

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM178103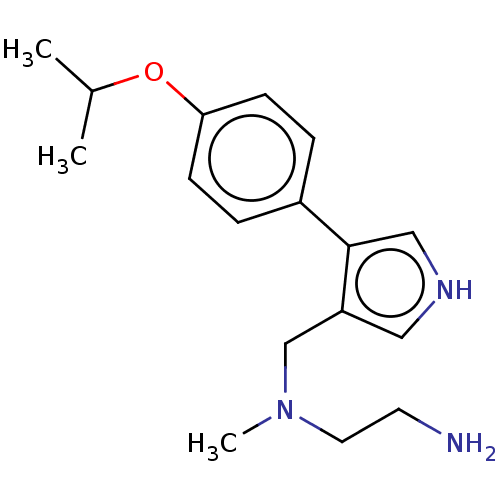 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.800 | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM178102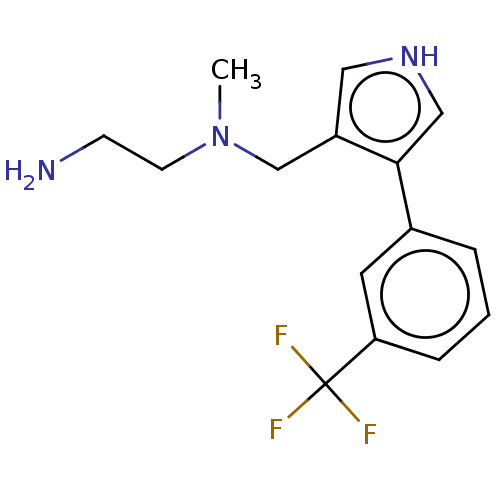 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50442103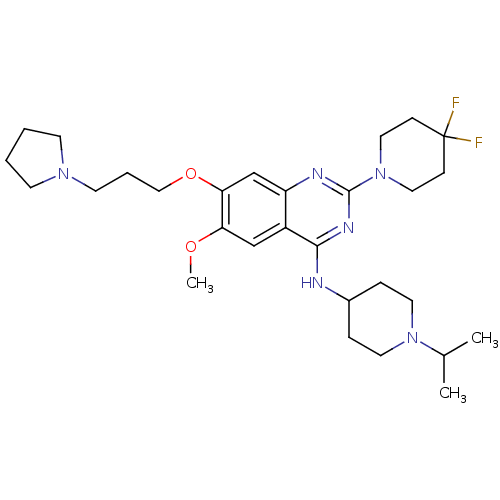 (CHEMBL2441082) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Non-competitive inhibition of lysine methyltransferase G9a (unknown origin) using SAM as substrate by Michaelis-Menten kinetic assay | J Med Chem 56: 8931-42 (2013) Article DOI: 10.1021/jm401480r BindingDB Entry DOI: 10.7270/Q2NZ892T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180955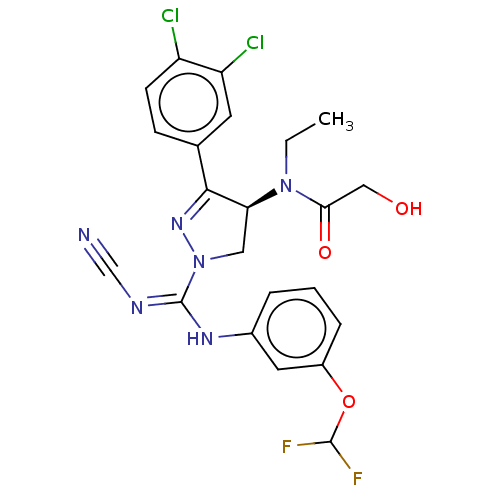 (CHEMBL3818617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Competitive inhibition of full length 6xHis-tagged SMYD2 (unknown origin) expressed in Escherichia coli using varying levels of Btn-Ahx-GSRAHSSHLKSKK... | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 11 | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50511923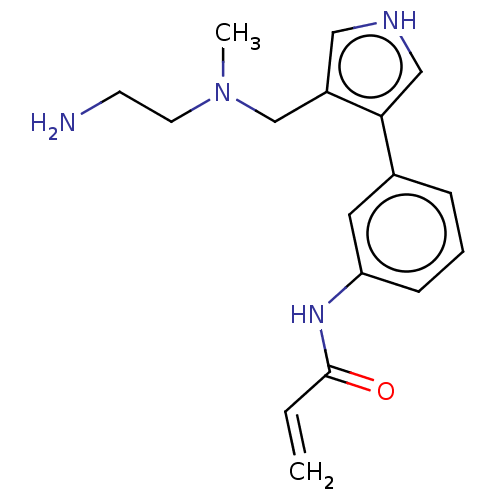 (CHEMBL4463793) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human PRMT6 pre-incubated for 1 to 30 mins using [3H]SAM as donor and [3H]methylated biotin-labeled peptide as substrate by scintillati... | J Med Chem 63: 5477-5487 (2020) Article DOI: 10.1021/acs.jmedchem.0c00406 BindingDB Entry DOI: 10.7270/Q2BG2S9V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 23 | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180955 (CHEMBL3818617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Uncompetitive inhibition of full length 6xHis-tagged SMYD2 (unknown origin) expressed in Escherichia coli using fixed levels of