 Found 5343 hits with Last Name = 'brown' and Initial = 'mf'
Found 5343 hits with Last Name = 'brown' and Initial = 'mf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0269 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.545 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.679 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human GST-tagged cytoplasmic ITK expressed in baculovirus expression system by KT236 probe based TR-FRET assay |
J Med Chem 60: 1971-1993 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01694
BindingDB Entry DOI: 10.7270/Q2TX3JC2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 32.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 62.3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D
| Assay Description
Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... |
ACS Chem Biol 11: 3442-3451 (2016)
Article DOI: 10.1021/acschembio.6b00677
BindingDB Entry DOI: 10.7270/Q2PN94F8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged TXK using GEPLYWSFPAKKK as substrate measured every 60 secs for 1 hr by PK/LDH coupled spectrop... |
J Med Chem 60: 1971-1993 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01694
BindingDB Entry DOI: 10.7270/Q2TX3JC2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human norepinephrine transporter |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged cytoplasmic BMX expressed in baculovirus expression system by KT236 probe based TR-FRET assay |
J Med Chem 60: 1971-1993 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01694
BindingDB Entry DOI: 10.7270/Q2TX3JC2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 679 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human His-tagged TEC by KT178 probe based TR-FRET assay |
J Med Chem 60: 1971-1993 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01694
BindingDB Entry DOI: 10.7270/Q2TX3JC2 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP12 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged cytoplasmic JAK3 catalytic domain (781 to 1124 residues) expressed in baculovirus expression system by KT2... |
J Med Chem 60: 1971-1993 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01694
BindingDB Entry DOI: 10.7270/Q2TX3JC2 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP13 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP7 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP3 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP1 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human TACE |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-26
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP26 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-25
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP25 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-24
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP24 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-20
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP20 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-15
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP15 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Stromelysin-2
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP10 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP8 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP14 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-16
(Homo sapiens (Human)) | BDBM50365344

(CHEMBL1956115)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccccc1)(C(=O)NO)S(C)(=O)=O |r| Show InChI InChI=1S/C18H21NO4S/c1-18(17(20)19-21,24(2,22)23)13-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,21H,12-13H2,1-2H3,(H,19,20)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP16 |
J Med Chem 55: 914-23 (2012)
Article DOI: 10.1021/jm2014748
BindingDB Entry DOI: 10.7270/Q2VX0H0W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged cytoplasmic BLK expressed in baculovirus expression system by KT236 probe based TR-FRET assay |
J Med Chem 60: 1971-1993 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01694
BindingDB Entry DOI: 10.7270/Q2TX3JC2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM209866

(PF-06651600 | US11111242, Example 5 | US2023034848...)Show SMILES C[C@H]1CC[C@H](CN1C(=O)C=C)Nc1ncnc2[nH]ccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 6.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged cytoplasmic BTK expressed in baculovirus expression system by KT236 probe based TR-FRET assay |
J Med Chem 60: 1971-1993 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01694
BindingDB Entry DOI: 10.7270/Q2TX3JC2 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM135525
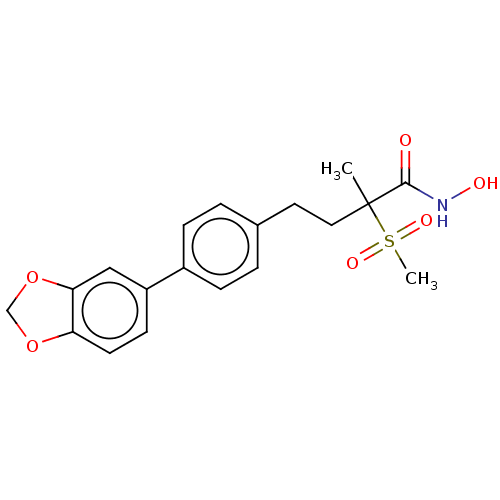
(US8853258, 176)Show SMILES CC(CCc1ccc(cc1)-c1ccc2OCOc2c1)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C19H21NO6S/c1-19(18(21)20-22,27(2,23)24)10-9-13-3-5-14(6-4-13)15-7-8-16-17(11-15)26-12-25-16/h3-8,11,22H,9-10,12H2,1-2H3,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0303 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Pfizer Inc.
