 Found 241 hits with Last Name = 'brown' and Initial = 'am'
Found 241 hits with Last Name = 'brown' and Initial = 'am' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50562760

(CHEMBL4750447)Show SMILES Cl.CCCCCCCCCn1ccc2cc(ccc12)-c1noc(n1)[C@@H]1CCCN1C(N)=N |r| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50562761

(CHEMBL4782021)Show SMILES Cl.CCCCCCCCCCn1ccc2cc(ccc12)-c1noc(n1)[C@@H]1CCCN1C(N)=N |r| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50562759

(CHEMBL4783605)Show SMILES Cl.CCCCCCCCCCCCn1cc(-c2noc(n2)[C@@H]2CCCN2C(N)=N)c2ccccc12 |r| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50562758

(CHEMBL4755938)Show SMILES Cl.CCCCCCCCCCn1cc(-c2noc(n2)[C@@H]2CCCN2C(N)=N)c2ccccc12 |r| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50562757
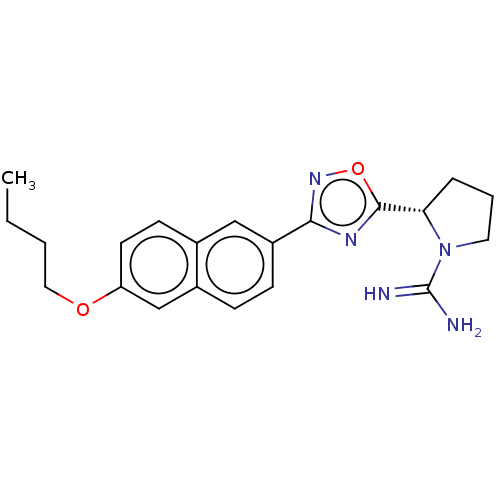
(CHEMBL3814580)Show SMILES Cl.CCCCOc1ccc2cc(ccc2c1)-c1noc(n1)[C@@H]1CCCN1C(N)=N |r| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50177006
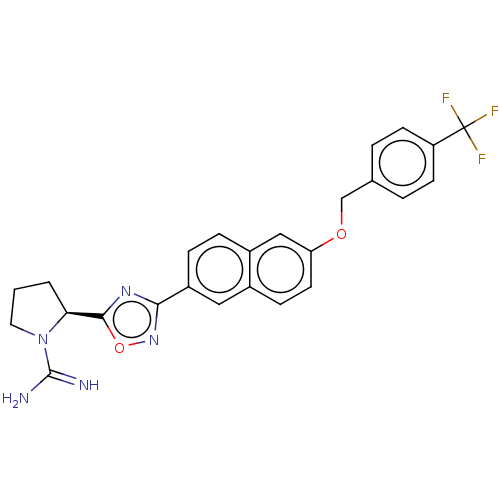
(CHEMBL3814849)Show SMILES Cl.NC(=N)N1CCC[C@H]1c1nc(no1)-c1ccc2cc(OCc3ccc(cc3)C(F)(F)F)ccc2c1 |r| Show InChI InChI=1S/C25H22F3N5O2.ClH/c26-25(27,28)19-8-3-15(4-9-19)14-34-20-10-7-16-12-18(6-5-17(16)13-20)22-31-23(35-32-22)21-2-1-11-33(21)24(29)30;/h3-10,12-13,21H,1-2,11,14H2,(H3,29,30);1H/t21-;/m0./s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Sphk2 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85097
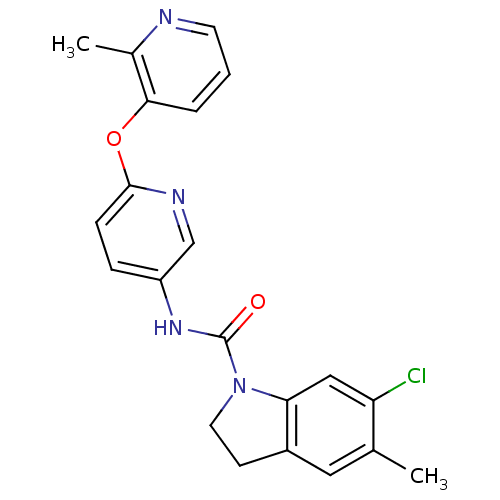
(CAS_181632-25-7 | CHEMBL14563 | SB 242084)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1Cl Show InChI InChI=1S/C21H19ClN4O2/c1-13-10-15-7-9-26(18(15)11-17(13)22)21(27)25-16-5-6-20(24-12-16)28-19-4-3-8-23-14(19)2/h3-6,8,10-12H,7,9H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 609-20 (1997)
Article DOI: 10.1016/s0028-3908(97)00038-5
BindingDB Entry DOI: 10.7270/Q2SN07GG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 124: 1300-6 (1998)
Article DOI: 10.1038/sj.bjp.0701946
BindingDB Entry DOI: 10.7270/Q269724V |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50041978

