 Found 88 hits with Last Name = 'brunsteiner' and Initial = 'm'
Found 88 hits with Last Name = 'brunsteiner' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 396 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using fluorescent acetylated substrate |
J Med Chem 52: 7003-13 (2009)
Article DOI: 10.1021/jm9005077
BindingDB Entry DOI: 10.7270/Q2ST7PWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Papain
(Carica papaya) | BDBM50437951
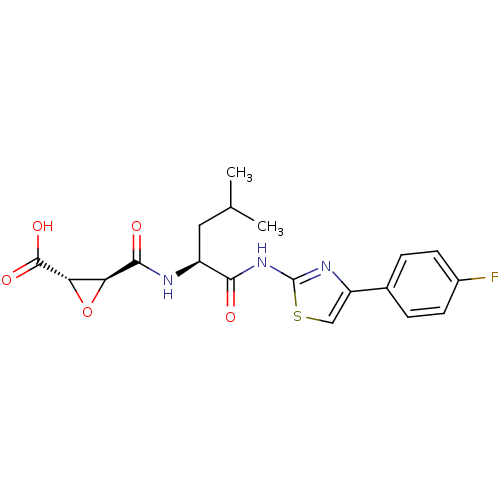
(CHEMBL2408922 | US9403843, 24a)Show SMILES CC(C)C[C@H](NC(=O)[C@H]1O[C@@H]1C(O)=O)C(=O)Nc1nc(cs1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H20FN3O5S/c1-9(2)7-12(21-17(25)14-15(28-14)18(26)27)16(24)23-19-22-13(8-29-19)10-3-5-11(20)6-4-10/h3-6,8-9,12,14-15H,7H2,1-2H3,(H,21,25)(H,26,27)(H,22,23,24)/t12-,14-,15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of papain after using SucLLVYAMC as substrate by FRET assay |
J Med Chem 56: 6054-68 (2013)
Article DOI: 10.1021/jm4006719
BindingDB Entry DOI: 10.7270/Q2VT1TG7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50299973

(CHEMBL572374 | Octanedioic Acid [4-(3-Azidophenyl)...)Show SMILES ONC(=O)CCCCCCC(=O)Nc1nc(cs1)-c1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C17H20N6O3S/c18-23-21-13-9-7-12(8-10-13)14-11-27-17(19-14)20-15(24)5-3-1-2-4-6-16(25)22-26/h7-11,26H,1-6H2,(H,22,25)(H,19,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using fluorescent acetylated substrate |
J Med Chem 52: 7003-13 (2009)
Article DOI: 10.1021/jm9005077
BindingDB Entry DOI: 10.7270/Q2ST7PWM |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Sus scrofa (pig)) | BDBM50437950
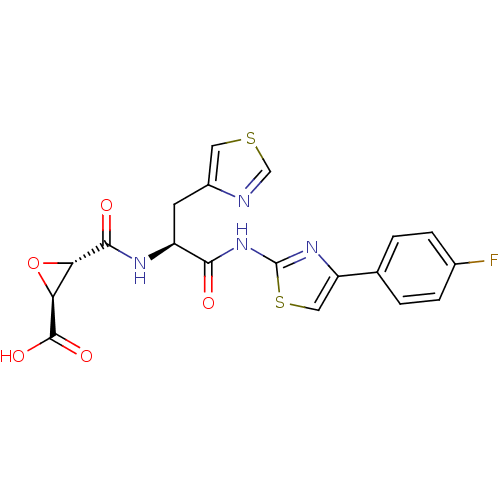
(CHEMBL2408917)Show SMILES OC(=O)[C@H]1O[C@@H]1C(=O)N[C@@H](Cc1cscn1)C(=O)Nc1nc(cs1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H15FN4O5S2/c20-10-3-1-9(2-4-10)13-7-31-19(23-13)24-16(25)12(5-11-6-30-8-21-11)22-17(26)14-15(29-14)18(27)28/h1-4,6-8,12,14-15H,5H2,(H,22,26)(H,27,28)(H,23,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of pig full length Cal1 after using SucLLVYAMC as substrate by FRET assay |
J Med Chem 56: 6054-68 (2013)
Article DOI: 10.1021/jm4006719
BindingDB Entry DOI: 10.7270/Q2VT1TG7 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Sus scrofa (pig)) | BDBM50437948
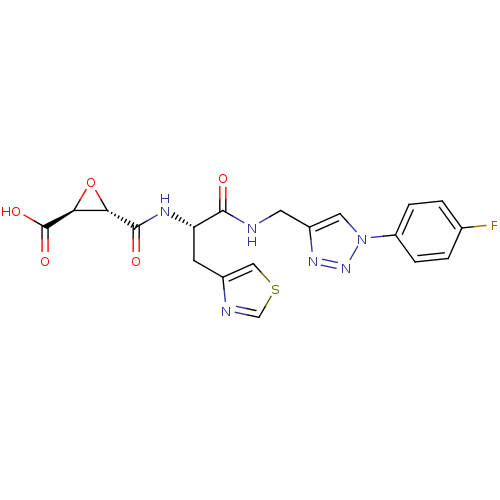
(CHEMBL2408899 | US9403843, 50)Show SMILES OC(=O)[C@H]1O[C@@H]1C(=O)N[C@@H](Cc1cscn1)C(=O)NCc1cn(nn1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H17FN6O5S/c20-10-1-3-13(4-2-10)26-7-12(24-25-26)6-21-17(27)14(5-11-8-32-9-22-11)23-18(28)15-16(31-15)19(29)30/h1-4,7-9,14-16H,5-6H2,(H,21,27)(H,23,28)(H,29,30)/t14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of pig full length Cal1 after using SucLLVYAMC as substrate by FRET assay |
J Med Chem 56: 6054-68 (2013)
Article DOI: 10.1021/jm4006719
BindingDB Entry DOI: 10.7270/Q2VT1TG7 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Sus scrofa (pig)) | BDBM50437949
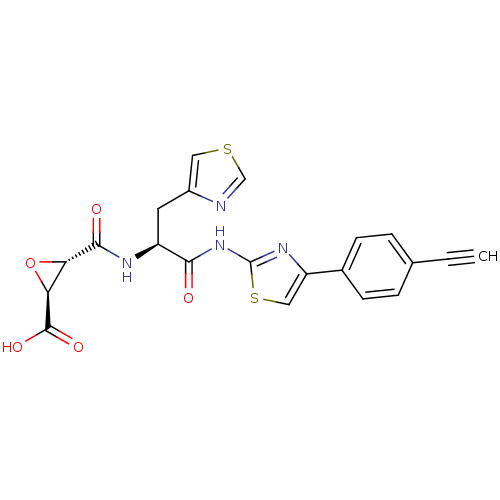
(CHEMBL2408918 | US9403843, 34)Show SMILES OC(=O)[C@H]1O[C@@H]1C(=O)N[C@@H](Cc1cscn1)C(=O)Nc1nc(cs1)-c1ccc(cc1)C#C |r| Show InChI InChI=1S/C21H16N4O5S2/c1-2-11-3-5-12(6-4-11)15-9-32-21(24-15)25-18(26)14(7-13-8-31-10-22-13)23-19(27)16-17(30-16)20(28)29/h1,3-6,8-10,14,16-17H,7H2,(H,23,27)(H,28,29)(H,24,25,26)/t14-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of pig full length Cal1 after using SucLLVYAMC as substrate by FRET assay |
J Med Chem 56: 6054-68 (2013)
Article DOI: 10.1021/jm4006719
BindingDB Entry DOI: 10.7270/Q2VT1TG7 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Sus scrofa (pig)) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of pig full length Cal1 after using SucLLVYAMC as substrate by FRET assay |
J Med Chem 56: 6054-68 (2013)
Article DOI: 10.1021/jm4006719
BindingDB Entry DOI: 10.7270/Q2VT1TG7 |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50157741