US Patent
| Assay Description
IC50 determination in the LpxC enzyme assay was carried out in a similar manner to that described by Malikzay et al in the 2006 Poster, Screening Lpx... |
US Patent US8853258 (2014)
BindingDB Entry DOI: 10.7270/Q25M64DP |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM135565
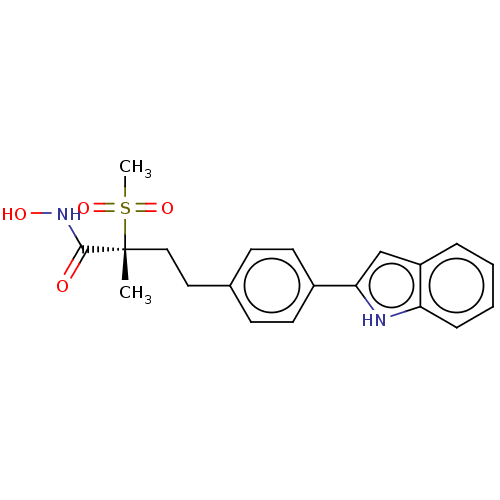
(US8853258, 218)Show SMILES C[C@@](CCc1ccc(cc1)-c1cc2ccccc2[nH]1)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C20H22N2O4S/c1-20(19(23)22-24,27(2,25)26)12-11-14-7-9-15(10-8-14)18-13-16-5-3-4-6-17(16)21-18/h3-10,13,21,24H,11-12H2,1-2H3,(H,22,23)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Pfizer Inc.
US Patent
| Assay Description
IC50 determination in the LpxC enzyme assay was carried out in a similar manner to that described by Malikzay et al in the 2006 Poster, Screening Lpx... |
US Patent US8853258 (2014)
BindingDB Entry DOI: 10.7270/Q25M64DP |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM135527
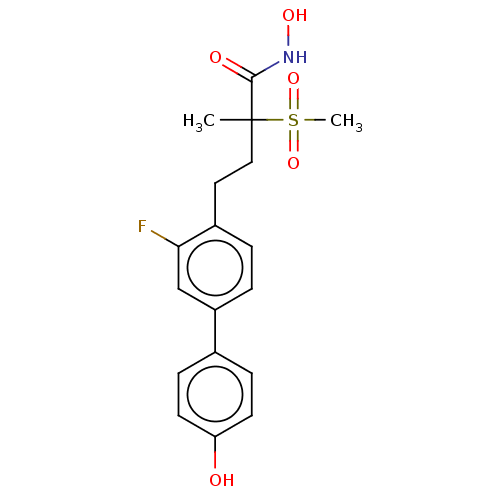
(US8853258, 178)Show SMILES CC(CCc1ccc(cc1F)-c1ccc(O)cc1)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C18H20FNO5S/c1-18(17(22)20-23,26(2,24)25)10-9-13-3-4-14(11-16(13)19)12-5-7-15(21)8-6-12/h3-8,11,21,23H,9-10H2,1-2H3,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0592 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Pfizer Inc.
US Patent
| Assay Description
IC50 determination in the LpxC enzyme assay was carried out in a similar manner to that described by Malikzay et al in the 2006 Poster, Screening Lpx... |
US Patent US8853258 (2014)
BindingDB Entry DOI: 10.7270/Q25M64DP |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM135477
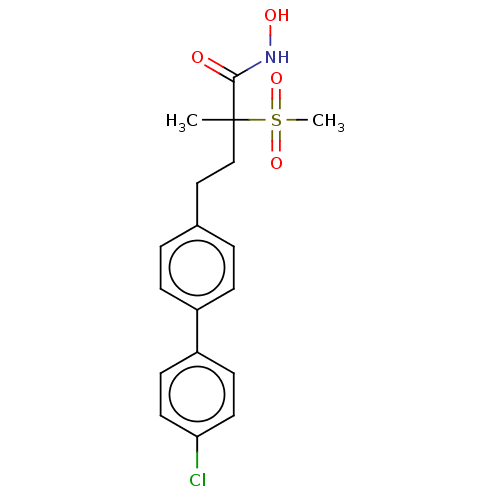
(US8853258, 44)Show SMILES CC(CCc1ccc(cc1)-c1ccc(Cl)cc1)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C18H20ClNO4S/c1-18(17(21)20-22,25(2,23)24)12-11-13-3-5-14(6-4-13)15-7-9-16(19)10-8-15/h3-10,22H,11-12H2,1-2H3,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0669 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Pfizer Inc.