(CHEMBL3134157)Show SMILES Cc1cc(CS(=O)(=O)c2ccccc2)cc(OCc2ccc(CN3CCC[C@@H]3CO)cc2)c1 |r| Show InChI InChI=1S/C27H31NO4S/c1-21-14-24(20-33(30,31)27-7-3-2-4-8-27)16-26(15-21)32-19-23-11-9-22(10-12-23)17-28-13-5-6-25(28)18-29/h2-4,7-12,14-16,25,29H,5-6,13,17-20H2,1H3/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human SphK1 expressed in baculovirus infected Sf9 cells assessed as decrease in [33P]SIP production using D-erythro-sphingo... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115941
BindingDB Entry DOI: 10.7270/Q23B63ST |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine kinase 1
(Mus musculus) | BDBM50041978

(CHEMBL3134157)Show SMILES Cc1cc(CS(=O)(=O)c2ccccc2)cc(OCc2ccc(CN3CCC[C@@H]3CO)cc2)c1 |r| Show InChI InChI=1S/C27H31NO4S/c1-21-14-24(20-33(30,31)27-7-3-2-4-8-27)16-26(15-21)32-19-23-11-9-22(10-12-23)17-28-13-5-6-25(28)18-29/h2-4,7-12,14-16,25,29H,5-6,13,17-20H2,1H3/t25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of SphK1 in mouse erythrocytes using sphingosine as substrate by luminescence assay |
ACS Med Chem Lett 7: 229-34 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00304
BindingDB Entry DOI: 10.7270/Q22B90ZN |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50041978

(CHEMBL3134157)Show SMILES Cc1cc(CS(=O)(=O)c2ccccc2)cc(OCc2ccc(CN3CCC[C@@H]3CO)cc2)c1 |r| Show InChI InChI=1S/C27H31NO4S/c1-21-14-24(20-33(30,31)27-7-3-2-4-8-27)16-26(15-21)32-19-23-11-9-22(10-12-23)17-28-13-5-6-25(28)18-29/h2-4,7-12,14-16,25,29H,5-6,13,17-20H2,1H3/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged SPHK1 expressed in fall armyworm sf21 cells using sphingosine as substrate after 1 hr by FITC-b... |
J Med Chem 60: 3933-3957 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00233
BindingDB Entry DOI: 10.7270/Q2DJ5J3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50041978

