 Found 287 hits with Last Name = 'brown' and Initial = 'gr'
Found 287 hits with Last Name = 'brown' and Initial = 'gr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Squalene synthase
(Rattus norvegicus) | BDBM50291312
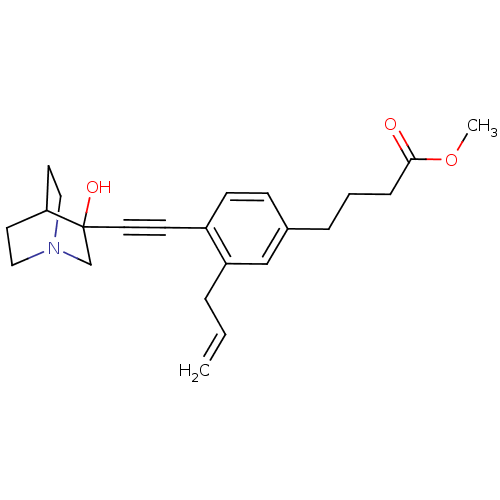
(4-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES COC(=O)CCCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:12:13:18.17:20.21,14:13:18.17:20.21,(20.76,-2.86,;19.42,-2.1,;18.08,-2.89,;18.1,-4.43,;16.75,-2.12,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C23H29NO3/c1-3-5-20-16-18(6-4-7-22(25)27-2)8-9-19(20)10-13-23(26)17-24-14-11-21(23)12-15-24/h3,8-9,16,21,26H,1,4-7,11-12,14-15,17H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50075719
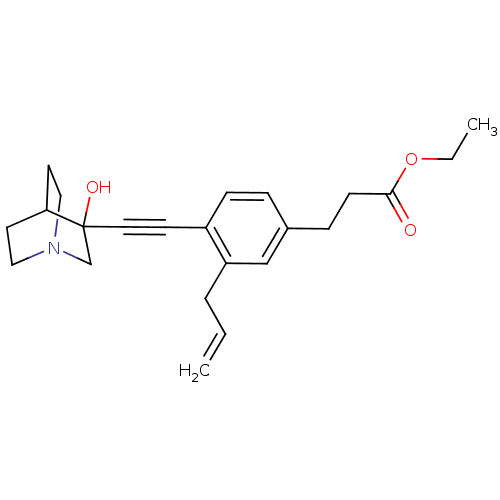
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CCOC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:12:13:18.17:20.21,14:13:18.17:20.21,(10.63,-1.41,;9.54,-2.5,;9.94,-3.98,;8.86,-5.08,;9.25,-6.57,;7.36,-4.68,;6.27,-5.77,;4.78,-5.38,;4.38,-3.89,;2.9,-3.49,;1.81,-4.59,;.33,-4.19,;-1.17,-3.79,;-2.66,-3.4,;-2.26,-1.9,;-3.89,-4.07,;-4.88,-2.49,;-6.75,-2.53,;-5.51,-1.8,;-3.62,-1.88,;-3.15,-.9,;-4.25,-1.27,;2.22,-6.06,;1.13,-7.15,;-.37,-6.75,;-1.45,-7.84,;3.7,-6.46,)| Show InChI InChI=1S/C23H29NO3/c1-3-5-20-16-18(7-9-22(25)27-4-2)6-8-19(20)10-13-23(26)17-24-14-11-21(23)12-15-24/h3,6,8,16,21,26H,1,4-5,7,9,11-12,14-15,17H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291315

(5-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES COC(=O)CCCCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:13:14:19.18:21.22,15:14:19.18:21.22,(22.09,-2.1,;20.76,-2.86,;19.42,-2.1,;19.42,-.56,;18.08,-2.89,;16.75,-2.12,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C24H31NO3/c1-3-6-21-17-19(7-4-5-8-23(26)28-2)9-10-20(21)11-14-24(27)18-25-15-12-22(24)13-16-25/h3,9-10,17,22,27H,1,4-8,12-13,15-16,18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291311

(6-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES COC(=O)CCCCCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:14:15:20.19:22.23,16:15:20.19:22.23,(23.43,-2.86,;22.09,-2.1,;20.76,-2.86,;20.76,-4.4,;19.42,-2.1,;18.08,-2.89,;16.75,-2.12,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C25H33NO3/c1-3-7-22-18-20(8-5-4-6-9-24(27)29-2)10-11-21(22)12-15-25(28)19-26-16-13-23(25)14-17-26/h3,10-11,18,23,28H,1,4-9,13-14,16-17,19H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291316
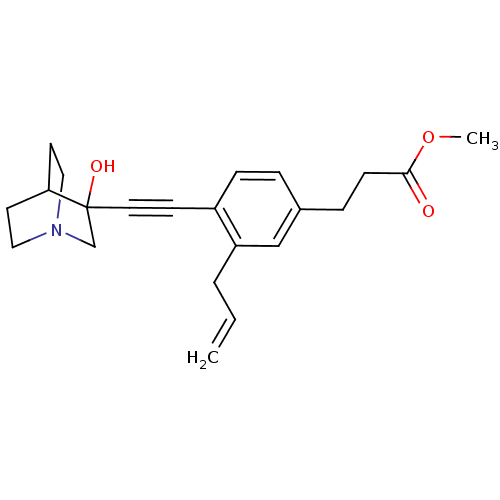
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES COC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:11:12:17.16:19.20,13:12:17.16:19.20,(19.42,-2.1,;18.08,-2.89,;16.75,-2.12,;16.75,-.58,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C22H27NO3/c1-3-4-19-15-17(6-8-21(24)26-2)5-7-18(19)9-12-22(25)16-23-13-10-20(22)11-14-23/h3,5,7,15,20,25H,1,4,6,8,10-11,13-14,16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Homo sapiens (Human)) | BDBM50075719

