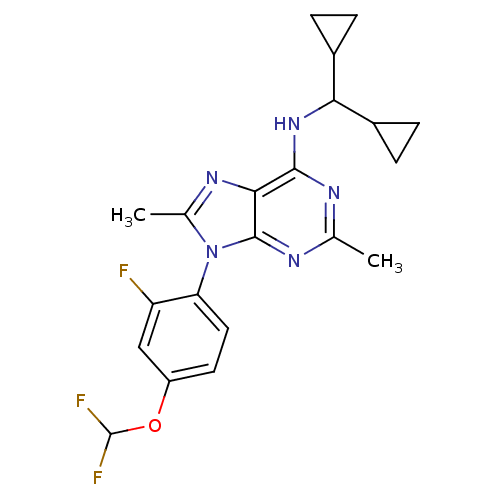
(CHEMBL1836948)Show SMILES Cc1nc2c(NC(C3CC3)C3CC3)nc(C)nc2n1-c1ccc(OC(F)F)cc1F |(42.35,-43.62,;40.81,-43.62,;39.9,-42.36,;38.42,-42.84,;37.09,-42.08,;37.08,-40.54,;35.75,-39.77,;35.74,-38.23,;36.51,-36.91,;34.97,-36.91,;34.42,-40.55,;32.88,-40.55,;33.65,-41.88,;35.76,-42.85,;35.76,-44.39,;34.42,-45.16,;37.09,-45.16,;38.42,-44.39,;39.9,-44.87,;40.69,-46.19,;39.94,-47.53,;40.73,-48.85,;42.27,-48.83,;43.06,-50.15,;42.32,-51.49,;43.11,-52.81,;40.78,-51.52,;43.02,-47.47,;42.22,-46.16,;42.97,-44.81,)| Show InChI InChI=1S/C21H22F3N5O/c1-10-25-19(28-17(12-3-4-12)13-5-6-13)18-20(26-10)29(11(2)27-18)16-8-7-14(9-15(16)22)30-21(23)24/h7-9,12-13,17,21H,3-6H2,1-2H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CRF1 receptor expressed in CHO cells assessed as inhibition of CRF-induced cAMP formation |
Bioorg Med Chem Lett 21: 6108-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.040
BindingDB Entry DOI: 10.7270/Q2F19046 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113798
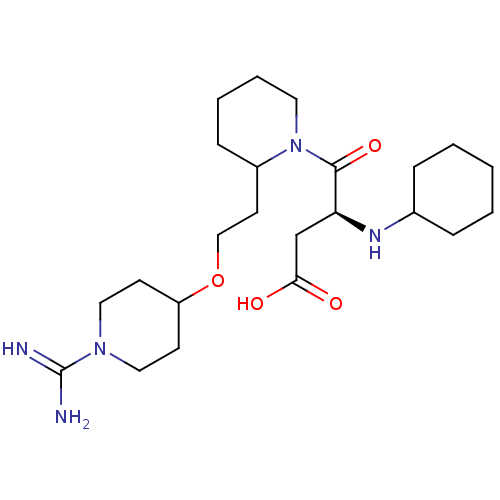
(CHEMBL82535 | RS-4-{2-[2-(1-Carbamimidoyl-piperidi...)Show SMILES NC(=N)N1CCC(CC1)OCCC1CCCCN1C(=O)[C@H](CC(O)=O)NC1CCCCC1 Show InChI InChI=1S/C23H41N5O4/c24-23(25)27-13-9-19(10-14-27)32-15-11-18-8-4-5-12-28(18)22(31)20(16-21(29)30)26-17-6-2-1-3-7-17/h17-20,26H,1-16H2,(H3,24,25)(H,29,30)/t18?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315681
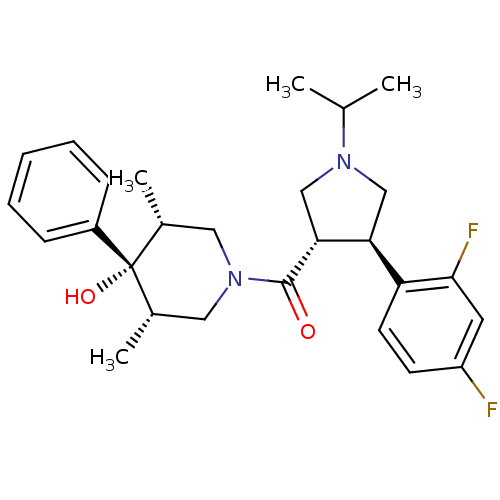
(((3S,4R)-4-(2,4-difluorophenyl)-1-isopropylpyrroli...)Show SMILES CC(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1C[C@H](C)[C@](O)([C@H](C)C1)c1ccccc1 |r| Show InChI InChI=1S/C27H34F2N2O2/c1-17(2)30-15-23(22-11-10-21(28)12-25(22)29)24(16-30)26(32)31-13-18(3)27(33,19(4)14-31)20-8-6-5-7-9-20/h5-12,17-19,23-24,33H,13-16H2,1-4H3/t18-,19+,23-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50378743