(CHEMBL3134157)Show SMILES Cc1cc(CS(=O)(=O)c2ccccc2)cc(OCc2ccc(CN3CCC[C@@H]3CO)cc2)c1 |r| Show InChI InChI=1S/C27H31NO4S/c1-21-14-24(20-33(30,31)27-7-3-2-4-8-27)16-26(15-21)32-19-23-11-9-22(10-12-23)17-28-13-5-6-25(28)18-29/h2-4,7-12,14-16,25,29H,5-6,13,17-20H2,1H3/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive-inhibition of recombinant human C-terminal His6-tagged Sphk1 expressed in baculovirus infected Sf21 insect cells using varying levels of ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 124: 1300-6 (1998)
Article DOI: 10.1038/sj.bjp.0701946
BindingDB Entry DOI: 10.7270/Q269724V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 124: 1300-6 (1998)
Article DOI: 10.1038/sj.bjp.0701946
BindingDB Entry DOI: 10.7270/Q269724V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 1413-20 (1998)
Article DOI: 10.1038/sj.bjp.0702201
BindingDB Entry DOI: 10.7270/Q2HM56ZD |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50562757

(CHEMBL3814580)Show SMILES Cl.CCCCOc1ccc2cc(ccc2c1)-c1noc(n1)[C@@H]1CCCN1C(N)=N |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50562759

(CHEMBL4783605)Show SMILES Cl.CCCCCCCCCCCCn1cc(-c2noc(n2)[C@@H]2CCCN2C(N)=N)c2ccccc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50562758

(CHEMBL4755938)Show SMILES Cl.CCCCCCCCCCn1cc(-c2noc(n2)[C@@H]2CCCN2C(N)=N)c2ccccc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50562760

(CHEMBL4750447)Show SMILES Cl.CCCCCCCCCn1ccc2cc(ccc12)-c1noc(n1)[C@@H]1CCCN1C(N)=N |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50562761

(CHEMBL4782021)Show SMILES Cl.CCCCCCCCCCn1ccc2cc(ccc12)-c1noc(n1)[C@@H]1CCCN1C(N)=N |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 1413-20 (1998)
Article DOI: 10.1038/sj.bjp.0702201
BindingDB Entry DOI: 10.7270/Q2HM56ZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 1413-20 (1998)
Article DOI: 10.1038/sj.bjp.0702201
BindingDB Entry DOI: 10.7270/Q2HM56ZD |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50177006

(CHEMBL3814849)Show SMILES Cl.NC(=N)N1CCC[C@H]1c1nc(no1)-c1ccc2cc(OCc3ccc(cc3)C(F)(F)F)ccc2c1 |r| Show InChI InChI=1S/C25H22F3N5O2.ClH/c26-25(27,28)19-8-3-15(4-9-19)14-34-20-10-7-16-12-18(6-5-17(16)13-20)22-31-23(35-32-22)21-2-1-11-33(21)24(29)30;/h3-10,12-13,21H,1-2,11,14H2,(H3,29,30);1H/t21-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human Sphk1 expressed in baculovirus infected Sf9 insect cells using d-erythro-sphingosine as substrate measured after 20 m... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113121
BindingDB Entry DOI: 10.7270/Q2F193FG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098550
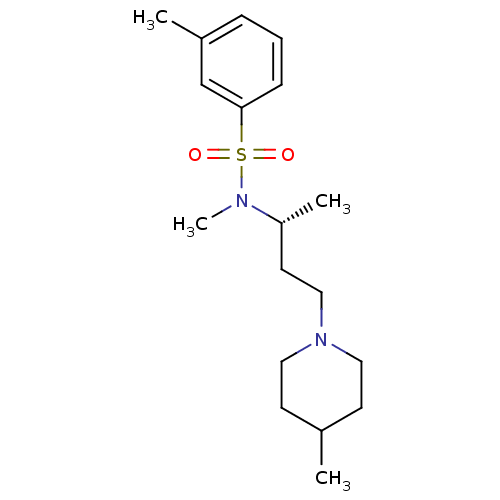
(3,N-Dimethyl-N-[(R)-1-methyl-3-(4-methyl-piperidin...)Show SMILES C[C@H](CCN1CCC(C)CC1)N(C)S(=O)(=O)c1cccc(C)c1 Show InChI InChI=1S/C18H30N2O2S/c1-15-8-11-20(12-9-15)13-10-17(3)19(4)23(21,22)18-7-5-6-16(2)14-18/h5-7,14-15,17H,8-13H2,1-4H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Med Chem 41: 655-7 (1998)
Article DOI: 10.1021/jm970519e
BindingDB Entry DOI: 10.7270/Q2GX4949 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098550