(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CCOC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:12:13:18.17:20.21,14:13:18.17:20.21,(10.63,-1.41,;9.54,-2.5,;9.94,-3.98,;8.86,-5.08,;9.25,-6.57,;7.36,-4.68,;6.27,-5.77,;4.78,-5.38,;4.38,-3.89,;2.9,-3.49,;1.81,-4.59,;.33,-4.19,;-1.17,-3.79,;-2.66,-3.4,;-2.26,-1.9,;-3.89,-4.07,;-4.88,-2.49,;-6.75,-2.53,;-5.51,-1.8,;-3.62,-1.88,;-3.15,-.9,;-4.25,-1.27,;2.22,-6.06,;1.13,-7.15,;-.37,-6.75,;-1.45,-7.84,;3.7,-6.46,)| Show InChI InChI=1S/C23H29NO3/c1-3-5-20-16-18(7-9-22(25)27-4-2)6-8-19(20)10-13-23(26)17-24-14-11-21(23)12-15-24/h3,6,8,16,21,26H,1,4-5,7,9,11-12,14-15,17H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against human microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291317
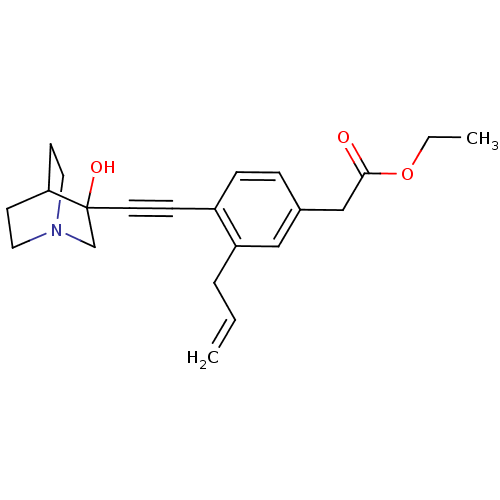
(CHEMBL154472 | [3-Allyl-4-(3-hydroxy-1-aza-bicyclo...)Show SMILES CCOC(=O)Cc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:11:12:17.16:19.20,13:12:17.16:19.20,(19.42,-2.1,;18.08,-2.89,;16.75,-2.12,;15.43,-2.9,;15.43,-4.44,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C22H27NO3/c1-3-5-19-14-17(15-21(24)26-4-2)6-7-18(19)8-11-22(25)16-23-12-9-20(22)10-13-23/h3,6-7,14,20,25H,1,4-5,9-10,12-13,15-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291318
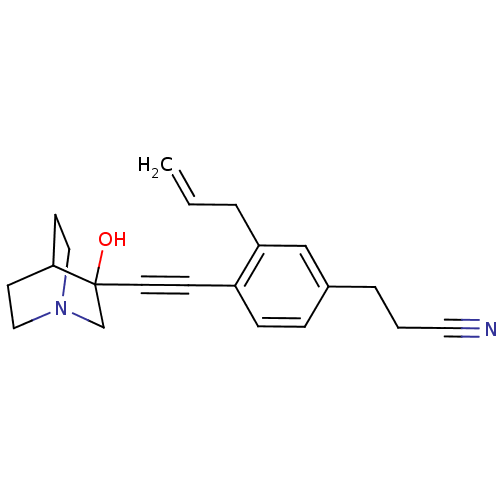
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES OC1(CN2CCC1CC2)C#Cc1ccc(CCC#N)cc1CC=C |THB:9:1:5.4:7.8,0:1:5.4:7.8,(7.67,-7.29,;6.19,-6.88,;6.4,-8.42,;4.96,-7.64,;3.26,-8.25,;3.14,-6.75,;4.78,-6.15,;4.96,-4.38,;5.37,-5.61,;7.45,-6.01,;8.78,-5.24,;10.12,-4.46,;11.45,-5.23,;12.77,-4.46,;12.77,-2.91,;14.1,-2.13,;15.44,-2.91,;16.76,-2.12,;18.1,-1.35,;11.43,-2.15,;10.09,-2.93,;8.77,-2.17,;7.44,-2.94,;6.1,-2.18,)| Show InChI InChI=1S/C21H24N2O/c1-2-4-19-15-17(5-3-12-22)6-7-18(19)8-11-21(24)16-23-13-9-20(21)10-14-23/h2,6-7,15,20,24H,1,3-5,9-10,13-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291313
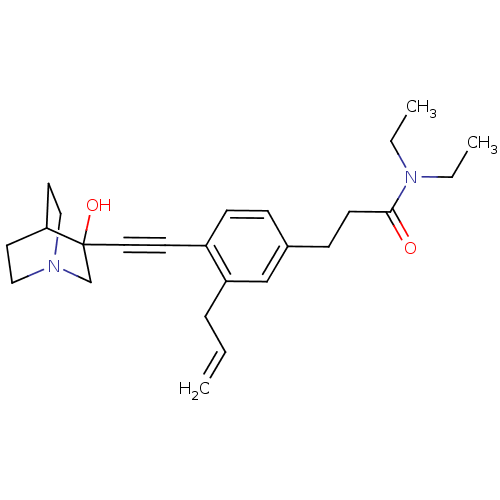
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CCN(CC)C(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:14:15:20.19:22.23,16:15:20.19:22.23,(19.46,-5.19,;18.12,-4.43,;18.1,-2.89,;19.44,-2.11,;20.78,-2.86,;16.76,-2.12,;16.76,-.58,;15.44,-2.91,;14.1,-2.13,;12.77,-2.91,;12.77,-4.46,;11.45,-5.23,;10.12,-4.46,;8.78,-5.24,;7.45,-6.01,;6.19,-6.88,;7.67,-7.29,;6.4,-8.42,;4.96,-7.64,;3.26,-8.25,;3.14,-6.75,;4.78,-6.15,;4.96,-4.38,;5.37,-5.61,;10.09,-2.93,;8.77,-2.17,;7.44,-2.94,;6.1,-2.18,;11.43,-2.15,)| Show InChI InChI=1S/C25H34N2O2/c1-4-7-22-18-20(9-11-24(28)27(5-2)6-3)8-10-21(22)12-15-25(29)19-26-16-13-23(25)14-17-26/h4,8,10,18,23,29H,1,5-7,9,11,13-14,16-17,19H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291319
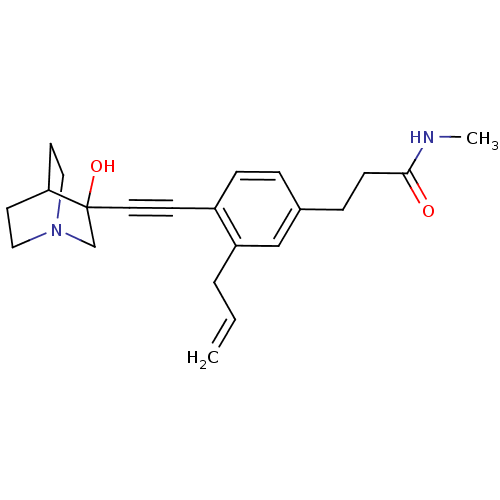
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CNC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:11:12:17.16:19.20,13:12:17.16:19.20,(19.42,-2.1,;18.08,-2.89,;16.75,-2.12,;16.75,-.58,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C22H28N2O2/c1-3-4-19-15-17(6-8-21(25)23-2)5-7-18(19)9-12-22(26)16-24-13-10-20(22)11-14-24/h3,5,7,15,20,26H,1,4,6,8,10-11,13-14,16H2,2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291314
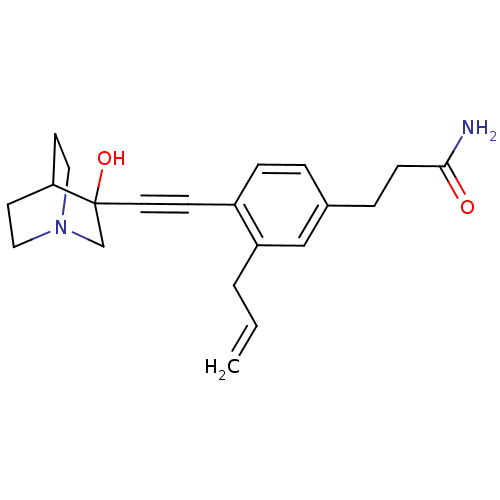
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES NC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:10:11:16.15:18.19,12:11:16.15:18.19,(18.09,-2.89,;16.75,-2.12,;16.75,-.58,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.24,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.64,;3.26,-8.24,;3.14,-6.74,;4.77,-6.15,;4.96,-4.38,;5.36,-5.61,;10.09,-2.93,;8.76,-2.17,;7.43,-2.94,;6.1,-2.18,;11.43,-2.15,)| Show InChI InChI=1S/C21H26N2O2/c1-2-3-18-14-16(5-7-20(22)24)4-6-17(18)8-11-21(25)15-23-12-9-19(21)10-13-23/h2,4,6,14,19,25H,1,3,5,7,9-10,12-13,15H2,(H2,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50029174
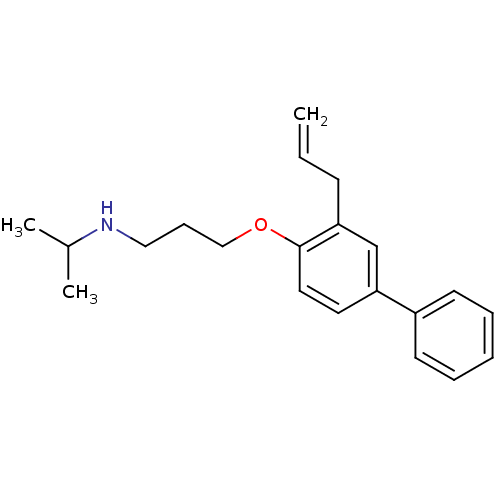
(CHEMBL131973 | N-(1-methylethyl)-3-[(3-prop-2-en-1...)Show InChI InChI=1S/C21H27NO/c1-4-9-20-16-19(18-10-6-5-7-11-18)12-13-21(20)23-15-8-14-22-17(2)3/h4-7,10-13,16-17,22H,1,8-9,14-15H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PubMed
| n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat microsomal squalene synthase |
J Med Chem 38: 4157-60 (1995)
BindingDB Entry DOI: 10.7270/Q21V5D0D |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50029177