US Patent
| Assay Description
IC50 determination in the LpxC enzyme assay was carried out in a similar manner to that described by Malikzay et al in the 2006 Poster, Screening Lpx... |
US Patent US8853258 (2014)
BindingDB Entry DOI: 10.7270/Q25M64DP |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM135531
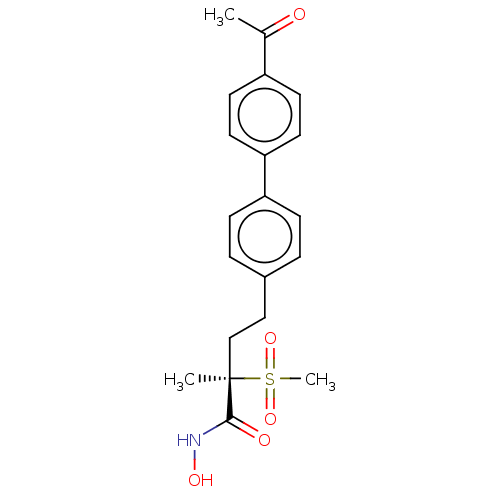
(US8853258, 182)Show SMILES CC(=O)c1ccc(cc1)-c1ccc(CC[C@](C)(C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C20H23NO5S/c1-14(22)16-8-10-18(11-9-16)17-6-4-15(5-7-17)12-13-20(2,19(23)21-24)27(3,25)26/h4-11,24H,12-13H2,1-3H3,(H,21,23)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0674 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Pfizer Inc.
US Patent
| Assay Description
IC50 determination in the LpxC enzyme assay was carried out in a similar manner to that described by Malikzay et al in the 2006 Poster, Screening Lpx... |
US Patent US8853258 (2014)
BindingDB Entry DOI: 10.7270/Q25M64DP |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM135562
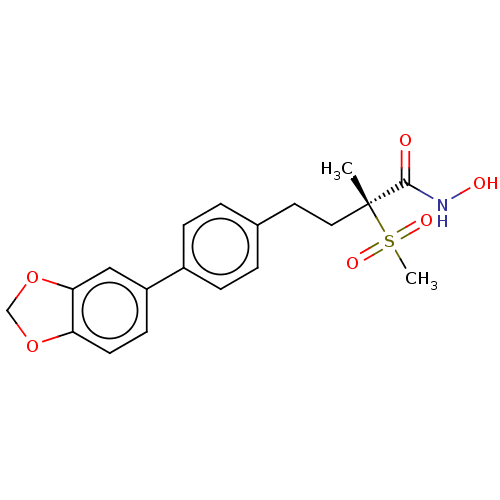
(US8853258, 215)Show SMILES C[C@@](CCc1ccc(cc1)-c1ccc2OCOc2c1)(C(=O)NO)S(C)(=O)=O Show InChI InChI=1S/C19H21NO6S/c1-19(18(21)20-22,27(2,23)24)10-9-13-3-5-14(6-4-13)15-7-8-16-17(11-15)26-12-25-16/h3-8,11,22H,9-10,12H2,1-2H3,(H,20,21)/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0842 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Pfizer Inc.
US Patent
| Assay Description
IC50 determination in the LpxC enzyme assay was carried out in a similar manner to that described by Malikzay et al in the 2006 Poster, Screening Lpx... |
US Patent US8853258 (2014)
BindingDB Entry DOI: 10.7270/Q25M64DP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM316711
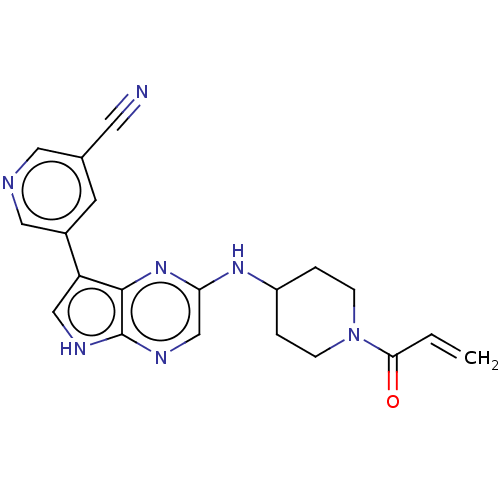
(5-{2-[(1-acryloylpiperidin-4-yl)amino]-5H-pyrrolo[...)Show SMILES C=CC(=O)N1CCC(CC1)Nc1cnc2[nH]cc(-c3cncc(c3)C#N)c2n1 Show InChI InChI=1S/C20H19N7O/c1-2-18(28)27-5-3-15(4-6-27)25-17-12-24-20-19(26-17)16(11-23-20)14-7-13(8-21)9-22-10-14/h2,7,9-12,15H,1,3-6H2,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Test article was solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. An 11-point half log dilution series was created in DMSO... |
US Patent US9617258 (2017)
BindingDB Entry DOI: 10.7270/Q2JD4ZWG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM316746