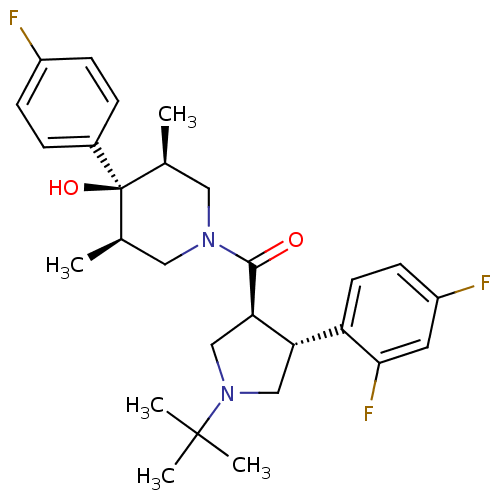
(CHEMBL1204061)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(F)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35F3N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019696
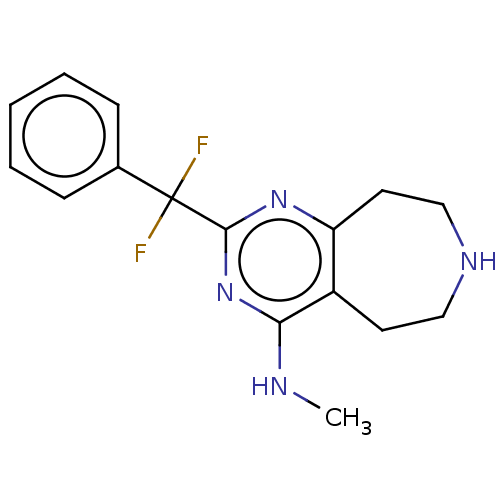
(CHEMBL3286557)Show InChI InChI=1S/C16H18F2N4/c1-19-14-12-7-9-20-10-8-13(12)21-15(22-14)16(17,18)11-5-3-2-4-6-11/h2-6,20H,7-10H2,1H3,(H,19,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50354979
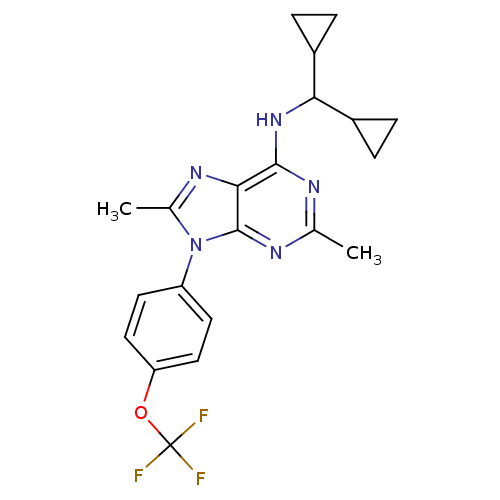
(CHEMBL1836943)Show SMILES Cc1nc2c(NC(C3CC3)C3CC3)nc(C)nc2n1-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C21H22F3N5O/c1-11-25-19(28-17(13-3-4-13)14-5-6-14)18-20(26-11)29(12(2)27-18)15-7-9-16(10-8-15)30-21(22,23)24/h7-10,13-14,17H,3-6H2,1-2H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human CRF1 receptor expressed in CHO cells assessed as inhibition of CRF-induced cAMP formation |
Bioorg Med Chem Lett 21: 6108-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.040
BindingDB Entry DOI: 10.7270/Q2F19046 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50257615
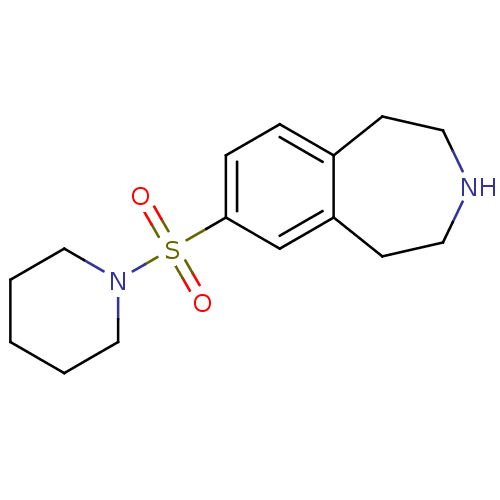
(7-(piperidin-1-ylsulfonyl)-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C15H22N2O2S/c18-20(19,17-10-2-1-3-11-17)15-5-4-13-6-8-16-9-7-14(13)12-15/h4-5,12,16H,1-3,6-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine form human recombinant 5HT2C receptor expressed in mouse Swiss 3T3 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 1871-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.071