Btn-Ahx-GSRAHSSHLKSKK... | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50431676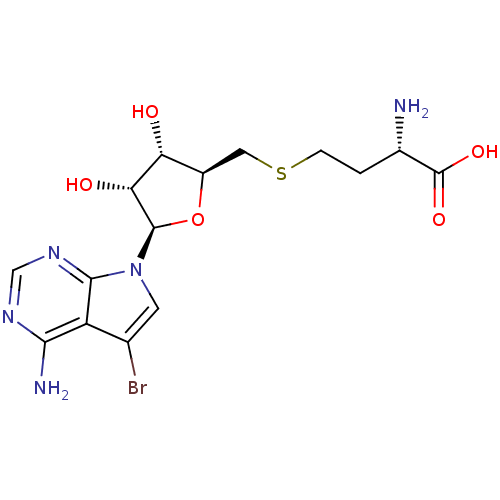 (CHEMBL2349526) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DOT1L (1 to 420 amino acid residues) overexpressed in Escherichia coli BL21 (DE3) using [3H]-SAM as subst... | Bioorg Med Chem 21: 1787-94 (2013) Article DOI: 10.1016/j.bmc.2013.01.049 BindingDB Entry DOI: 10.7270/Q24F1S41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50201571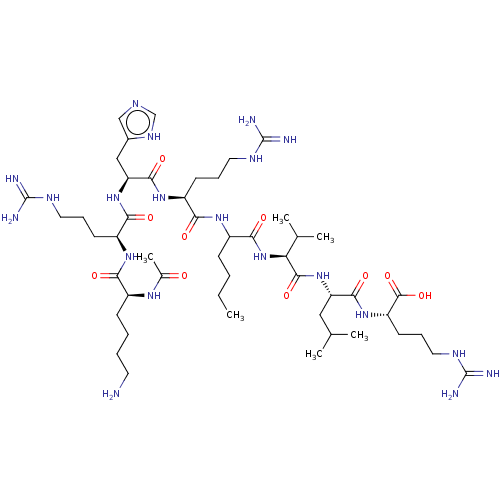 (CHEMBL3934996) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human SETD8 (186 to 352 residues) using biotin-labeled H4K20 (1 to 24 residues) as substrate after 1 hr in presence of 3H-S... | ACS Med Chem Lett 7: 1102-1106 (2016) Article DOI: 10.1021/acsmedchemlett.6b00303 BindingDB Entry DOI: 10.7270/Q2319XVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 55 | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50194756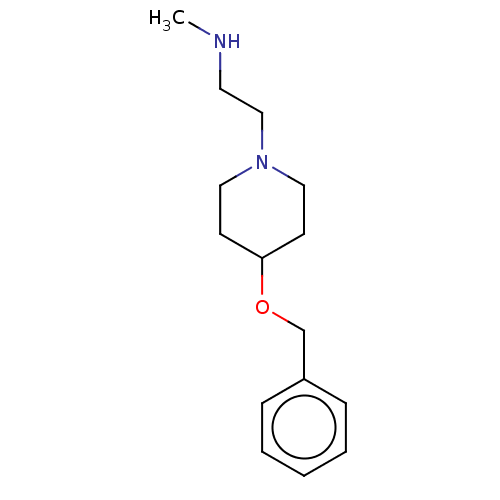 (CHEMBL3961701) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Displacement of [3H]pentazocine from guinea pig sigma1 receptor | J Med Chem 59: 9124-9139 (2016) Article DOI: 10.1021/acs.jmedchem.6b01033 BindingDB Entry DOI: 10.7270/Q2028TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194756 (CHEMBL3961701) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Displacement of [3H]alpha-methylhistamine from human histamine H3 receptor expressed HEK Flp-In cell membranes | J Med Chem 59: 9124-9139 (2016) Article DOI: 10.1021/acs.jmedchem.6b01033 BindingDB Entry DOI: 10.7270/Q2028TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM178101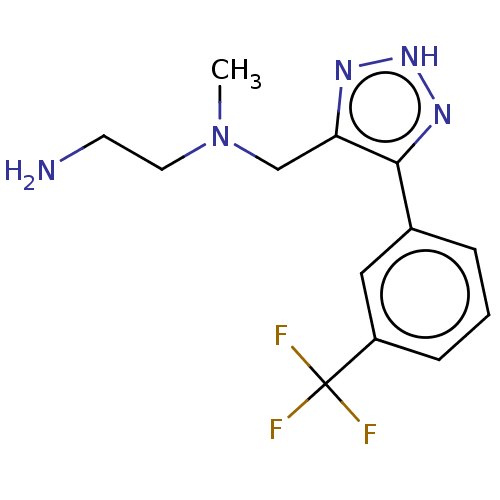 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase G2 (Pseudomonas aeruginosa) | BDBM50074672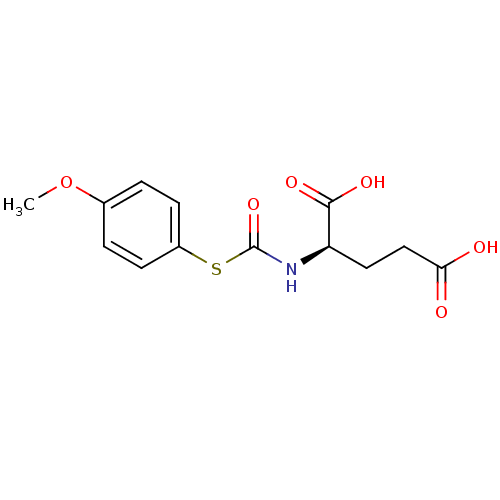 ((R)-2-(4-Methoxy-phenylsulfanylcarbonylamino)-pent...