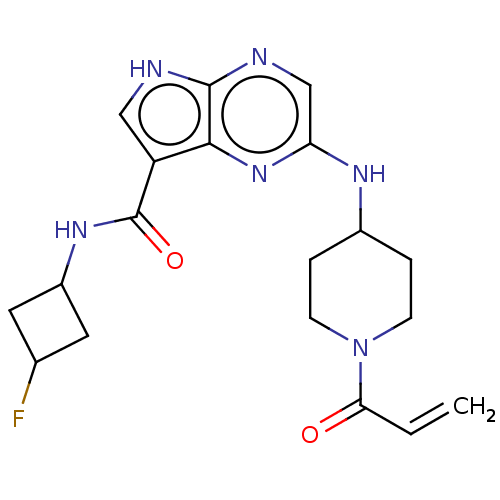
(2-[(1-acryloylpiperidin-4-yl)amino]-N-(3-fluorocyc...)Show SMILES FC1CC(C1)NC(=O)c1c[nH]c2ncc(NC3CCN(CC3)C(=O)C=C)nc12 |(-8.91,2.66,;-7.82,1.57,;-6.28,1.57,;-6.28,.03,;-7.82,.03,;-5.19,-1.06,;-3.7,-.66,;-3.3,.83,;-2.61,-1.75,;-2.77,-3.28,;-1.37,-3.91,;-.33,-2.76,;1.21,-2.76,;1.98,-1.43,;1.21,-.09,;1.98,1.24,;3.52,1.24,;4.29,-.09,;5.83,-.09,;6.6,1.24,;5.83,2.57,;4.29,2.57,;8.14,1.24,;8.91,-.09,;8.91,2.57,;8.14,3.91,;-.33,-.09,;-1.1,-1.43,)| Show InChI InChI=1S/C19H23FN6O2/c1-2-16(27)26-5-3-12(4-6-26)23-15-10-22-18-17(25-15)14(9-21-18)19(28)24-13-7-11(20)8-13/h2,9-13H,1,3-8H2,(H,21,22)(H,23,25)(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
JAK Caliper Enzyme Assay at 4 μM: Test article was solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. An 11-point half ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29026XR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM316774

(2-[(1-acryloylpiperidin-4-yl)amino]-N-(cyclopentyl...)Show SMILES C=CC(=O)N1CCC(CC1)Nc1cnc2[nH]cc(C(=O)NCC3CCCC3)c2n1 Show InChI InChI=1S/C21H28N6O2/c1-2-18(28)27-9-7-15(8-10-27)25-17-13-23-20-19(26-17)16(12-22-20)21(29)24-11-14-5-3-4-6-14/h2,12-15H,1,3-11H2,(H,22,23)(H,24,29)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Test article was solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. An 11-point half log dilution series was created in DMSO... |
US Patent US9617258 (2017)
BindingDB Entry DOI: 10.7270/Q2JD4ZWG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM316739
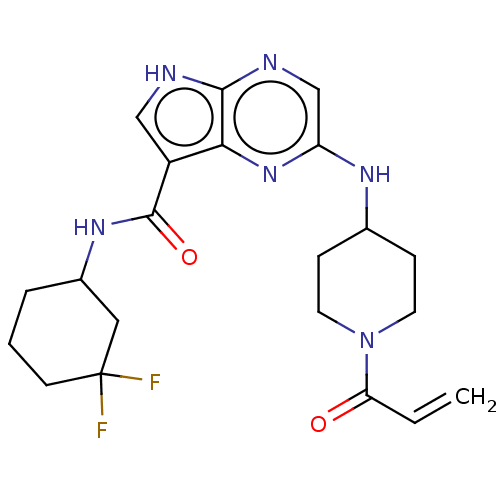
(2-[(1-acryloylpiperidin-4-yl)amino]-N-(3,3-difluor...)Show SMILES FC1(F)CCCC(C1)NC(=O)c1c[nH]c2ncc(NC3CCN(CC3)C(=O)C=C)nc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Test article was solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. An 11-point half log dilution series was created in DMSO... |
US Patent US9617258 (2017)
BindingDB Entry DOI: 10.7270/Q2JD4ZWG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM316743
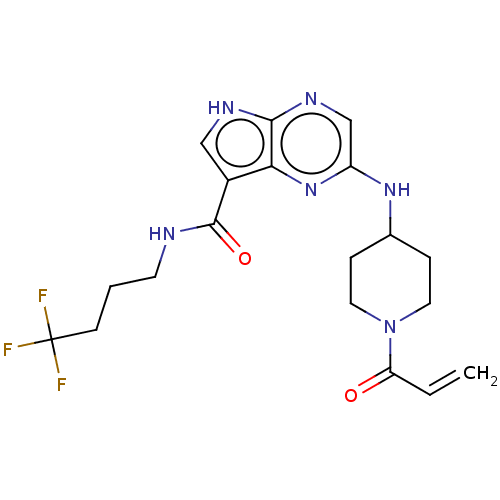
(2-[(1-acryloylpiperidin-4-yl)amino]-N-(4,4,4-trifl...)Show SMILES FC(F)(F)CCCNC(=O)c1c[nH]c2ncc(NC3CCN(CC3)C(=O)C=C)nc12 Show InChI InChI=1S/C19H23F3N6O2/c1-2-15(29)28-8-4-12(5-9-28)26-14-11-25-17-16(27-14)13(10-24-17)18(30)23-7-3-6-19(20,21)22/h2,10-12H,1,3-9H2,(H,23,30)(H,24,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Test article was solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. An 11-point half log dilution series was created in DMSO... |