BindingDB Entry DOI: 10.7270/Q2GB23XH |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50302574
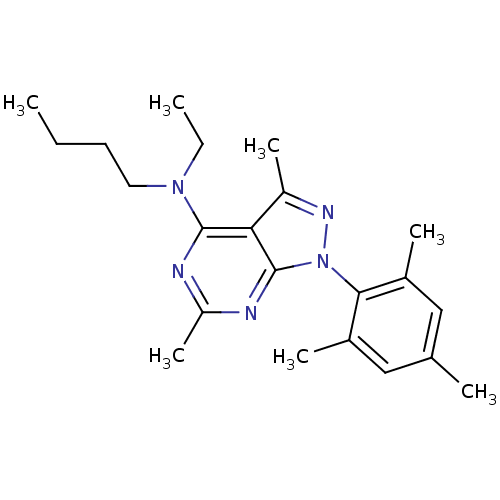
(CHEMBL576049 | N-butyl-N-ethyl-1-mesityl-3,6-dimet...)Show SMILES CCCCN(CC)c1nc(C)nc2n(nc(C)c12)-c1c(C)cc(C)cc1C |(-8.73,6.43,;-8.72,4.89,;-7.38,4.13,;-7.38,2.59,;-6.04,1.82,;-4.71,2.59,;-4.72,4.13,;-6.04,.28,;-7.37,-.49,;-7.37,-2.03,;-8.7,-2.8,;-6.04,-2.81,;-4.7,-2.04,;-3.22,-2.51,;-2.31,-1.25,;-3.23,-0,;-2.76,1.47,;-4.7,-.48,;-2.47,-3.86,;-.93,-3.87,;-.15,-2.54,;-.18,-5.21,;-.97,-6.54,;-.22,-7.88,;-2.51,-6.51,;-3.26,-5.17,;-4.8,-5.14,)| Show InChI InChI=1S/C22H31N5/c1-8-10-11-26(9-2)21-19-17(6)25-27(22(19)24-18(7)23-21)20-15(4)12-14(3)13-16(20)5/h12-13H,8-11H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CRF1 receptor expressed in CHO cells assessed as inhibition of CRF-induced cAMP production |
Bioorg Med Chem Lett 19: 6144-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.016
BindingDB Entry DOI: 10.7270/Q2FT8M46 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315679

(((3R,4S)-1-cyclopropyl-4-(2,4-difluorophenyl)pyrro...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CC1 |r| Show InChI InChI=1S/C27H32F2N2O2/c1-17-13-31(14-18(2)27(17,33)19-6-4-3-5-7-19)26(32)24-16-30(21-9-10-21)15-23(24)22-11-8-20(28)12-25(22)29/h3-8,11-12,17-18,21,23-24,33H,9-10,13-16H2,1-2H3/t17-,18+,23-,24+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50113796
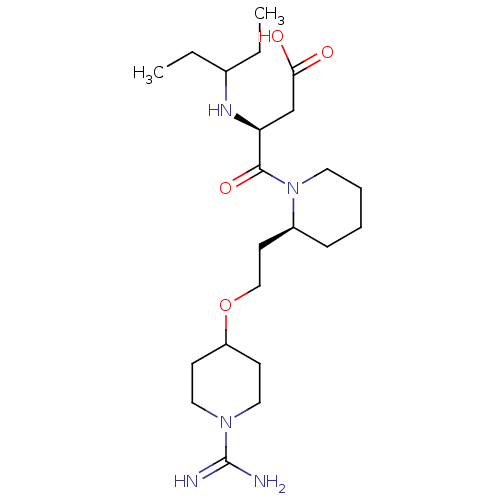