(3,N-Dimethyl-N-[(R)-1-methyl-3-(4-methyl-piperidin...)Show SMILES C[C@H](CCN1CCC(C)CC1)N(C)S(=O)(=O)c1cccc(C)c1 Show InChI InChI=1S/C18H30N2O2S/c1-15-8-11-20(12-9-15)13-10-17(3)19(4)23(21,22)18-7-5-6-16(2)14-18/h5-7,14-15,17H,8-13H2,1-4H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 124: 1300-6 (1998)
Article DOI: 10.1038/sj.bjp.0701946
BindingDB Entry DOI: 10.7270/Q269724V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 124: 1300-6 (1998)
Article DOI: 10.1038/sj.bjp.0701946
BindingDB Entry DOI: 10.7270/Q269724V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 1413-20 (1998)
Article DOI: 10.1038/sj.bjp.0702201
BindingDB Entry DOI: 10.7270/Q2HM56ZD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 1413-20 (1998)
Article DOI: 10.1038/sj.bjp.0702201
BindingDB Entry DOI: 10.7270/Q2HM56ZD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 1413-20 (1998)
Article DOI: 10.1038/sj.bjp.0702201
BindingDB Entry DOI: 10.7270/Q2HM56ZD |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50077147
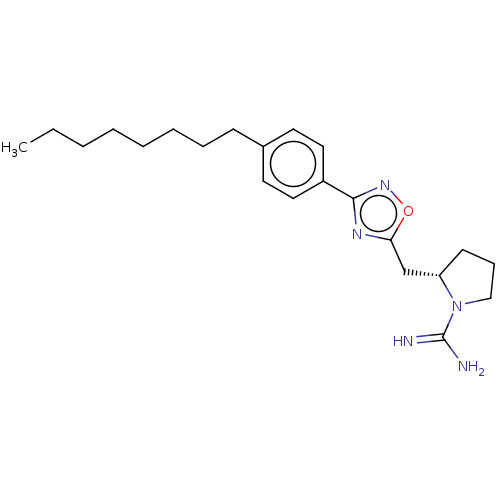
(CHEMBL3416846)Show SMILES Cl.CCCCCCCCc1ccc(cc1)-c1noc(C[C@@H]2CCCN2C(N)=N)n1 |r| Show InChI InChI=1S/C22H33N5O/c1-2-3-4-5-6-7-9-17-11-13-18(14-12-17)21-25-20(28-26-21)16-19-10-8-15-27(19)22(23)24/h11-14,19H,2-10,15-16H2,1H3,(H3,23,24)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human SphK1 expressed in baculovirus infected Sf9 cells assessed as decrease in [33P]SIP production using D-erythro-sphingo... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115941
BindingDB Entry DOI: 10.7270/Q23B63ST |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 1413-20 (1998)
Article DOI: 10.1038/sj.bjp.0702201
BindingDB Entry DOI: 10.7270/Q2HM56ZD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 124: 1300-6 (1998)
Article DOI: 10.1038/sj.bjp.0701946
BindingDB Entry DOI: 10.7270/Q269724V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 1413-20 (1998)
Article DOI: 10.1038/sj.bjp.0702201
BindingDB Entry DOI: 10.7270/Q2HM56ZD |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50514473

(CHEMBL4453931)Show SMILES Cl.NC(=N)N1CCC[C@H]1c1nc(no1)-c1ccc(OCc2ccc(cc2)C(F)(F)F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C22H19F6N5O2.ClH/c23-21(24,25)14-6-3-12(4-7-14)11-34-17-8-5-13(10-15(17)22(26,27)28)18-31-19(35-32-18)16-2-1-9-33(16)20(29)30;/h3-8,10,16H,1-2,9,11H2,(H3,29,30);1H/t16-;/m0./s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SphK2 expressed in baculovirus infected Sf9 cells assessed as decrease in [33P]S1P production using varying level of ... |
J Med Chem 63: 1178-1198 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01508
BindingDB Entry DOI: 10.7270/Q2WH2T9K |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50514473