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College of Science Curated by ChEMBL | Assay Description Inhibitory activity against bacterial carboxypeptidase G2 by Dixon plot assay | J Med Chem 42: 951-6 (1999) Article DOI: 10.1021/jm990004i BindingDB Entry DOI: 10.7270/Q2J965KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396981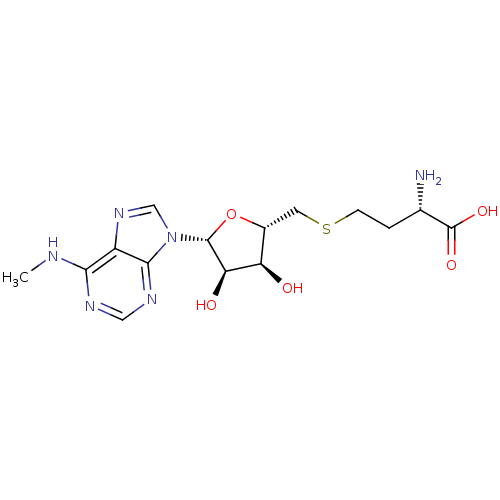 (CHEMBL2171174) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DOT1L (1 to 472 amino acid residues) expressed in Escherichia coli BL21 (DE3) using [3H]-SAM assessed as ... | Bioorg Med Chem 21: 1787-94 (2013) Article DOI: 10.1016/j.bmc.2013.01.049 BindingDB Entry DOI: 10.7270/Q24F1S41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 550 | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide N-methyltransferase (Homo sapiens (Human)) | BDBM50431676 (CHEMBL2349526) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length human NNMT measured for 30 mins by SAHH-coupled fluorescence assay | Bioorg Med Chem 21: 1787-94 (2013) Article DOI: 10.1016/j.bmc.2013.01.049 BindingDB Entry DOI: 10.7270/Q24F1S41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50431676 (CHEMBL2349526) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of DNMT1 (unknown origin) using [3H]-SAM assessed as inhibition of dsDNA methylation after 1 hr by scintillation proximity assay | Bioorg Med Chem 21: 1787-94 (2013) Article DOI: 10.1016/j.bmc.2013.01.049 BindingDB Entry DOI: 10.7270/Q24F1S41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase G2 (Pseudomonas aeruginosa) | BDBM50074680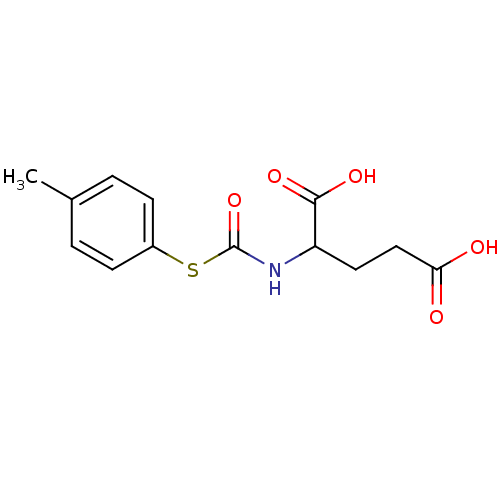 (2-p-Tolylsulfanylcarbonylamino-pentanedioic acid |...