(CHEMBL134337 | Propionic acid 3-allyl-4-(3-isoprop...)Show InChI InChI=1S/C18H27NO3/c1-5-8-15-13-16(22-18(20)6-2)9-10-17(15)21-12-7-11-19-14(3)4/h5,9-10,13-14,19H,1,6-8,11-12H2,2-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat microsomal squalene synthase |
J Med Chem 38: 4157-60 (1995)
BindingDB Entry DOI: 10.7270/Q21V5D0D |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50029166
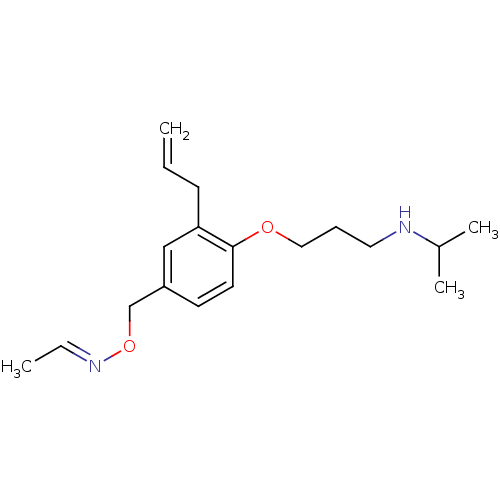
(Acetaldehyde O-[3-allyl-4-(3-isopropylamino-propox...)Show InChI InChI=1S/C18H28N2O2/c1-5-8-17-13-16(14-22-20-6-2)9-10-18(17)21-12-7-11-19-15(3)4/h5-6,9-10,13,15,19H,1,7-8,11-12,14H2,2-4H3/b20-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat microsomal squalene synthase |
J Med Chem 38: 4157-60 (1995)
BindingDB Entry DOI: 10.7270/Q21V5D0D |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50029159
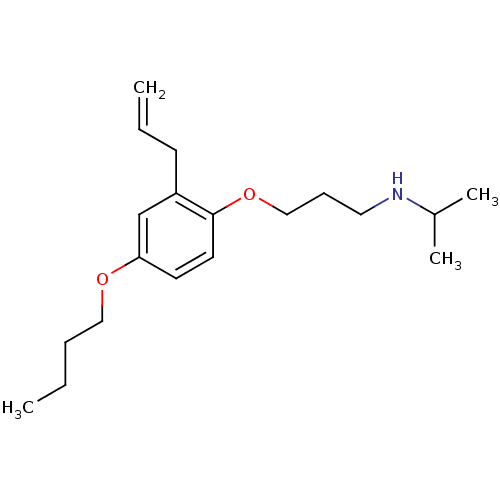
(CHEMBL132881 | [3-(2-Allyl-4-butoxy-phenoxy)-propy...)Show InChI InChI=1S/C19H31NO2/c1-5-7-13-21-18-10-11-19(17(15-18)9-6-2)22-14-8-12-20-16(3)4/h6,10-11,15-16,20H,2,5,7-9,12-14H2,1,3-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat microsomal squalene synthase |
J Med Chem 38: 4157-60 (1995)
BindingDB Entry DOI: 10.7270/Q21V5D0D |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50029171

(CHEMBL341371 | N-[3-Benzyl-4-(3-isopropylamino-pro...)Show InChI InChI=1S/C22H30N2O2/c1-4-22(25)24-20-11-12-21(26-14-8-13-23-17(2)3)19(16-20)15-18-9-6-5-7-10-18/h5-7,9-12,16-17,23H,4,8,13-15H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat microsomal squalene synthase |
J Med Chem 38: 4157-60 (1995)
BindingDB Entry DOI: 10.7270/Q21V5D0D |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50029179
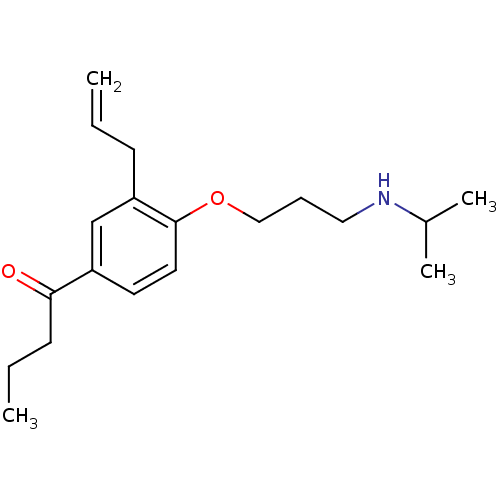
(1-[3-Allyl-4-(3-isopropylamino-propoxy)-phenyl]-bu...)Show InChI InChI=1S/C19H29NO2/c1-5-8-17-14-16(18(21)9-6-2)10-11-19(17)22-13-7-12-20-15(3)4/h5,10-11,14-15,20H,1,6-9,12-13H2,2-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat microsomal squalene synthase |
J Med Chem 38: 4157-60 (1995)
BindingDB Entry DOI: 10.7270/Q21V5D0D |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50029163
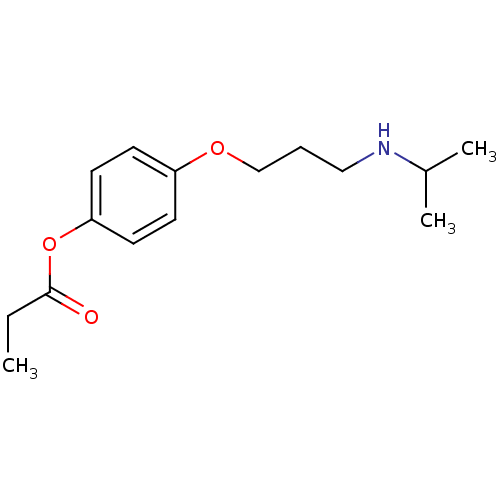
(CHEMBL340992 | Propionic acid 4-(3-isopropylamino-...)Show InChI InChI=1S/C15H23NO3/c1-4-15(17)19-14-8-6-13(7-9-14)18-11-5-10-16-12(2)3/h6-9,12,16H,4-5,10-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat microsomal squalene synthase |
J Med Chem 38: 4157-60 (1995)
BindingDB Entry DOI: 10.7270/Q21V5D0D |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052343
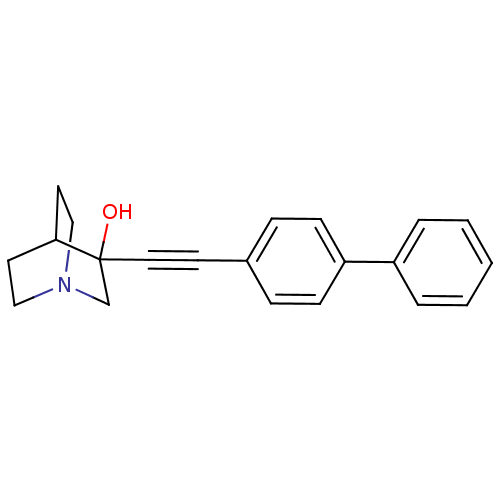
(3-Biphenyl-4-ylethynyl-1-aza-bicyclo[2.2.2]octan-3...)Show SMILES OC1(CN2CCC1CC2)C#Cc1ccc(cc1)-c1ccccc1 |THB:9:1:7.8:5.4,0:1:7.8:5.4,(8.8,-5.03,;8.54,-7.02,;8.83,-8.52,;7.36,-7.82,;5.68,-8.55,;5.47,-7.05,;7.08,-6.36,;7.08,-4.51,;7.64,-5.76,;10.08,-7.02,;10.91,-8.3,;11.75,-9.56,;13.23,-9.22,;14.27,-10.37,;13.78,-11.83,;12.28,-12.18,;11.24,-11.02,;14.83,-12.95,;16.33,-12.63,;17.37,-13.77,;16.92,-15.25,;15.39,-15.56,;14.38,-14.43,)| Show InChI InChI=1S/C21H21NO/c23-21(16-22-14-11-20(21)12-15-22)13-10-17-6-8-19(9-7-17)18-4-2-1-3-5-18/h1-9,20,23H,11-12,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052350
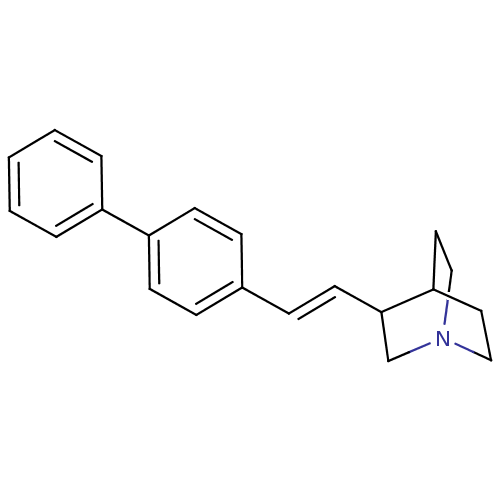
(3-((E)-2-Biphenyl-4-yl-vinyl)-1-aza-bicyclo[2.2.2]...)Show SMILES C1CN2CCC1C(C2)\C=C\c1ccc(cc1)-c1ccccc1 |THB:8:6:4.3:0.1,(9.53,-12.11,;9.74,-13.61,;11.4,-12.88,;11.68,-10.82,;11.12,-9.57,;11.12,-11.41,;12.6,-12.04,;12.88,-13.58,;13.78,-11.1,;15.21,-11.68,;16.43,-10.72,;16.11,-9.22,;17.24,-8.17,;18.7,-8.65,;19.05,-10.15,;17.9,-11.17,;19.85,-7.61,;19.53,-6.11,;20.65,-5.07,;22.14,-5.54,;22.47,-7.04,;21.32,-8.09,)| Show InChI InChI=1S/C21H23N/c1-2-4-18(5-3-1)19-9-6-17(7-10-19)8-11-21-16-22-14-12-20(21)13-15-22/h1-11,20-21H,12-16H2/b11-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50029170
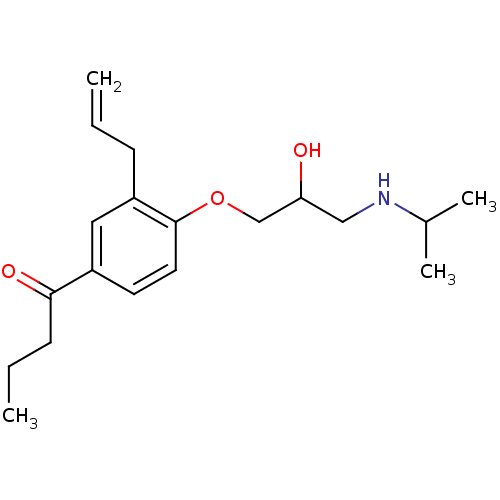
(1-[3-Allyl-4-(2-hydroxy-3-isopropylamino-propoxy)-...)Show InChI InChI=1S/C19H29NO3/c1-5-7-16-11-15(18(22)8-6-2)9-10-19(16)23-13-17(21)12-20-14(3)4/h5,9-11,14,17,20-21H,1,6-8,12-13H2,2-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat microsomal squalene synthase |
J Med Chem 38: 4157-60 (1995)
BindingDB Entry DOI: 10.7270/Q21V5D0D |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50029161
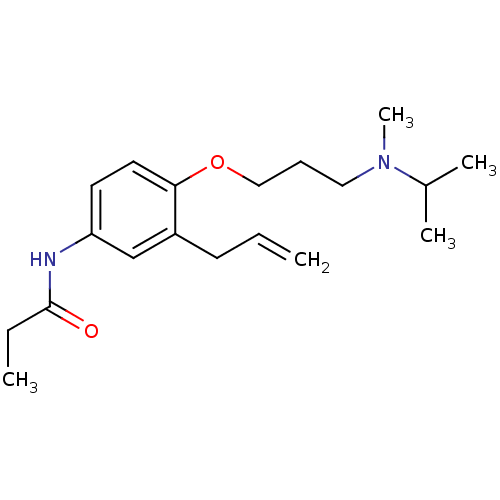
(CHEMBL433864 | N-{3-Allyl-4-[3-(isopropyl-methyl-a...)Show InChI InChI=1S/C19H30N2O2/c1-6-9-16-14-17(20-19(22)7-2)10-11-18(16)23-13-8-12-21(5)15(3)4/h6,10-11,14-15H,1,7-9,12-13H2,2-5H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat microsomal squalene synthase |
J Med Chem 38: 4157-60 (1995)
BindingDB Entry DOI: 10.7270/Q21V5D0D |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052342

