

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50428854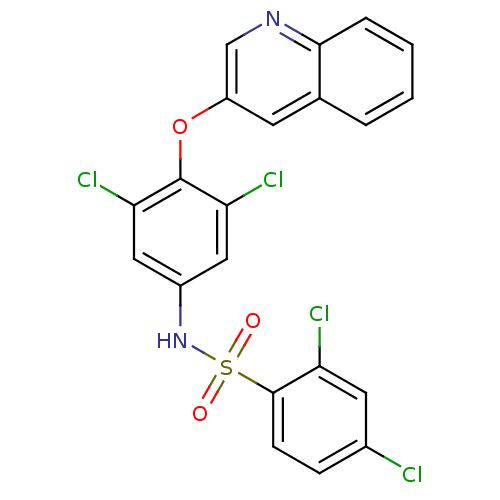 (CHEMBL1236924) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from human PPARgamma LBD expressed in HEK293 cells by filtration assay | J Med Chem 60: 4584-4593 (2017) Article DOI: 10.1021/acs.jmedchem.6b01727 BindingDB Entry DOI: 10.7270/Q23J3G4S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523302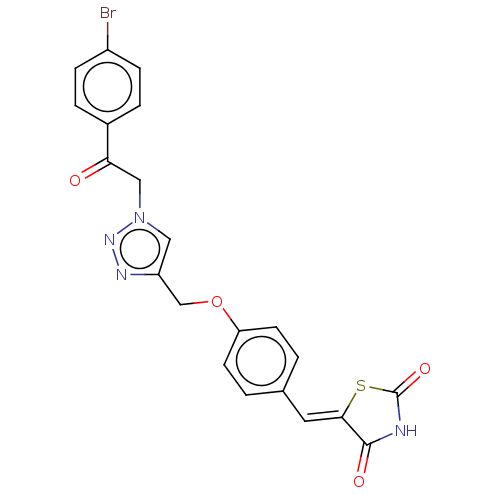 (CHEMBL4589007) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523300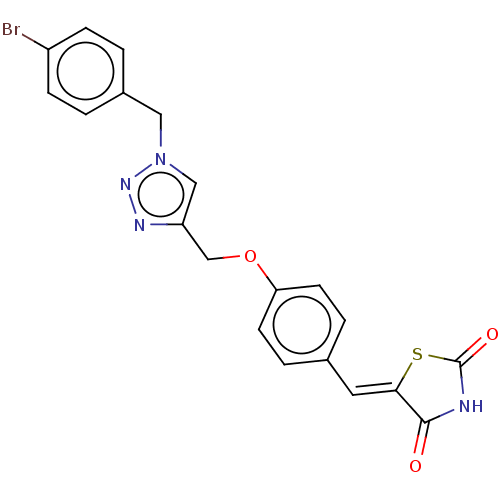 (CHEMBL4475190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523290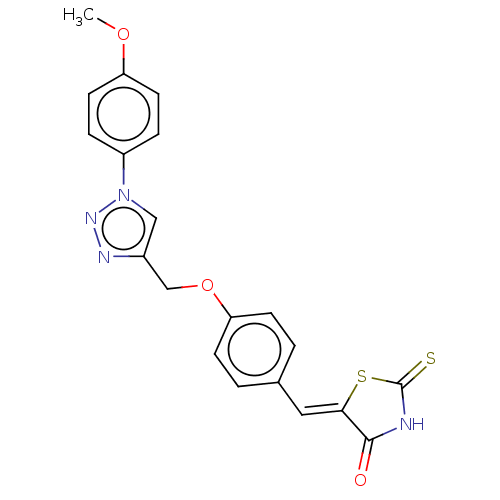 (CHEMBL4474290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523294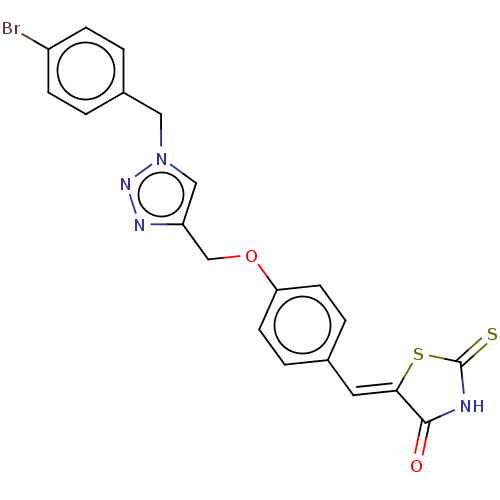 (CHEMBL4579799) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523283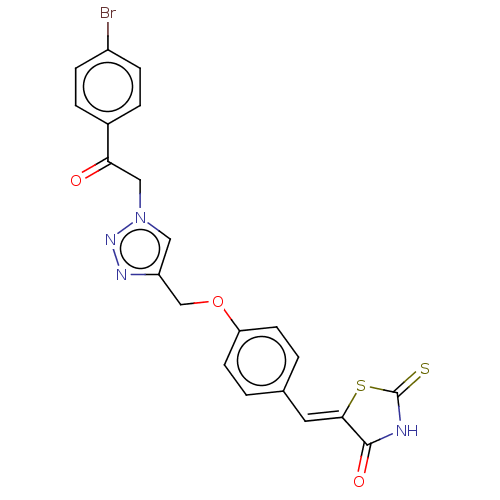 (CHEMBL4438502) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523304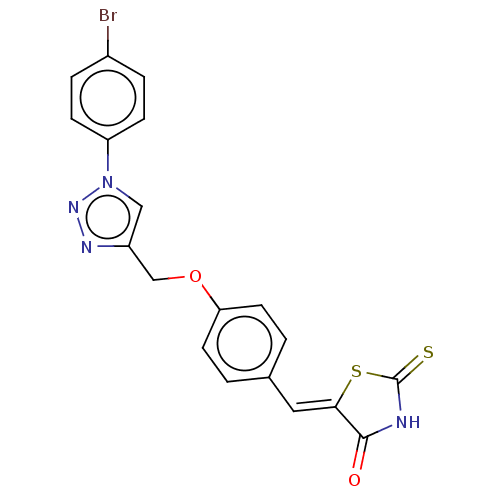 (CHEMBL4542774) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523287 (CHEMBL4447565) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523284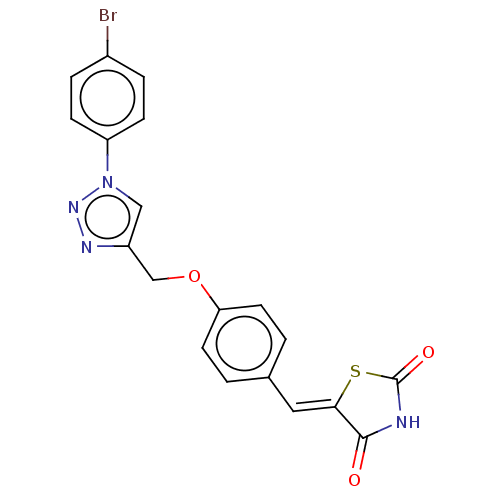 (CHEMBL4461022) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523303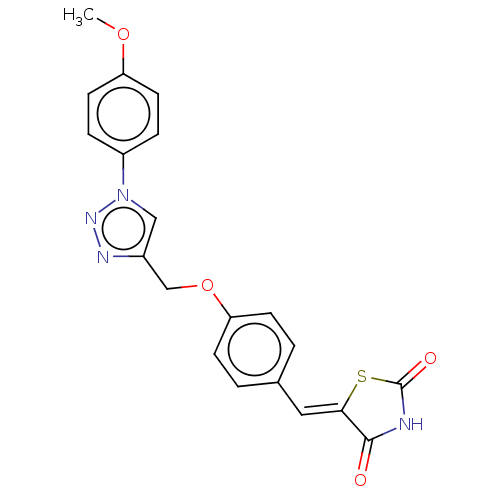 (CHEMBL4472170) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523289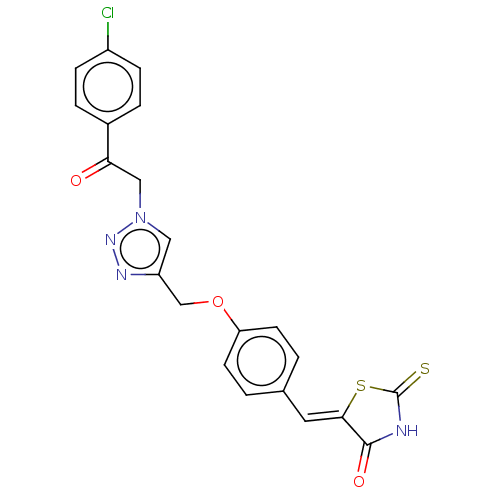 (CHEMBL4436095) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523292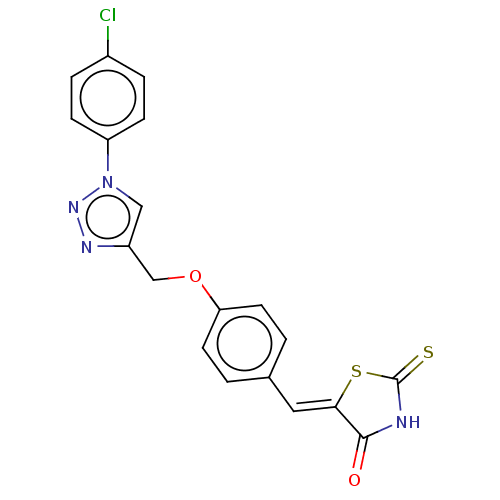 (CHEMBL4530072) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523288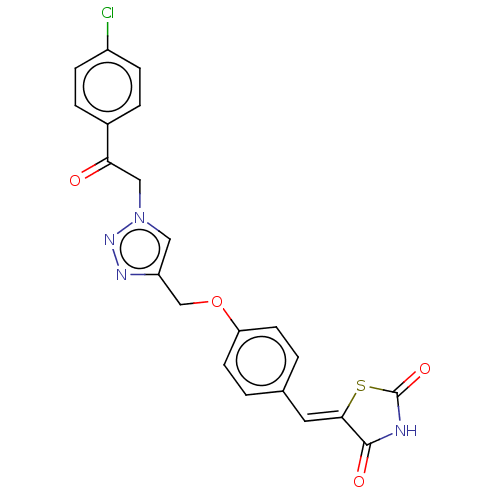 (CHEMBL4521668) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523286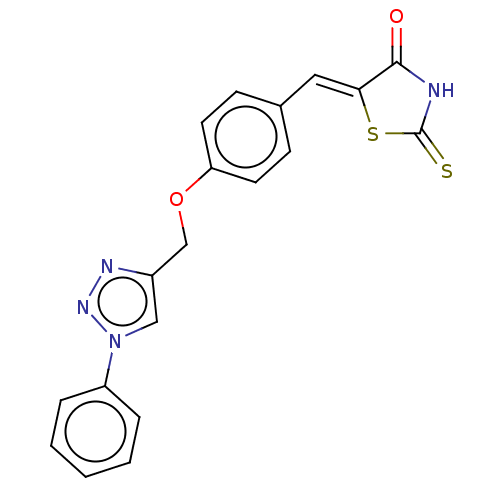 (CHEMBL4559991) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523299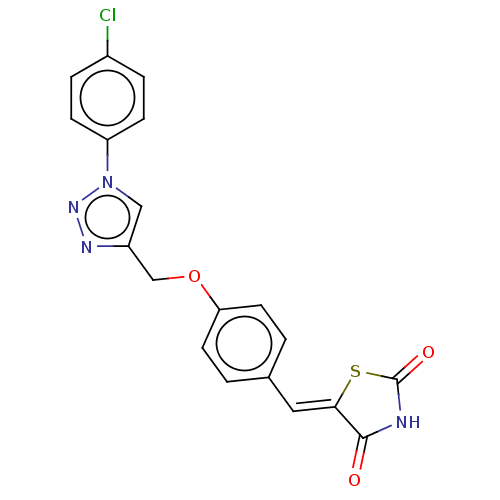 (CHEMBL4544031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523301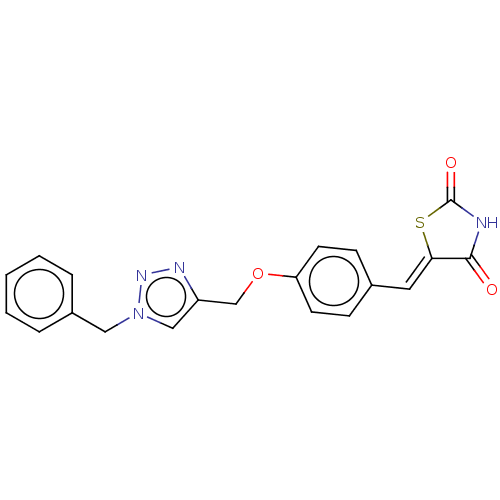 (CHEMBL4570970) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523296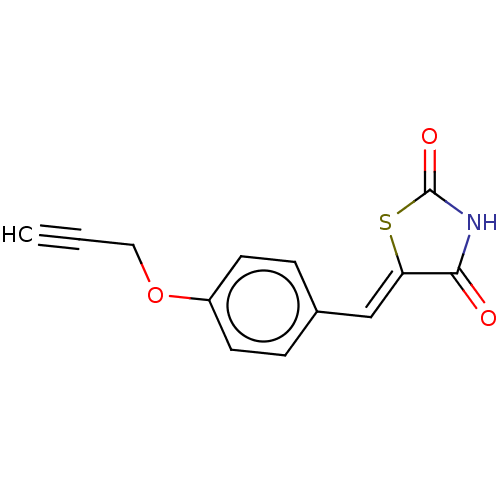 (CHEMBL4454251) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523295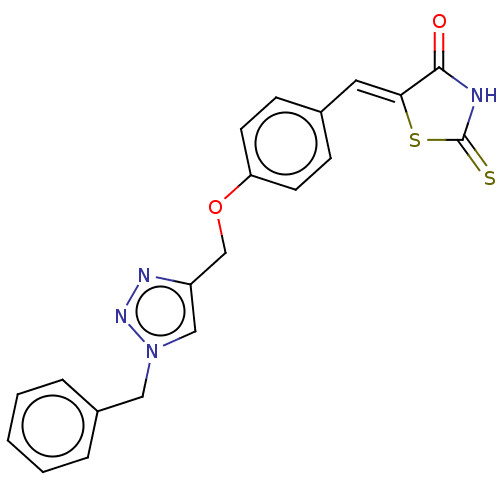 (CHEMBL4449234) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523285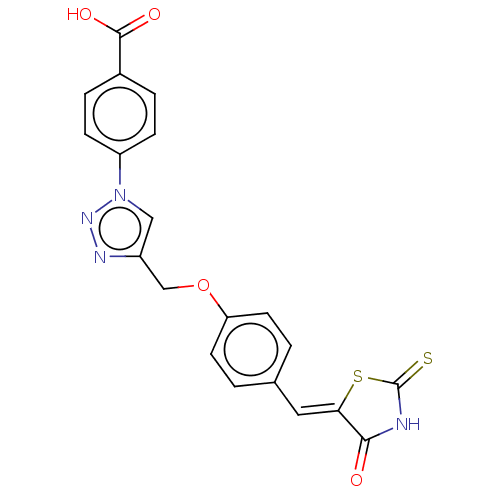 (CHEMBL4445953) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523293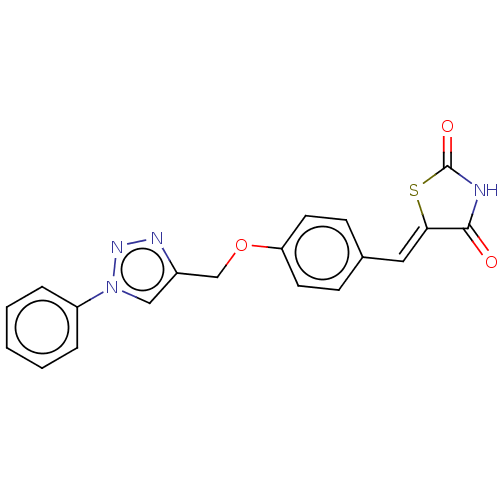 (CHEMBL4439919) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523298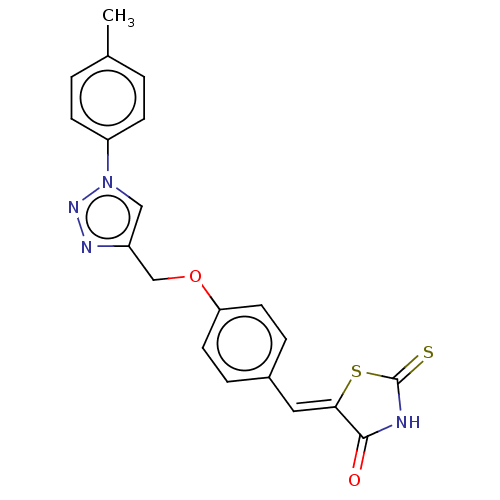 (CHEMBL4454204) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523297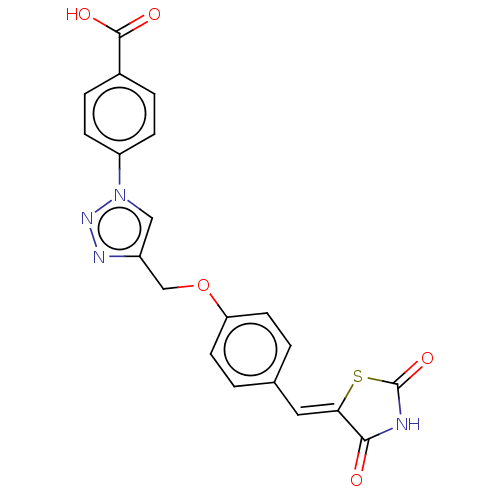 (CHEMBL4535128) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50523291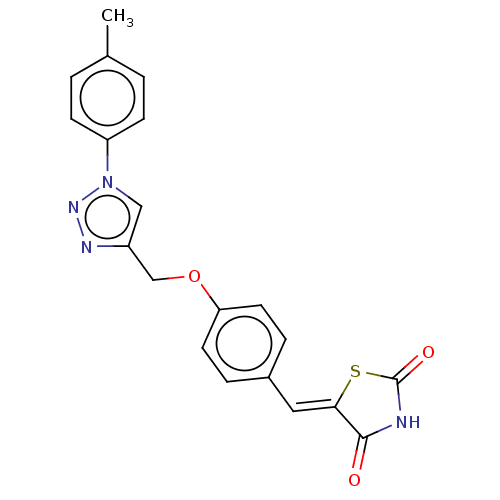 (CHEMBL4450483) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50174201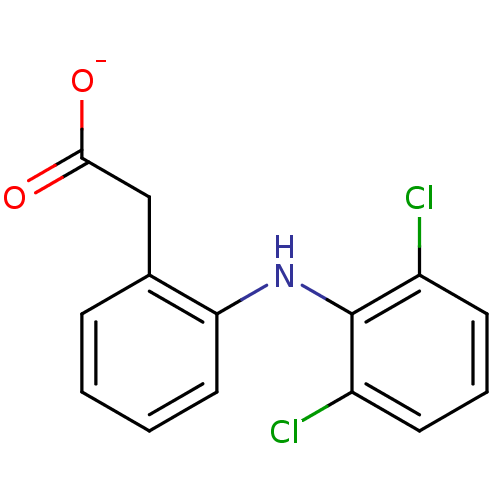 (ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50174201 (ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523297 (CHEMBL4535128) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523291 (CHEMBL4450483) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523293 (CHEMBL4439919) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523286 (CHEMBL4559991) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523296 (CHEMBL4454251) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523299 (CHEMBL4544031) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523298 (CHEMBL4454204) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523285 (CHEMBL4445953) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523295 (CHEMBL4449234) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523292 (CHEMBL4530072) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523301 (CHEMBL4570970) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523303 (CHEMBL4472170) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523304 (CHEMBL4542774) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523288 (CHEMBL4521668) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523287 (CHEMBL4447565) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523284 (CHEMBL4461022) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523289 (CHEMBL4436095) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523290 (CHEMBL4474290) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523294 (CHEMBL4579799) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523302 (CHEMBL4589007) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50523283 (CHEMBL4438502) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 BindingDB Entry DOI: 10.7270/Q2DN48FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 65 total ) | Next | Last >> |