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College of Science Curated by ChEMBL | Assay Description Inhibitory activity against bacterial carboxypeptidase G2 by Dixon plot assay | J Med Chem 42: 951-6 (1999) Article DOI: 10.1021/jm990004i BindingDB Entry DOI: 10.7270/Q2J965KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase G2 (Pseudomonas aeruginosa) | BDBM50074674 (2-(3-Methoxy-phenylsulfanylcarbonylamino)-pentaned...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College of Science Curated by ChEMBL | Assay Description Inhibitory activity against bacterial carboxypeptidase G2 by Dixon plot assay | J Med Chem 42: 951-6 (1999) Article DOI: 10.1021/jm990004i BindingDB Entry DOI: 10.7270/Q2J965KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50431676 (CHEMBL2349526) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of PRMT5 (unknown origin) using [3H]SAM after 1 hr by scintillation proximity assay | Bioorg Med Chem 21: 1787-94 (2013) Article DOI: 10.1016/j.bmc.2013.01.049 BindingDB Entry DOI: 10.7270/Q24F1S41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM50431676 (CHEMBL2349526) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Inhibition of PRMT3 (unknown origin) using [3H]SAM assessed as inhibition of biotinylated-H4 (1 to 24 amino acid residues) methylation after 1 hr by ... | Bioorg Med Chem 21: 1787-94 (2013) Article DOI: 10.1016/j.bmc.2013.01.049 BindingDB Entry DOI: 10.7270/Q24F1S41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178101 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+3 | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051116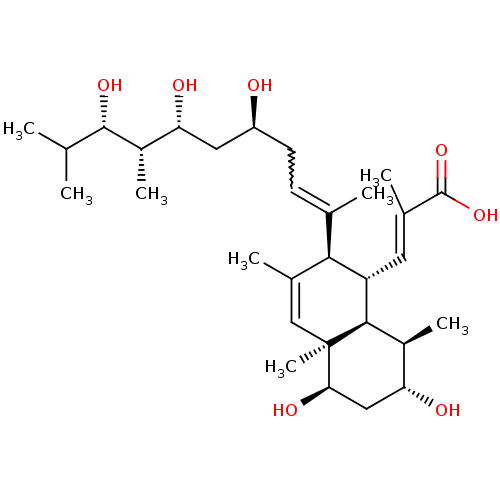 (CHEMBL3318284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Competitive inhibition of SETD8 (unknown origin) using biotin-labeled H4 (1 to 24 residues) as substrate after 1 hr in presence of varying levels of ... | ACS Med Chem Lett 7: 1102-1106 (2016) Article DOI: 10.1021/acsmedchemlett.6b00303 BindingDB Entry DOI: 10.7270/Q2319XVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase G2 (Pseudomonas aeruginosa) | BDBM50074673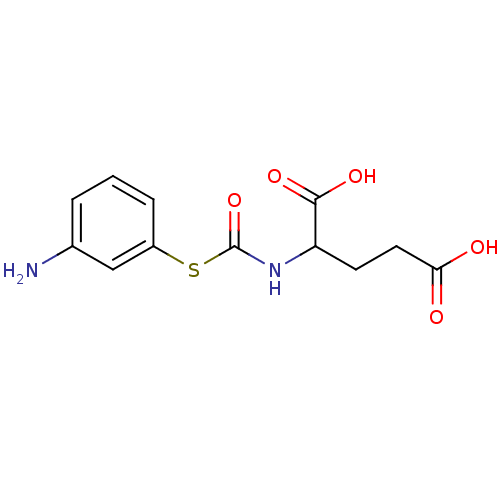 (2-(3-Amino-phenylsulfanylcarbonylamino)-pentanedio...