(3-(2-Biphenyl-4-yl-ethyl)-1-aza-bicyclo[2.2.2]octa...)Show SMILES C(Cc1ccc(cc1)-c1ccccc1)C1CN2CCC1CC2 |TLB:0:14:20.21:18.17,(13.67,-10.95,;15.1,-11.51,;16.32,-10.57,;16.07,-9.06,;17.28,-8.09,;18.72,-8.65,;18.93,-10.18,;17.74,-11.15,;19.93,-7.7,;19.68,-6.18,;20.88,-5.23,;22.31,-5.78,;22.55,-7.32,;21.33,-8.27,;12.48,-11.91,;12.76,-13.42,;11.29,-12.73,;11.57,-10.67,;11.01,-9.42,;11.01,-11.26,;9.42,-11.96,;9.63,-13.46,)| Show InChI InChI=1S/C21H25N/c1-2-4-18(5-3-1)19-9-6-17(7-10-19)8-11-21-16-22-14-12-20(21)13-15-22/h1-7,9-10,20-21H,8,11-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50075719

(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CCOC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:12:13:18.17:20.21,14:13:18.17:20.21,(10.63,-1.41,;9.54,-2.5,;9.94,-3.98,;8.86,-5.08,;9.25,-6.57,;7.36,-4.68,;6.27,-5.77,;4.78,-5.38,;4.38,-3.89,;2.9,-3.49,;1.81,-4.59,;.33,-4.19,;-1.17,-3.79,;-2.66,-3.4,;-2.26,-1.9,;-3.89,-4.07,;-4.88,-2.49,;-6.75,-2.53,;-5.51,-1.8,;-3.62,-1.88,;-3.15,-.9,;-4.25,-1.27,;2.22,-6.06,;1.13,-7.15,;-.37,-6.75,;-1.45,-7.84,;3.7,-6.46,)| Show InChI InChI=1S/C23H29NO3/c1-3-5-20-16-18(7-9-22(25)27-4-2)6-8-19(20)10-13-23(26)17-24-14-11-21(23)12-15-24/h3,6,8,16,21,26H,1,4-5,7,9,11-12,14-15,17H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was measured for the inhibition of rat microsomal squalene synthase enzyme |
J Med Chem 42: 1306-11 (1999)
Article DOI: 10.1021/jm990038q
BindingDB Entry DOI: 10.7270/Q23X85TQ |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052353