(CHEMBL418923 | S-4-{2-[2-(1-Carbamimidoyl-piperidi...)Show SMILES CCC(CC)N[C@@H](CC(O)=O)C(=O)N1CCCC[C@H]1CCOC1CCN(CC1)C(N)=N Show InChI InChI=1S/C22H41N5O4/c1-3-16(4-2)25-19(15-20(28)29)21(30)27-11-6-5-7-17(27)10-14-31-18-8-12-26(13-9-18)22(23)24/h16-19,25H,3-15H2,1-2H3,(H3,23,24)(H,28,29)/t17-,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
J Med Chem 45: 2432-53 (2002)
BindingDB Entry DOI: 10.7270/Q2S181VR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315691
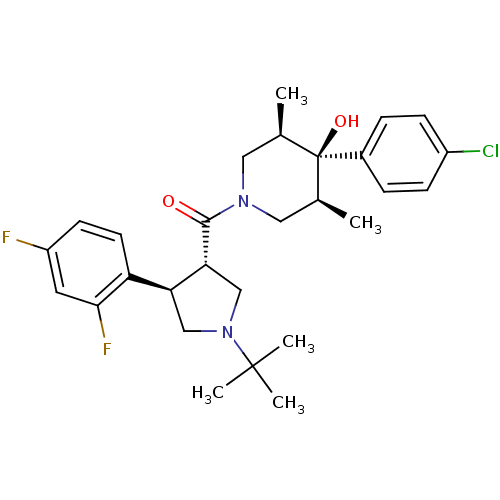
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(Cl)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35ClF2N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315692
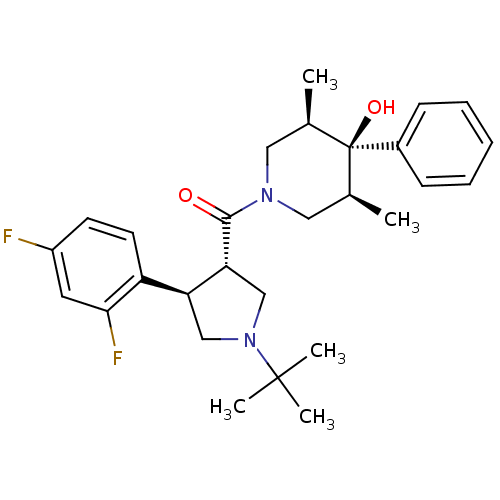
(CHEMBL1090488 | CHEMBL1204059)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H36F2N2O2/c1-18-14-31(15-19(2)28(18,34)20-9-7-6-8-10-20)26(33)24-17-32(27(3,4)5)16-23(24)22-12-11-21(29)13-25(22)30/h6-13,18-19,23-24,34H,14-17H2,1-5H3/t18-,19+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315673
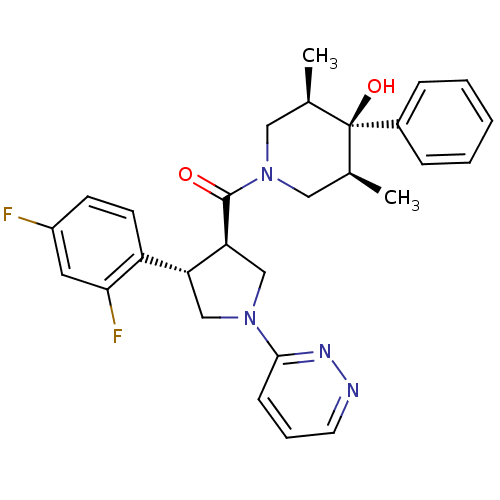
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridazin-3-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C28H30F2N4O2/c1-18-14-34(15-19(2)28(18,36)20-7-4-3-5-8-20)27(35)24-17-33(26-9-6-12-31-32-26)16-23(24)22-11-10-21(29)13-25(22)30/h3-13,18-19,23-24,36H,14-17H2,1-2H3/t18-,19+,23-,24+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50378748
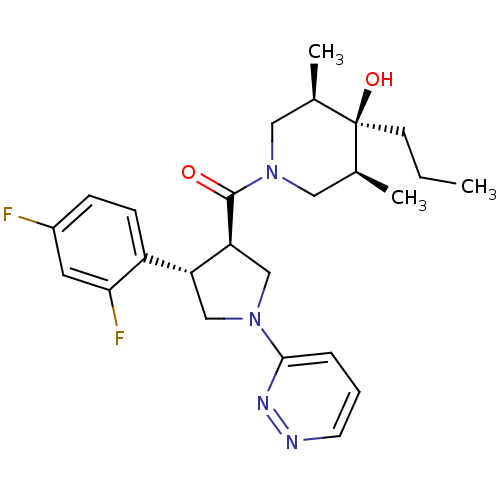
(CHEMBL1204054)Show SMILES CCC[C@]1(O)[C@@H](C)CN(C[C@H]1C)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C25H32F2N4O2/c1-4-9-25(33)16(2)12-31(13-17(25)3)24(32)21-15-30(23-6-5-10-28-29-23)14-20(21)19-8-7-18(26)11-22(19)27/h5-8,10-11,16-17,20-21,33H,4,9,12-15H2,1-3H3/t16-,17+,20-,21+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data