(CHEMBL4453931)Show SMILES Cl.NC(=N)N1CCC[C@H]1c1nc(no1)-c1ccc(OCc2ccc(cc2)C(F)(F)F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C22H19F6N5O2.ClH/c23-21(24,25)14-6-3-12(4-7-14)11-34-17-8-5-13(10-15(17)22(26,27)28)18-31-19(35-32-18)16-2-1-9-33(16)20(29)30;/h3-8,10,16H,1-2,9,11H2,(H3,29,30);1H/t16-;/m0./s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human SphK2 assessed as decrease in [33P]SIP production using sphingosine as substrate in presence of [gamma33P]-ATP by sci... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115941
BindingDB Entry DOI: 10.7270/Q23B63ST |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50266429

(CHEMBL4074731)Show SMILES Cl.[H][C@@]1(Cc2nc(no2)-c2ccc(Nc3nc(cs3)-c3ccc(cc3)-c3ccccc3)cc2)CCCN1C(N)=N |r| Show InChI InChI=1S/C29H27N7OS.ClH/c30-28(31)36-16-4-7-24(36)17-26-34-27(35-37-26)22-12-14-23(15-13-22)32-29-33-25(18-38-29)21-10-8-20(9-11-21)19-5-2-1-3-6-19;/h1-3,5-6,8-15,18,24H,4,7,16-17H2,(H3,30,31)(H,32,33);1H/t24-;/m0./s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SPHK2 at using sphingosine as substrate after 30 mins in presence of gamma-[32P]-ATP by liquid scintillation counting |
J Med Chem 60: 3933-3957 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00233
BindingDB Entry DOI: 10.7270/Q2DJ5J3Q |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50266426

(CHEMBL4076808)Show SMILES Cl.[H][C@@]1(Cc2nc(no2)-c2ccc(Nc3nc(cs3)-c3cccc(c3)C(F)(F)F)cc2)CCCN1C(N)=N |r| Show InChI InChI=1S/C24H22F3N7OS.ClH/c25-24(26,27)16-4-1-3-15(11-16)19-13-36-23(31-19)30-17-8-6-14(7-9-17)21-32-20(35-33-21)12-18-5-2-10-34(18)22(28)29;/h1,3-4,6-9,11,13,18H,2,5,10,12H2,(H3,28,29)(H,30,31);1H/t18-;/m0./s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SPHK2 at using sphingosine as substrate after 30 mins in presence of gamma-[32P]-ATP by liquid scintillation counting |
J Med Chem 60: 3933-3957 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00233
BindingDB Entry DOI: 10.7270/Q2DJ5J3Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85097

(CAS_181632-25-7 | CHEMBL14563 | SB 242084)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1Cl Show InChI InChI=1S/C21H19ClN4O2/c1-13-10-15-7-9-26(18(15)11-17(13)22)21(27)25-16-5-6-20(24-12-16)28-19-4-3-8-23-14(19)2/h3-6,8,10-12H,7,9H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 609-20 (1997)
Article DOI: 10.1016/s0028-3908(97)00038-5
BindingDB Entry DOI: 10.7270/Q2SN07GG |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50266426