(3-(4-Benzothiazol-2-yl-phenyl)-1-aza-bicyclo[2.2.2...)Show SMILES OC1(CN2CCC1CC2)c1ccc(cc1)-c1nc2ccccc2s1 |THB:0:1:5.4:7.8,9:1:5.4:7.8,(12.16,-7.72,;11.82,-9.91,;12.13,-11.4,;10.64,-10.73,;9,-11.44,;8.79,-9.95,;10.36,-9.25,;10.36,-7.42,;10.92,-8.69,;13.36,-9.94,;14.12,-8.6,;15.65,-8.6,;16.42,-9.94,;15.66,-11.25,;14.12,-11.25,;17.94,-9.91,;18.81,-8.49,;20.29,-9.14,;21.61,-8.35,;22.96,-9.12,;22.96,-10.67,;21.64,-11.44,;20.3,-10.67,;18.84,-11.18,)| Show InChI InChI=1S/C20H20N2OS/c23-20(13-22-11-9-16(20)10-12-22)15-7-5-14(6-8-15)19-21-17-3-1-2-4-18(17)24-19/h1-8,16,23H,9-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052355
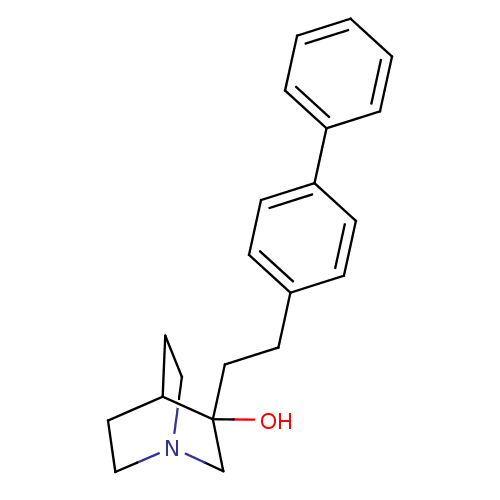
(3-(2-Biphenyl-4-yl-ethyl)-1-aza-bicyclo[2.2.2]octa...)Show SMILES OC1(CCc2ccc(cc2)-c2ccccc2)CN2CCC1CC2 |THB:2:1:21.22:19.18,0:1:21.22:19.18,(12.01,-8.04,;11.74,-9.93,;13.28,-9.93,;14.12,-11.22,;15.68,-11.12,;16.49,-12.41,;18.05,-12.34,;18.73,-10.94,;17.89,-9.65,;16.35,-9.75,;20.26,-10.86,;21.13,-12.15,;22.66,-12.06,;23.36,-10.66,;22.5,-9.37,;20.96,-9.47,;12.05,-11.43,;10.56,-10.75,;8.88,-11.47,;8.67,-9.96,;10.28,-9.27,;10.28,-7.41,;10.84,-8.69,)| Show InChI InChI=1S/C21H25NO/c23-21(16-22-14-11-20(21)12-15-22)13-10-17-6-8-19(9-7-17)18-4-2-1-3-5-18/h1-9,20,23H,10-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052377
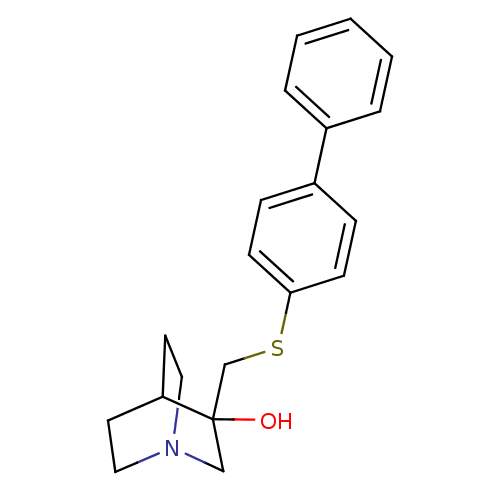
(3-(Biphenyl-4-ylsulfanylmethyl)-1-aza-bicyclo[2.2....)Show SMILES OC1(CSc2ccc(cc2)-c2ccccc2)CN2CCC1CC2 |THB:2:1:21.22:19.18,0:1:21.22:19.18,(12.09,-8,;11.74,-10.14,;13.28,-10.14,;14.12,-11.43,;15.68,-11.32,;16.49,-12.62,;18.05,-12.55,;18.73,-11.15,;17.89,-9.86,;16.35,-9.96,;20.26,-11.07,;21.12,-12.36,;22.66,-12.27,;23.36,-10.87,;22.5,-9.58,;20.96,-9.68,;12.05,-11.64,;10.56,-10.96,;8.88,-11.67,;8.67,-10.17,;10.28,-9.47,;10.28,-7.62,;10.84,-8.9,)| Show InChI InChI=1S/C20H23NOS/c22-20(14-21-12-10-18(20)11-13-21)15-23-19-8-6-17(7-9-19)16-4-2-1-3-5-16/h1-9,18,22H,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50153846
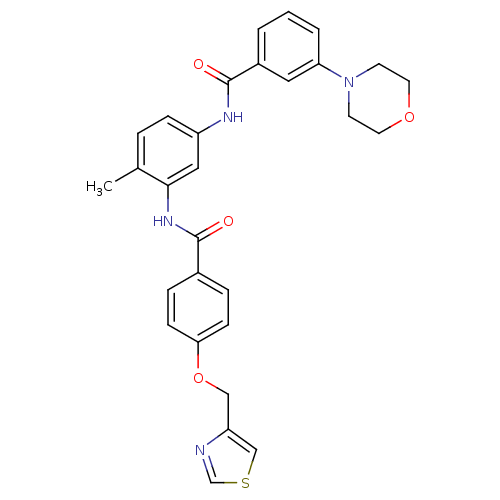
(4-methyl-1-[3-(1,4-oxazinan-4-yl)phenylcarboxamido...)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)N2CCOCC2)cc1NC(=O)c1ccc(OCc2cscn2)cc1 Show InChI InChI=1S/C29H28N4O4S/c1-20-5-8-23(31-29(35)22-3-2-4-25(15-22)33-11-13-36-14-12-33)16-27(20)32-28(34)21-6-9-26(10-7-21)37-17-24-18-38-19-30-24/h2-10,15-16,18-19H,11-14,17H2,1H3,(H,31,35)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Concentration required to inhibit human mitogen activated protein kinase p38 activity |
Bioorg Med Chem Lett 14: 5383-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.006
BindingDB Entry DOI: 10.7270/Q28G8K57 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50034767
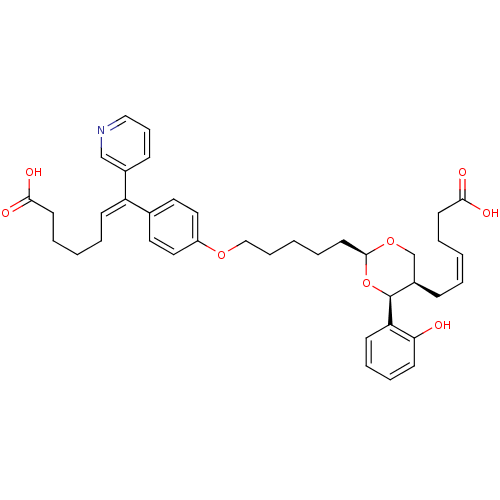
((E)-7-(4-{5-[(2S,4S,5R)-5-((Z)-5-Carboxy-pent-2-en...)Show SMILES OC(=O)CCCC\C=C(/c1ccc(OCCCCC[C@H]2OC[C@@H](C\C=C/CCC(O)=O)[C@H](O2)c2ccccc2O)cc1)c1cccnc1 Show InChI InChI=1S/C39H47NO8/c41-35-17-10-9-16-34(35)39-31(13-4-1-6-18-36(42)43)28-47-38(48-39)20-8-3-11-26-46-32-23-21-29(22-24-32)33(30-14-12-25-40-27-30)15-5-2-7-19-37(44)45/h1,4,9-10,12,14-17,21-25,27,31,38-39,41H,2-3,5-8,11,13,18-20,26,28H2,(H,42,43)(H,44,45)/b4-1-,33-15+/t31-,38+,39+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human microsomal thromboxane synthase. |
J Med Chem 38: 1608-28 (1995)
BindingDB Entry DOI: 10.7270/Q2319TWW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50153832
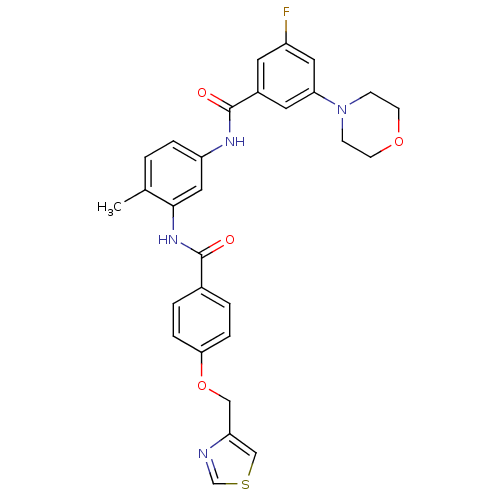
(3-Fluoro-N-{4-methyl-3-[4-(thiazol-4-ylmethoxy)-be...)Show SMILES Cc1ccc(NC(=O)c2cc(F)cc(c2)N2CCOCC2)cc1NC(=O)c1ccc(OCc2cscn2)cc1 Show InChI InChI=1S/C29H27FN4O4S/c1-19-2-5-23(32-29(36)21-12-22(30)14-25(13-21)34-8-10-37-11-9-34)15-27(19)33-28(35)20-3-6-26(7-4-20)38-16-24-17-39-18-31-24/h2-7,12-15,17-18H,8-11,16H2,1H3,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Concentration required to inhibit human mitogen activated protein kinase p38 activity |
Bioorg Med Chem Lett 14: 5383-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.006
BindingDB Entry DOI: 10.7270/Q28G8K57 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50034758