US Patent US9617258 (2017)
BindingDB Entry DOI: 10.7270/Q2JD4ZWG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM316745
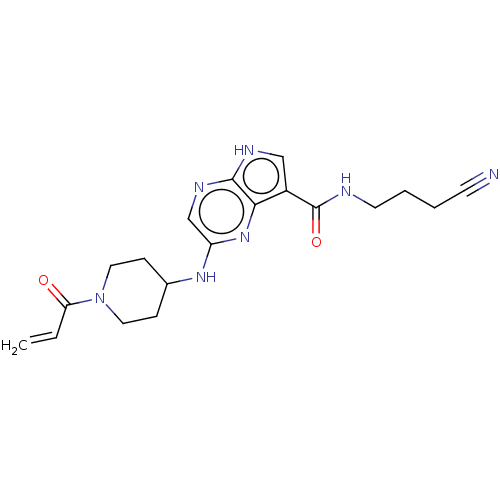
(2-[(1-acryloylpiperidin-4-yl)amino]-N-(3-cyanoprop...)Show SMILES C=CC(=O)N1CCC(CC1)Nc1cnc2[nH]cc(C(=O)NCCCC#N)c2n1 Show InChI InChI=1S/C19H23N7O2/c1-2-16(27)26-9-5-13(6-10-26)24-15-12-23-18-17(25-15)14(11-22-18)19(28)21-8-4-3-7-20/h2,11-13H,1,3-6,8-10H2,(H,21,28)(H,22,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Test article was solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. An 11-point half log dilution series was created in DMSO... |
US Patent US9617258 (2017)
BindingDB Entry DOI: 10.7270/Q2JD4ZWG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM316746

(2-[(1-acryloylpiperidin-4-yl)amino]-N-(3-fluorocyc...)Show SMILES FC1CC(C1)NC(=O)c1c[nH]c2ncc(NC3CCN(CC3)C(=O)C=C)nc12 |(-8.91,2.66,;-7.82,1.57,;-6.28,1.57,;-6.28,.03,;-7.82,.03,;-5.19,-1.06,;-3.7,-.66,;-3.3,.83,;-2.61,-1.75,;-2.77,-3.28,;-1.37,-3.91,;-.33,-2.76,;1.21,-2.76,;1.98,-1.43,;1.21,-.09,;1.98,1.24,;3.52,1.24,;4.29,-.09,;5.83,-.09,;6.6,1.24,;5.83,2.57,;4.29,2.57,;8.14,1.24,;8.91,-.09,;8.91,2.57,;8.14,3.91,;-.33,-.09,;-1.1,-1.43,)| Show InChI InChI=1S/C19H23FN6O2/c1-2-16(27)26-5-3-12(4-6-26)23-15-10-22-18-17(25-15)14(9-21-18)19(28)24-13-7-11(20)8-13/h2,9-13H,1,3-8H2,(H,21,22)(H,23,25)(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Test article was solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. An 11-point half log dilution series was created in DMSO... |
US Patent US9617258 (2017)
BindingDB Entry DOI: 10.7270/Q2JD4ZWG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM316711

(5-{2-[(1-acryloylpiperidin-4-yl)amino]-5H-pyrrolo[...)Show SMILES C=CC(=O)N1CCC(CC1)Nc1cnc2[nH]cc(-c3cncc(c3)C#N)c2n1 Show InChI InChI=1S/C20H19N7O/c1-2-18(28)27-5-3-15(4-6-27)25-17-12-24-20-19(26-17)16(11-23-20)14-7-13(8-21)9-22-10-14/h2,7,9-12,15H,1,3-6H2,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