(CHEMBL4076808)Show SMILES Cl.[H][C@@]1(Cc2nc(no2)-c2ccc(Nc3nc(cs3)-c3cccc(c3)C(F)(F)F)cc2)CCCN1C(N)=N |r| Show InChI InChI=1S/C24H22F3N7OS.ClH/c25-24(26,27)16-4-1-3-15(11-16)19-13-36-23(31-19)30-17-8-6-14(7-9-17)21-32-20(35-33-21)12-18-5-2-10-34(18)22(28)29;/h1,3-4,6-9,11,13,18H,2,5,10,12H2,(H3,28,29)(H,30,31);1H/t18-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SPHK1 using sphingosine as substrate after 30 mins in presence of gamma-[32P]-ATP by liquid scintillation counting |
J Med Chem 60: 3933-3957 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00233
BindingDB Entry DOI: 10.7270/Q2DJ5J3Q |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 1413-20 (1998)
Article DOI: 10.1038/sj.bjp.0702201
BindingDB Entry DOI: 10.7270/Q2HM56ZD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 1413-20 (1998)
Article DOI: 10.1038/sj.bjp.0702201
BindingDB Entry DOI: 10.7270/Q2HM56ZD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Br J Pharmacol 125: 1413-20 (1998)
Article DOI: 10.1038/sj.bjp.0702201
BindingDB Entry DOI: 10.7270/Q2HM56ZD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85097

(CAS_181632-25-7 | CHEMBL14563 | SB 242084)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1Cl Show InChI InChI=1S/C21H19ClN4O2/c1-13-10-15-7-9-26(18(15)11-17(13)22)21(27)25-16-5-6-20(24-12-16)28-19-4-3-8-23-14(19)2/h3-6,8,10-12H,7,9H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 609-20 (1997)
Article DOI: 10.1016/s0028-3908(97)00038-5
BindingDB Entry DOI: 10.7270/Q2SN07GG |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50266428
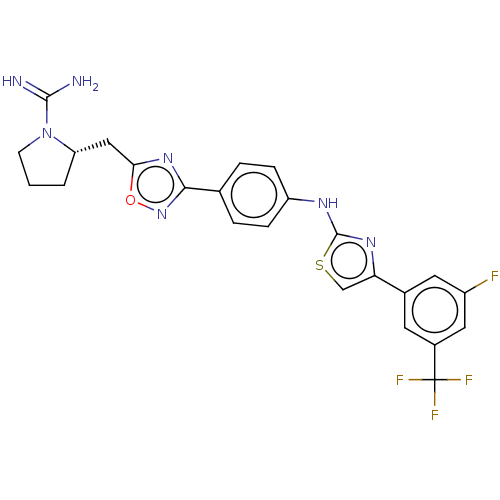
(CHEMBL4082275)Show SMILES Cl.[H][C@@]1(Cc2nc(no2)-c2ccc(Nc3nc(cs3)-c3cc(F)cc(c3)C(F)(F)F)cc2)CCCN1C(N)=N |r| Show InChI InChI=1S/C24H21F4N7OS.ClH/c25-16-9-14(8-15(10-16)24(26,27)28)19-12-37-23(32-19)31-17-5-3-13(4-6-17)21-33-20(36-34-21)11-18-2-1-7-35(18)22(29)30;/h3-6,8-10,12,18H,1-2,7,11H2,(H3,29,30)(H,31,32);1H/t18-;/m0./s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SPHK2 at using sphingosine as substrate after 30 mins in presence of gamma-[32P]-ATP by liquid scintillation counting |
J Med Chem 60: 3933-3957 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00233
BindingDB Entry DOI: 10.7270/Q2DJ5J3Q |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50266427