((E)-7-(3-{4-[(2S,4S,5R)-5-((Z)-5-Carboxy-pent-2-en...)Show SMILES OC(=O)CCCC\C=C(\c1cccnc1)c1cccc(OCc2ccc(cc2)[C@H]2OC[C@@H](C\C=C/CCC(O)=O)[C@H](O2)c2ccccc2O)c1 Show InChI InChI=1S/C41H43NO8/c43-37-17-8-7-16-36(37)40-33(11-3-1-5-18-38(44)45)28-49-41(50-40)30-22-20-29(21-23-30)27-48-34-14-9-12-31(25-34)35(32-13-10-24-42-26-32)15-4-2-6-19-39(46)47/h1,3,7-10,12-17,20-26,33,40-41,43H,2,4-6,11,18-19,27-28H2,(H,44,45)(H,46,47)/b3-1-,35-15+/t33-,40+,41+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human microsomal thromboxane synthase. |
J Med Chem 38: 1608-28 (1995)
BindingDB Entry DOI: 10.7270/Q2319TWW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50153848

(CHEMBL184012 | N-{4-Methyl-3-[4-(pyridin-2-ylmetho...)Show SMILES Cc1ccc(NC(=O)c2ccnc(c2)N2CCOCC2)cc1NC(=O)c1ccc(OCc2ccccn2)cc1 Show InChI InChI=1S/C30H29N5O4/c1-21-5-8-24(33-30(37)23-11-13-32-28(18-23)35-14-16-38-17-15-35)19-27(21)34-29(36)22-6-9-26(10-7-22)39-20-25-4-2-3-12-31-25/h2-13,18-19H,14-17,20H2,1H3,(H,33,37)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Concentration required to inhibit human mitogen activated protein kinase p38 activity |
Bioorg Med Chem Lett 14: 5383-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.006
BindingDB Entry DOI: 10.7270/Q28G8K57 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50034762
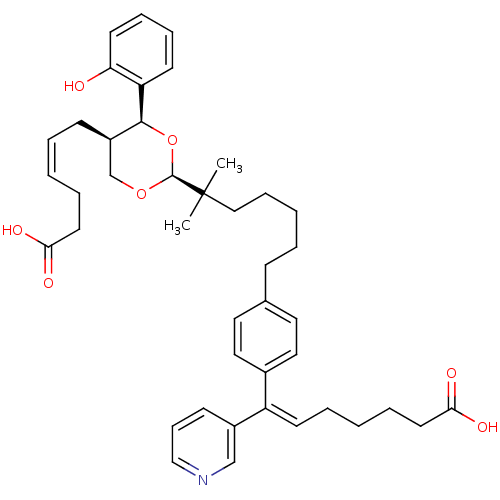
((E)-7-(4-{6-[(2S,4S,5R)-5-((Z)-5-Carboxy-pent-2-en...)Show SMILES CC(C)(CCCCCc1ccc(cc1)C(=C/CCCCC(O)=O)\c1cccnc1)[C@H]1OC[C@@H](C\C=C/CCC(O)=O)[C@H](O1)c1ccccc1O Show InChI InChI=1S/C42H53NO7/c1-42(2,41-49-30-34(16-7-3-9-21-38(45)46)40(50-41)36-19-11-12-20-37(36)44)27-13-5-6-15-31-23-25-32(26-24-31)35(33-17-14-28-43-29-33)18-8-4-10-22-39(47)48/h3,7,11-12,14,17-20,23-26,28-29,34,40-41,44H,4-6,8-10,13,15-16,21-22,27,30H2,1-2H3,(H,45,46)(H,47,48)/b7-3-,35-18+/t34-,40+,41+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human microsomal thromboxane synthase. |
J Med Chem 38: 1608-28 (1995)
BindingDB Entry DOI: 10.7270/Q2319TWW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50153821
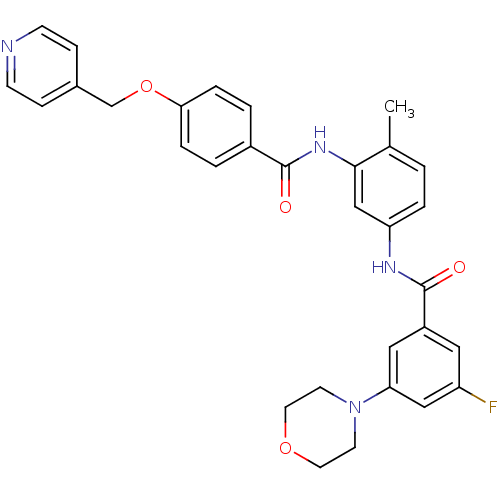
(3-Fluoro-N-{4-methyl-3-[4-(pyridin-4-ylmethoxy)-be...)Show SMILES Cc1ccc(NC(=O)c2cc(F)cc(c2)N2CCOCC2)cc1NC(=O)c1ccc(OCc2ccncc2)cc1 Show InChI InChI=1S/C31H29FN4O4/c1-21-2-5-26(34-31(38)24-16-25(32)18-27(17-24)36-12-14-39-15-13-36)19-29(21)35-30(37)23-3-6-28(7-4-23)40-20-22-8-10-33-11-9-22/h2-11,16-19H,12-15,20H2,1H3,(H,34,38)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Concentration required to inhibit human mitogen activated protein kinase p38 activity |
Bioorg Med Chem Lett 14: 5383-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.006
BindingDB Entry DOI: 10.7270/Q28G8K57 |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052376
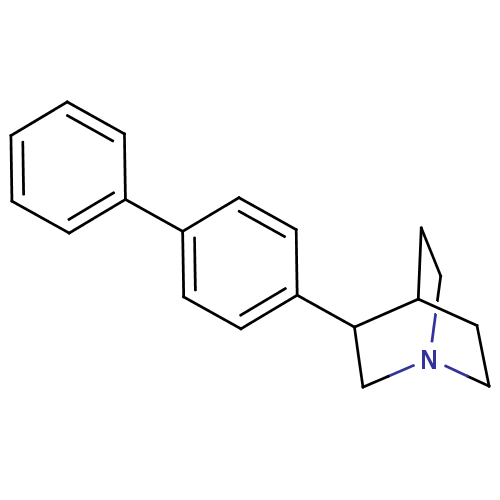
(3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]octane | CHEMB...)Show SMILES C1CN2CCC1C(C2)c1ccc(cc1)-c1ccccc1 |TLB:8:6:4.3:0.1,(10.23,-6.77,;10.66,-7.87,;10.41,-9.76,;8.89,-10.44,;8.69,-9.06,;10.15,-8.42,;11.5,-9.02,;11.79,-10.41,;13.04,-9.02,;13.81,-10.36,;15.35,-10.36,;16.12,-9.03,;15.35,-7.68,;13.81,-7.68,;17.66,-9.03,;18.43,-7.68,;19.97,-7.68,;20.74,-9.02,;19.97,-10.34,;18.43,-10.34,)| Show InChI InChI=1S/C19H21N/c1-2-4-15(5-3-1)16-6-8-17(9-7-16)19-14-20-12-10-18(19)11-13-20/h1-9,18-19H,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50036368
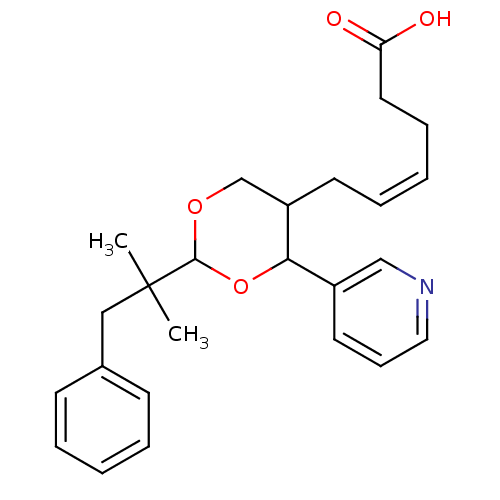
((Z)-6-[2-(1,1-Dimethyl-2-phenyl-ethyl)-4-pyridin-3...)Show SMILES CC(C)(Cc1ccccc1)C1OCC(C\C=C/CCC(O)=O)C(O1)c1cccnc1 Show InChI InChI=1S/C25H31NO4/c1-25(2,16-19-10-5-3-6-11-19)24-29-18-21(12-7-4-8-14-22(27)28)23(30-24)20-13-9-15-26-17-20/h3-7,9-11,13,15,17,21,23-24H,8,12,14,16,18H2,1-2H3,(H,27,28)/b7-4- | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro Inhibition of thromboxane synthase from human blood platelet microsomes |
J Med Chem 38: 686-94 (1995)
BindingDB Entry DOI: 10.7270/Q24Q7T2K |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50029172
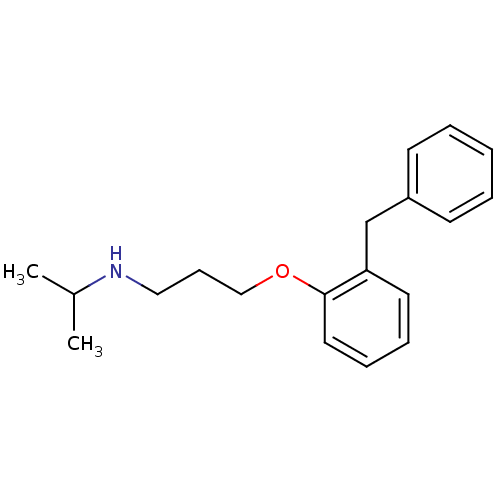
(CHEMBL131930 | [3-(2-Benzyl-phenoxy)-propyl]-isopr...)Show InChI InChI=1S/C19H25NO/c1-16(2)20-13-8-14-21-19-12-7-6-11-18(19)15-17-9-4-3-5-10-17/h3-7,9-12,16,20H,8,13-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat microsomal squalene synthase |
J Med Chem 38: 4157-60 (1995)
BindingDB Entry DOI: 10.7270/Q21V5D0D |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052358