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College of Science Curated by ChEMBL | Assay Description Inhibitory activity against bacterial carboxypeptidase G2 by Dixon plot assay | J Med Chem 42: 951-6 (1999) Article DOI: 10.1021/jm990004i BindingDB Entry DOI: 10.7270/Q2J965KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase G2 (Pseudomonas aeruginosa) | BDBM50074679 (2-(4-Amino-phenylsulfanylcarbonylamino)-pentanedio...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College of Science Curated by ChEMBL | Assay Description Inhibitory activity against bacterial carboxypeptidase G2 by Dixon plot assay | J Med Chem 42: 951-6 (1999) Article DOI: 10.1021/jm990004i BindingDB Entry DOI: 10.7270/Q2J965KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM178101 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM178101 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.75E+4 | n/a | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM178101 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase G2 (Pseudomonas aeruginosa) | BDBM50074678 (2-(1-Oxy-pyridin-4-ylsulfanylcarbonylamino)-pentan...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College of Science Curated by ChEMBL | Assay Description Inhibitory activity against bacterial carboxypeptidase G2 by Dixon plot assay | J Med Chem 42: 951-6 (1999) Article DOI: 10.1021/jm990004i BindingDB Entry DOI: 10.7270/Q2J965KN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50396023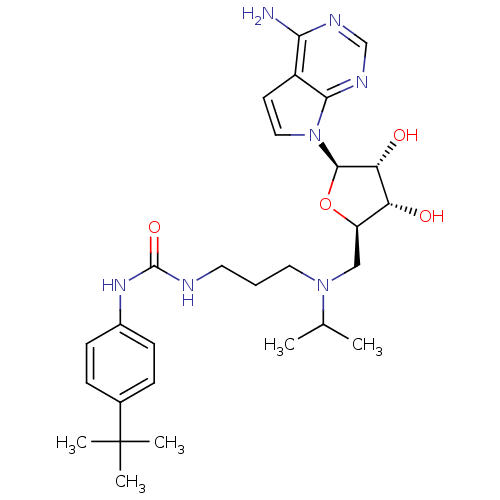 (CHEMBL2169919) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc. Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DOT1L (1 to 416 amino acid residues) using [3H]-SAM assessed as inhibition of nucleosome methylation incu... | Bioorg Med Chem 21: 1787-94 (2013) Article DOI: 10.1016/j.bmc.2013.01.049 BindingDB Entry DOI: 10.7270/Q24F1S41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50446378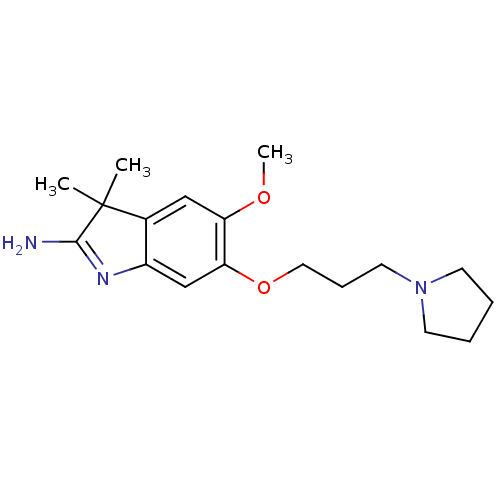 (CHEMBL3109639) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) using biotinylated-histone H3(1-21) peptide as substrate after 3 hrs by AlphaLISA assay | ACS Med Chem Lett 5: 205-9 (2014) Article DOI: 10.1021/ml400496h BindingDB Entry DOI: 10.7270/Q2FT8NJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| WD repeat-containing protein 5 (Homo sapiens (Human)) | BDBM50164787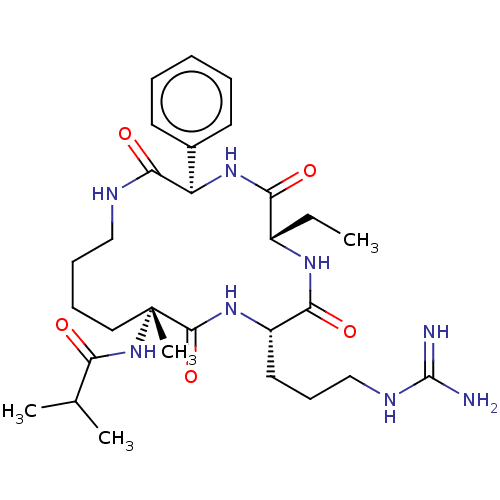 (CHEMBL3798088) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of SUMO-His-tagged WDR5 (unknown origin) interaction with MLL1 assessed as displacement of fluorescence labelled Ac-ARA peptide substrate ... | J Med Chem 59: 2478-96 (2016) Article DOI: 10.1021/acs.jmedchem.5b01630 BindingDB Entry DOI: 10.7270/Q2VT1V0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50446386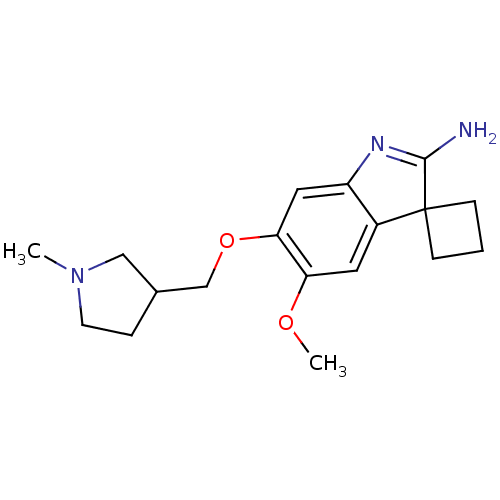 (CHEMBL3109631) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) using biotinylated-histone H3(1-21) peptide as substrate after 3 hrs by AlphaLISA assay | ACS Med Chem Lett 5: 205-9 (2014) Article DOI: 10.1021/ml400496h BindingDB Entry DOI: 10.7270/Q2FT8NJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50353121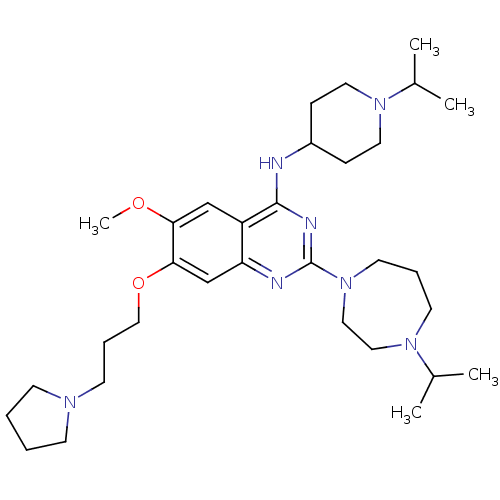 (CHEMBL1829295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of G9a assessed as hydrolysis of S-adenosyl-L-homocysteine after 2 mins by SAHH-coupled fluorescence assay | J Med Chem 54: 6139-50 (2011) Article DOI: 10.1021/jm200903z BindingDB Entry