(CHEMBL4071762)Show SMILES Cl.[H][C@@]1(Cc2nc(no2)-c2ccc(Nc3nc(cs3)-c3ccc(F)c(c3)C(F)(F)F)cc2)CCCN1C(N)=N |r| Show InChI InChI=1S/C24H21F4N7OS.ClH/c25-18-8-5-14(10-17(18)24(26,27)28)19-12-37-23(32-19)31-15-6-3-13(4-7-15)21-33-20(36-34-21)11-16-2-1-9-35(16)22(29)30;/h3-8,10,12,16H,1-2,9,11H2,(H3,29,30)(H,31,32);1H/t16-;/m0./s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SPHK2 at using sphingosine as substrate after 30 mins in presence of gamma-[32P]-ATP by liquid scintillation counting |
J Med Chem 60: 3933-3957 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00233
BindingDB Entry DOI: 10.7270/Q2DJ5J3Q |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50514475
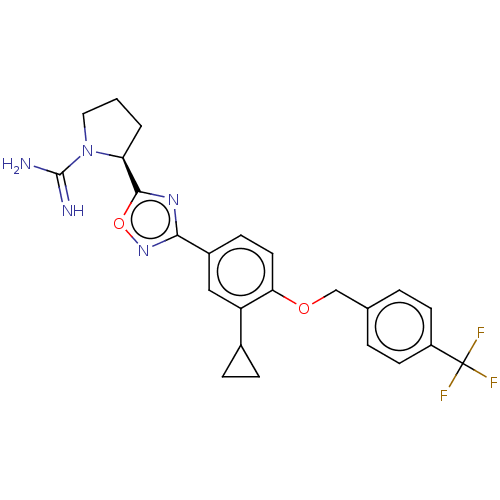
(CHEMBL4456325)Show SMILES Cl.NC(=N)N1CCC[C@H]1c1nc(no1)-c1ccc(OCc2ccc(cc2)C(F)(F)F)c(c1)C1CC1 |r| Show InChI InChI=1S/C24H24F3N5O2.ClH/c25-24(26,27)17-8-3-14(4-9-17)13-33-20-10-7-16(12-18(20)15-5-6-15)21-30-22(34-31-21)19-2-1-11-32(19)23(28)29;/h3-4,7-10,12,15,19H,1-2,5-6,11,13H2,(H3,28,29);1H/t19-;/m0./s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SphK2 expressed in baculovirus infected Sf9 cells assessed as decrease in [33P]S1P production using varying level of ... |
J Med Chem 63: 1178-1198 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01508
BindingDB Entry DOI: 10.7270/Q2WH2T9K |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50514477
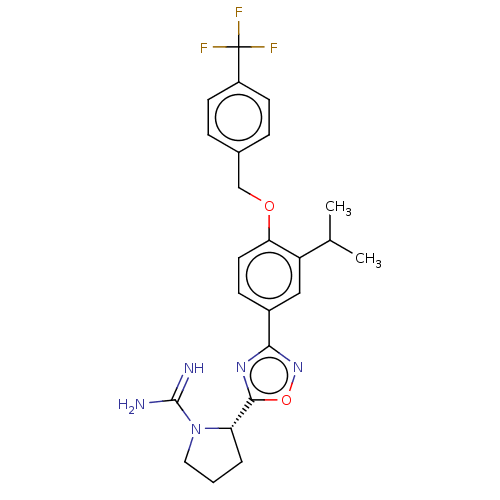
(CHEMBL4593983)Show SMILES Cl.CC(C)c1cc(ccc1OCc1ccc(cc1)C(F)(F)F)-c1noc(n1)[C@@H]1CCCN1C(N)=N |r| Show InChI InChI=1S/C24H26F3N5O2.ClH/c1-14(2)18-12-16(21-30-22(34-31-21)19-4-3-11-32(19)23(28)29)7-10-20(18)33-13-15-5-8-17(9-6-15)24(25,26)27;/h5-10,12,14,19H,3-4,11,13H2,1-2H3,(H3,28,29);1H/t19-;/m0./s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SphK2 expressed in baculovirus infected Sf9 cells assessed as decrease in [33P]S1P production using varying level of ... |
J Med Chem 63: 1178-1198 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01508
BindingDB Entry DOI: 10.7270/Q2WH2T9K |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50514478
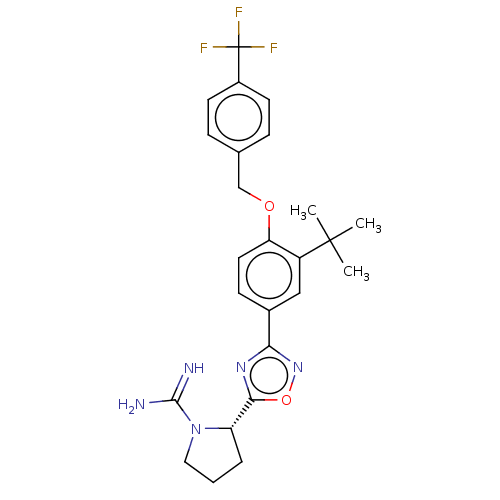