(3-(Biphenyl-4-yloxymethyl)-1-aza-bicyclo[2.2.2]oct...)Show SMILES OC1(COc2ccc(cc2)-c2ccccc2)CN2CCC1CC2 |THB:2:1:21.22:19.18,0:1:21.22:19.18,(12.09,-8,;11.74,-9.89,;13.28,-9.89,;14.12,-11.17,;15.68,-11.08,;16.49,-12.37,;18.05,-12.29,;18.73,-10.91,;17.89,-9.61,;16.35,-9.71,;20.26,-10.8,;21.13,-12.09,;22.66,-12.01,;23.36,-10.63,;22.5,-9.34,;20.96,-9.44,;12.05,-11.4,;10.56,-10.7,;8.88,-11.43,;8.67,-9.93,;10.28,-9.23,;10.28,-7.38,;10.84,-8.64,)| Show InChI InChI=1S/C20H23NO2/c22-20(14-21-12-10-18(20)11-13-21)15-23-19-8-6-17(7-9-19)16-4-2-1-3-5-16/h1-9,18,22H,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50036355
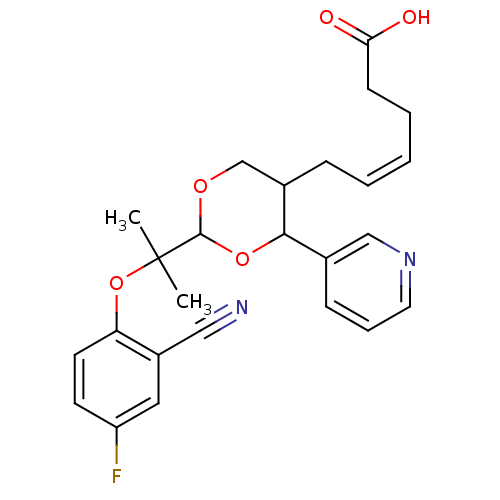
((Z)-6-{2-[1-(2-Cyano-4-fluoro-phenoxy)-1-methyl-et...)Show SMILES CC(C)(Oc1ccc(F)cc1C#N)C1OCC(C\C=C/CCC(O)=O)C(O1)c1cccnc1 Show InChI InChI=1S/C25H27FN2O5/c1-25(2,33-21-11-10-20(26)13-19(21)14-27)24-31-16-18(7-4-3-5-9-22(29)30)23(32-24)17-8-6-12-28-15-17/h3-4,6,8,10-13,15,18,23-24H,5,7,9,16H2,1-2H3,(H,29,30)/b4-3- | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro Inhibition of thromboxane synthase from human blood platelet microsomes |
J Med Chem 38: 686-94 (1995)
BindingDB Entry DOI: 10.7270/Q24Q7T2K |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052351
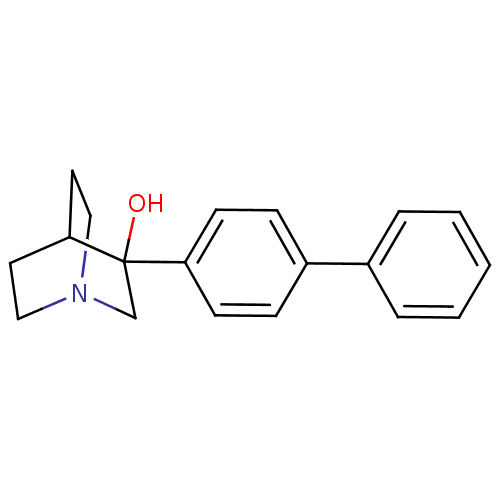
(3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]octan-3-ol | 3...)Show SMILES OC1(CN2CCC1CC2)c1ccc(cc1)-c1ccccc1 |(10.58,-8.89,;10.59,-10.42,;10.59,-11.97,;9.25,-12.74,;7.92,-11.97,;7.92,-10.42,;9.25,-9.65,;8.51,-11,;10,-11.41,;11.92,-9.66,;13.25,-10.43,;14.59,-9.66,;14.59,-8.12,;13.25,-7.35,;11.92,-8.12,;15.92,-7.35,;17.25,-8.12,;18.58,-7.36,;18.58,-5.81,;17.24,-5.04,;15.91,-5.82,)| Show InChI InChI=1S/C19H21NO/c21-19(14-20-12-10-18(19)11-13-20)17-8-6-16(7-9-17)15-4-2-1-3-5-15/h1-9,18,21H,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was measured for the inhibition of rat microsomal squalene synthase enzyme |
J Med Chem 42: 1306-11 (1999)
Article DOI: 10.1021/jm990038q
BindingDB Entry DOI: 10.7270/Q23X85TQ |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052351

(3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]octan-3-ol | 3...)Show SMILES OC1(CN2CCC1CC2)c1ccc(cc1)-c1ccccc1 |(10.58,-8.89,;10.59,-10.42,;10.59,-11.97,;9.25,-12.74,;7.92,-11.97,;7.92,-10.42,;9.25,-9.65,;8.51,-11,;10,-11.41,;11.92,-9.66,;13.25,-10.43,;14.59,-9.66,;14.59,-8.12,;13.25,-7.35,;11.92,-8.12,;15.92,-7.35,;17.25,-8.12,;18.58,-7.36,;18.58,-5.81,;17.24,-5.04,;15.91,-5.82,)| Show InChI InChI=1S/C19H21NO/c21-19(14-20-12-10-18(19)11-13-20)17-8-6-16(7-9-17)15-4-2-1-3-5-15/h1-9,18,21H,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50010960
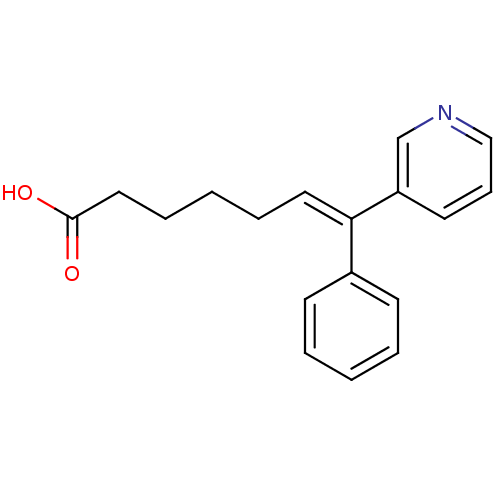
((E)-7-Phenyl-7-pyridin-3-yl-hept-6-enoic acid | 7-...)Show InChI InChI=1S/C18H19NO2/c20-18(21)12-6-2-5-11-17(15-8-3-1-4-9-15)16-10-7-13-19-14-16/h1,3-4,7-11,13-14H,2,5-6,12H2,(H,20,21)/b17-11+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro Inhibition of thromboxane synthase from human blood platelet microsomes |
J Med Chem 38: 686-94 (1995)
BindingDB Entry DOI: 10.7270/Q24Q7T2K |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052366