DOI: 10.7270/Q237793P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 21 (Homo sapiens (Human)) | CHEMBL5279102 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50040583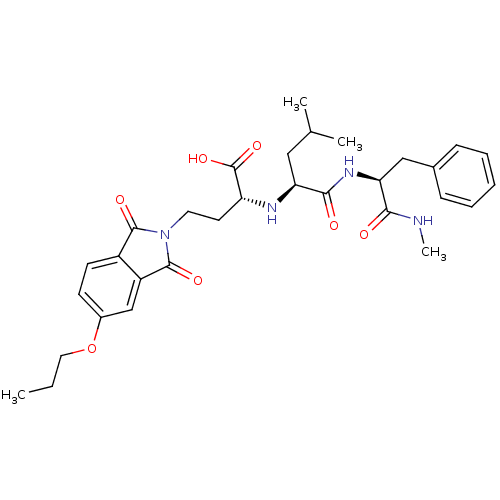 ((R)-4-(1,3-Dioxo-5-propoxy-1,3-dihydro-isoindol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Incorporated Research Institute Curated by ChEMBL | Assay Description Activity against human gelatinase (MMP-9). | J Med Chem 37: 674-88 (1994) BindingDB Entry DOI: 10.7270/Q2RV0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50095537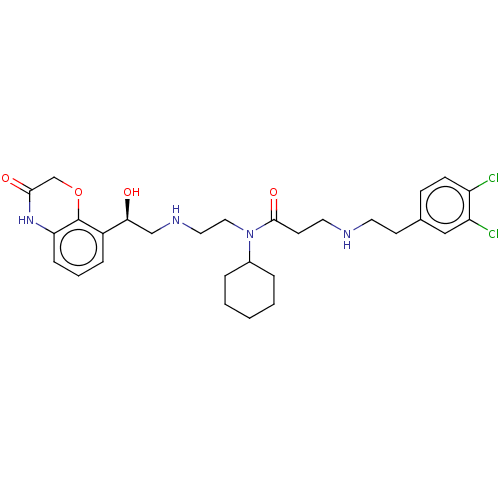 (CHEMBL3590526 | US9598381, 1a (S enantiomer)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of SMYD2 (unknown origin) using biotinylated GSRAHSSHLKSKKGQSTSRH as substrate assessed as incorporation of tritium labeled methyl group f... | ACS Med Chem Lett 6: 695-700 (2015) Article DOI: 10.1021/acsmedchemlett.5b00124 BindingDB Entry DOI: 10.7270/Q2KP83X5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| WD repeat-containing protein 5 (Homo sapiens (Human)) | BDBM200712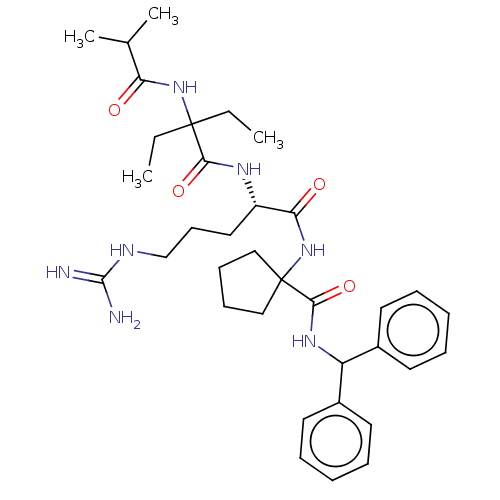 (US9233086, 10A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged WDR5 (24 to 334 residues) (unknown origin) interaction with MLL1 assessed as displacement of 5-Lys-FAM peptide su... | J Med Chem 59: 2478-96 (2016) Article DOI: 10.1021/acs.jmedchem.5b01630 BindingDB Entry DOI: 10.7270/Q2VT1V0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50446376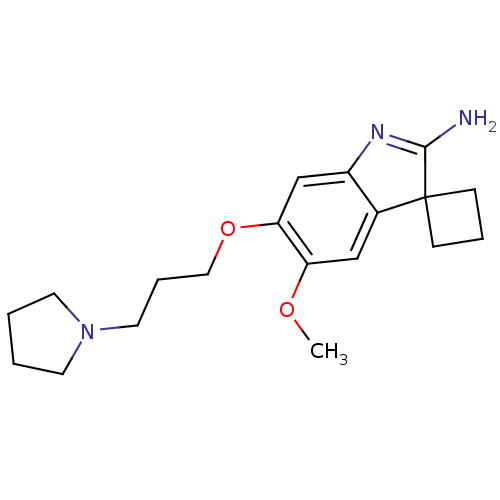 (CHEMBL3109630) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin)-mediated incorporation of methyl group from [3H]-SAM into peptide substrate by scintillation proximity assay | ACS Med Chem Lett 5: 205-9 (2014) Article DOI: 10.1021/ml400496h BindingDB Entry DOI: 