(CHEMBL4570134)Show SMILES Cl.CC(C)(C)c1cc(ccc1OCc1ccc(cc1)C(F)(F)F)-c1noc(n1)[C@@H]1CCCN1C(N)=N |r| Show InChI InChI=1S/C25H28F3N5O2.ClH/c1-24(2,3)18-13-16(21-31-22(35-32-21)19-5-4-12-33(19)23(29)30)8-11-20(18)34-14-15-6-9-17(10-7-15)25(26,27)28;/h6-11,13,19H,4-5,12,14H2,1-3H3,(H3,29,30);1H/t19-;/m0./s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SphK2 expressed in baculovirus infected Sf9 cells assessed as decrease in [33P]S1P production using varying level of ... |
J Med Chem 63: 1178-1198 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01508
BindingDB Entry DOI: 10.7270/Q2WH2T9K |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50514474
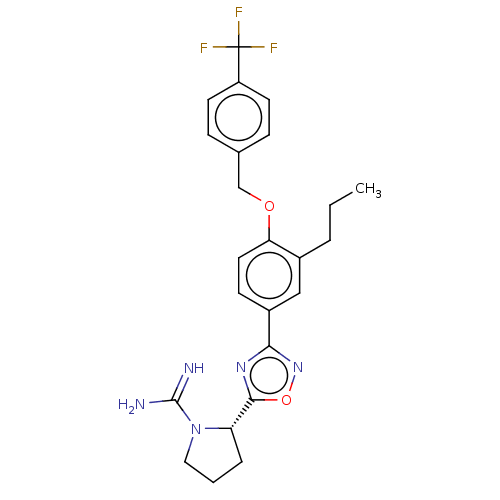
(CHEMBL4462152)Show SMILES Cl.CCCc1cc(ccc1OCc1ccc(cc1)C(F)(F)F)-c1noc(n1)[C@@H]1CCCN1C(N)=N |r| Show InChI InChI=1S/C24H26F3N5O2.ClH/c1-2-4-16-13-17(21-30-22(34-31-21)19-5-3-12-32(19)23(28)29)8-11-20(16)33-14-15-6-9-18(10-7-15)24(25,26)27;/h6-11,13,19H,2-5,12,14H2,1H3,(H3,28,29);1H/t19-;/m0./s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Tech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SphK2 expressed in baculovirus infected Sf9 cells assessed as decrease in [33P]S1P production using varying level of ... |
J Med Chem 63: 1178-1198 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01508
BindingDB Entry DOI: 10.7270/Q2WH2T9K |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50266436

(CHEMBL4093841)Show SMILES Cl.[H][C@@]1(Cc2nc(no2)-c2ccc(Nc3nc(cs3)-c3ccc(OC(F)(F)F)cc3)cc2)CCCN1C(N)=N |r| Show InChI InChI=1S/C24H22F3N7O2S.ClH/c25-24(26,27)35-18-9-5-14(6-10-18)19-13-37-23(31-19)30-16-7-3-15(4-8-16)21-32-20(36-33-21)12-17-2-1-11-34(17)22(28)29;/h3-10,13,17H,1-2,11-12H2,(H3,28,29)(H,30,31);1H/t17-;/m0./s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human SPHK2 at using sphingosine as substrate after 30 mins in presence of gamma-[32P]-ATP by liquid scintillation counting |
J Med Chem 60: 3933-3957 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00233
BindingDB Entry DOI: 10.7270/Q2DJ5J3Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data