JAK Caliper Enzyme Assay at 4 μM: Test article was solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. An 11-point half ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29026XR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM316515
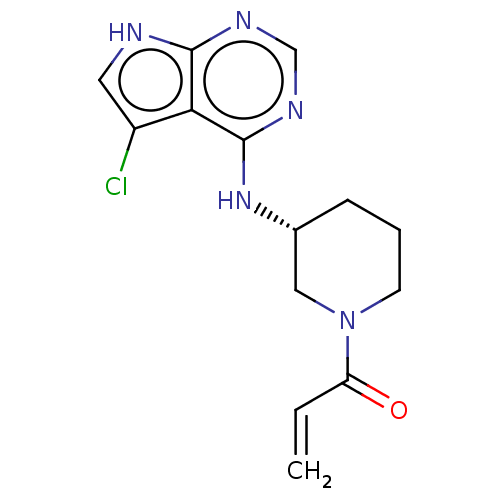
(US11111242, Example 3 | US9617258, Example 3)Show SMILES Clc1c[nH]c2ncnc(N[C@@H]3CCCN(C3)C(=O)C=C)c12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
JAK Caliper Enzyme Assay at 4 μM: Test article was solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. An 11-point half ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29026XR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM316840
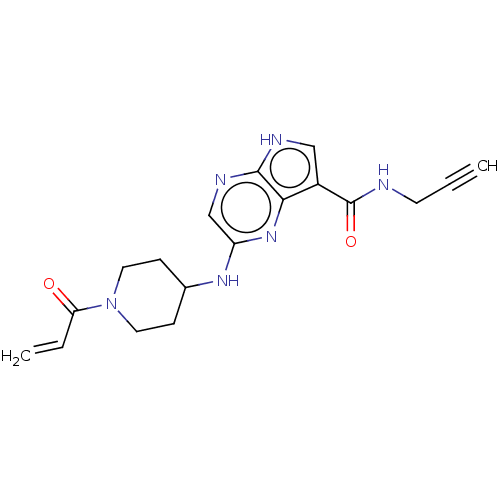
(US11111242, Example 343 | US9617258, Example 343)Show SMILES C=CC(=O)N1CCC(CC1)Nc1cnc2[nH]cc(C(=O)NCC#C)c2n1 Show InChI InChI=1S/C18H20N6O2/c1-3-7-19-18(26)13-10-20-17-16(13)23-14(11-21-17)22-12-5-8-24(9-6-12)15(25)4-2/h1,4,10-12H,2,5-9H2,(H,19,26)(H,20,21)(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Test article was solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. An 11-point half log dilution series was created in DMSO... |
US Patent US9617258 (2017)
BindingDB Entry DOI: 10.7270/Q2JD4ZWG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM316840

(US11111242, Example 343 | US9617258, Example 343)Show SMILES C=CC(=O)N1CCC(CC1)Nc1cnc2[nH]cc(C(=O)NCC#C)c2n1 Show InChI InChI=1S/C18H20N6O2/c1-3-7-19-18(26)13-10-20-17-16(13)23-14(11-21-17)22-12-5-8-24(9-6-12)15(25)4-2/h1,4,10-12H,2,5-9H2,(H,19,26)(H,20,21)(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
JAK Caliper Enzyme Assay at 4 μM: Test article was solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. An 11-point half ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29026XR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM316774

(2-[(1-acryloylpiperidin-4-yl)amino]-N-(cyclopentyl...)Show SMILES C=CC(=O)N1CCC(CC1)Nc1cnc2[nH]cc(C(=O)NCC3CCCC3)c2n1 Show InChI InChI=1S/C21H28N6O2/c1-2-18(28)27-9-7-15(8-10-27)25-17-13-23-20-19(26-17)16(12-22-20)21(29)24-11-14-5-3-4-6-14/h2,12-15H,1,3-11H2,(H,22,23)(H,24,29)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
JAK Caliper Enzyme Assay at 4 μM: Test article was solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. An 11-point half ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29026XR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data