10.7270/Q2FT8NJ0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50442106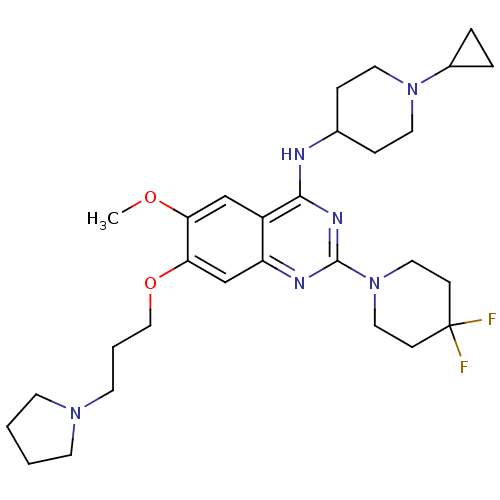 (CHEMBL2441078) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of lysine methyltransferase G9a (unknown origin) using [3H]-SAM as substrate after 0.25 hrs by scintillation proximity assay | J Med Chem 56: 8931-42 (2013) Article DOI: 10.1021/jm401480r BindingDB Entry DOI: 10.7270/Q2NZ892T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50446376 (CHEMBL3109630) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) using biotinylated-histone H3(1-21) peptide as substrate after 3 hrs by AlphaLISA assay | ACS Med Chem Lett 5: 205-9 (2014) Article DOI: 10.1021/ml400496h BindingDB Entry DOI: 10.7270/Q2FT8NJ0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50446380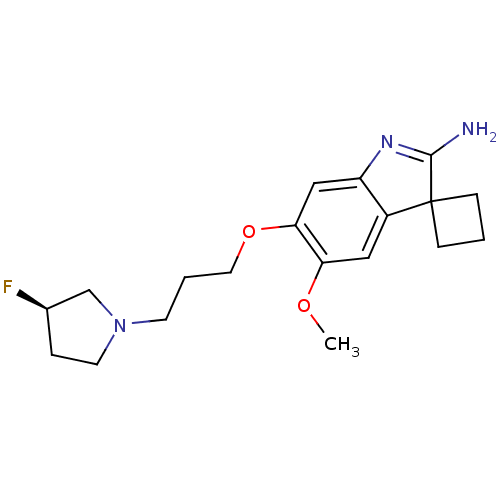 (CHEMBL3109637) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) using biotinylated-histone H3(1-21) peptide as substrate after 3 hrs by AlphaLISA assay | ACS Med Chem Lett 5: 205-9 (2014) Article DOI: 10.1021/ml400496h BindingDB Entry DOI: 10.7270/Q2FT8NJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50040607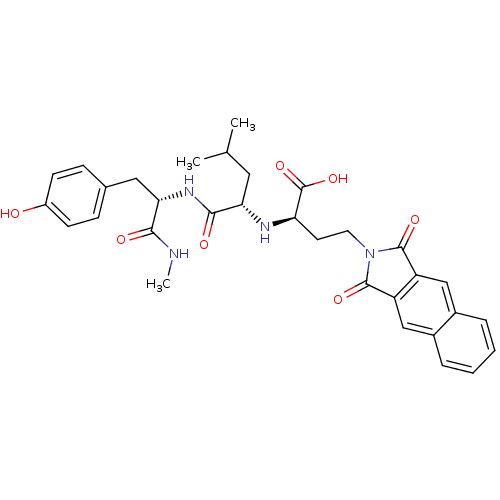 ((R)-4-(1,3-Dioxo-1,3-dihydro-benzo[f]isoindol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Incorporated Research Institute Curated by ChEMBL | Assay Description Activity against human gelatinase (MMP-9). | J Med Chem 37: 674-88 (1994) BindingDB Entry DOI: 10.7270/Q2RV0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human PRMT4 assessed as inhibition of methylation activity using biotin-labeled peptide as substrate and [3H]-SAM by scintillation prox... | J Med Chem 59: 9124-9139 (2016) Article DOI: 10.1021/acs.jmedchem.6b01033 BindingDB Entry DOI: 10.7270/Q2028TGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2027 total ) | Next | Last >> |