(3-(4'-Fluoro-biphenyl-4-yl)-1-aza-bicyclo[2.2.2]oc...)Show SMILES OC1(CN2CCC1CC2)c1ccc(cc1)-c1ccc(F)cc1 |THB:9:1:5.4:7.8,0:1:5.4:7.8,(12.37,-8.38,;12.18,-10.06,;12.47,-11.56,;10.99,-10.89,;9.32,-11.6,;9.11,-10.09,;10.71,-9.39,;10.71,-7.54,;11.27,-8.82,;13.73,-10.08,;14.5,-11.39,;16.04,-11.39,;16.81,-10.08,;16.04,-8.73,;14.5,-8.73,;18.38,-10.06,;19.12,-8.73,;20.66,-8.73,;21.46,-10.06,;23.01,-10.06,;20.69,-11.39,;19.12,-11.39,)| Show InChI InChI=1S/C19H20FNO/c20-18-7-3-15(4-8-18)14-1-5-16(6-2-14)19(22)13-21-11-9-17(19)10-12-21/h1-8,17,22H,9-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50052352

(3-Biphenyl-4-yl-1-aza-bicyclo[2.2.2]oct-2-ene | CH...)Show SMILES C1CN2CCC1C(=C2)c1ccc(cc1)-c1ccccc1 |c:7,THB:8:6:0.1:4.3,(8.69,-9.06,;8.89,-10.44,;10.41,-9.76,;10.66,-7.87,;10.23,-6.77,;10.15,-8.42,;11.52,-9.02,;11.79,-10.41,;13.06,-9.02,;13.81,-10.36,;15.35,-10.36,;16.12,-9.03,;15.35,-7.68,;13.81,-7.68,;17.66,-9.03,;18.43,-7.68,;19.97,-7.68,;20.74,-9.02,;19.97,-10.34,;18.43,-10.34,)| Show InChI InChI=1S/C19H19N/c1-2-4-15(5-3-1)16-6-8-17(9-7-16)19-14-20-12-10-18(19)11-13-20/h1-9,14,18H,10-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition against rat microsomal squalene synthase (SS) |
J Med Chem 39: 2971-9 (1996)
Article DOI: 10.1021/jm950907l
BindingDB Entry DOI: 10.7270/Q24J0D7G |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50036354
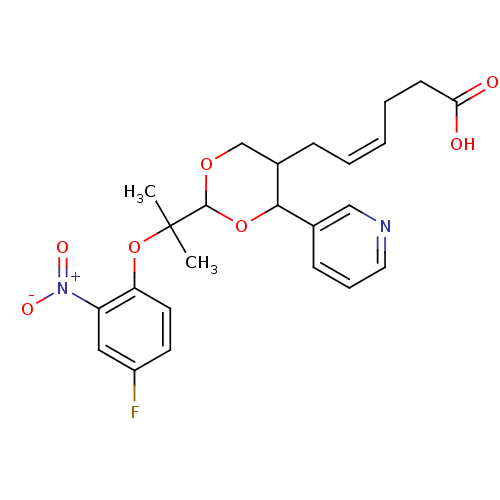
((Z)-6-{2-[1-(4-Fluoro-2-nitro-phenoxy)-1-methyl-et...)Show SMILES CC(C)(Oc1ccc(F)cc1[N+]([O-])=O)C1OCC(C\C=C/CCC(O)=O)C(O1)c1cccnc1 Show InChI InChI=1S/C24H27FN2O7/c1-24(2,34-20-11-10-18(25)13-19(20)27(30)31)23-32-15-17(7-4-3-5-9-21(28)29)22(33-23)16-8-6-12-26-14-16/h3-4,6,8,10-14,17,22-23H,5,7,9,15H2,1-2H3,(H,28,29)/b4-3- | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro Inhibition of thromboxane synthase from human blood platelet microsomes |
J Med Chem 38: 686-94 (1995)
BindingDB Entry DOI: 10.7270/Q24Q7T2K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50153847
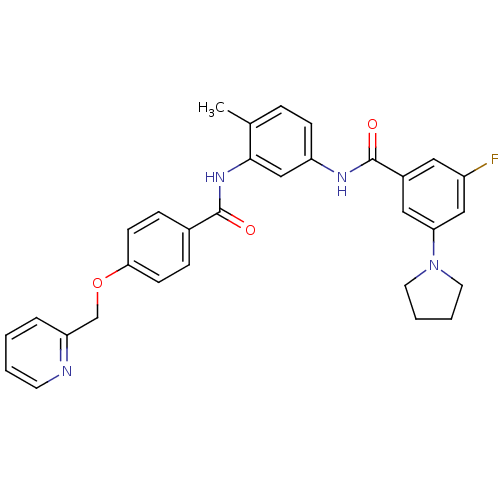
(3-Fluoro-N-{4-methyl-3-[4-(pyridin-2-ylmethoxy)-be...)Show SMILES Cc1ccc(NC(=O)c2cc(F)cc(c2)N2CCCC2)cc1NC(=O)c1ccc(OCc2ccccn2)cc1 Show InChI InChI=1S/C31H29FN4O3/c1-21-7-10-25(34-31(38)23-16-24(32)18-27(17-23)36-14-4-5-15-36)19-29(21)35-30(37)22-8-11-28(12-9-22)39-20-26-6-2-3-13-33-26/h2-3,6-13,16-19H,4-5,14-15,20H2,1H3,(H,34,38)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Concentration required to inhibit human mitogen activated protein kinase p38 activity |
Bioorg Med Chem Lett 14: 5383-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.006
BindingDB Entry DOI: 10.7270/Q28G8K57 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50153833
(CHEMBL365688 | N-{4-Methyl-3-[4-(pyridin-2-ylmetho...)Show SMILES Cc1ccc(NC(=O)c2cc(cc(c2)C(F)(F)F)N2CCOCC2)cc1NC(=O)c1ccc(OCc2ccccn2)cc1 Show InChI InChI=1S/C32H29F3N4O4/c1-21-5-8-25(37-31(41)23-16-24(32(33,34)35)18-27(17-23)39-12-14-42-15-13-39)19-29(21)38-30(40)22-6-9-28(10-7-22)43-20-26-4-2-3-11-36-26/h2-11,16-19H,12-15,20H2,1H3,(H,37,41)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Concentration required to inhibit human mitogen activated protein kinase p38 activity |
Bioorg Med Chem Lett 14: 5383-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.006
BindingDB Entry DOI: 10.7270/Q28G8K57 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50288601

((Z)-6-[(2S,4S,5R)-2-(4-Cyano-phenyl)-4-pyridin-3-y...)Show SMILES OC(=O)CC\C=C/C[C@@H]1CO[C@@H](O[C@@H]1c1cccnc1)c1ccc(cc1)C#N Show InChI InChI=1S/C22H22N2O4/c23-13-16-8-10-17(11-9-16)22-27-15-19(5-2-1-3-7-20(25)26)21(28-22)18-6-4-12-24-14-18/h1-2,4,6,8-12,14,19,21-22H,3,5,7,15H2,(H,25,26)/b2-1-/t19-,21-,22+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for thromboxane TXA2 synthase inhibitory activity using human platelet |
Bioorg Med Chem Lett 6: 273-278 (1996)
Article DOI: 10.1016/0960-894X(96)00004-2
BindingDB Entry DOI: 10.7270/Q2PG1RR0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM13336
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Concentration required to inhibit human mitogen activated protein kinase p38 activity |
Bioorg Med Chem Lett 14: 5383-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.006
BindingDB Entry DOI: 10.7270/Q28G8K57 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50153845
(4-methyl-3-[4-(2-methyl-1,3-thiazol-4-ylmethoxy)ph...)Show SMILES Cc1nc(COc2ccc(cc2)C(=O)Nc2cc(NC(=O)c3cccc(c3)N3CCOCC3)ccc2C)cs1 Show InChI InChI=1S/C30H30N4O4S/c1-20-6-9-24(32-30(36)23-4-3-5-26(16-23)34-12-14-37-15-13-34)17-28(20)33-29(35)22-7-10-27(11-8-22)38-18-25-19-39-21(2)31-25/h3-11,16-17,19H,12-15,18H2,1-2H3,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Concentration required to inhibit human mitogen activated protein kinase p38 activity |
Bioorg Med Chem Lett 14: 5383-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.006
BindingDB Entry DOI: 10.7270/Q28G8K57 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data