(CHEMBL374508 | E-64 | E64)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H]-1-[#8]-[#6@@H]-1-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C15H27N5O5/c1-8(2)7-9(20-13(22)10-11(25-10)14(23)24)12(21)18-5-3-4-6-19-15(16)17/h8-11H,3-7H2,1-2H3,(H,18,21)(H,20,22)(H,23,24)(H4,16,17,19)/t9-,10-,11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of papain after using SucLLVYAMC as substrate by FRET assay |
J Med Chem 56: 6054-68 (2013)
Article DOI: 10.1021/jm4006719
BindingDB Entry DOI: 10.7270/Q2VT1TG7 |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50437950

(CHEMBL2408917)Show SMILES OC(=O)[C@H]1O[C@@H]1C(=O)N[C@@H](Cc1cscn1)C(=O)Nc1nc(cs1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H15FN4O5S2/c20-10-3-1-9(2-4-10)13-7-31-19(23-13)24-16(25)12(5-11-6-30-8-21-11)22-17(26)14-15(29-14)18(27)28/h1-4,6-8,12,14-15H,5H2,(H,22,26)(H,27,28)(H,23,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of papain after using SucLLVYAMC as substrate by FRET assay |
J Med Chem 56: 6054-68 (2013)
Article DOI: 10.1021/jm4006719
BindingDB Entry DOI: 10.7270/Q2VT1TG7 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Sus scrofa (pig)) | BDBM50437951

(CHEMBL2408922 | US9403843, 24a)Show SMILES CC(C)C[C@H](NC(=O)[C@H]1O[C@@H]1C(O)=O)C(=O)Nc1nc(cs1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H20FN3O5S/c1-9(2)7-12(21-17(25)14-15(28-14)18(26)27)16(24)23-19-22-13(8-29-19)10-3-5-11(20)6-4-10/h3-6,8-9,12,14-15H,7H2,1-2H3,(H,21,25)(H,26,27)(H,22,23,24)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of pig full length Cal1 after using SucLLVYAMC as substrate by FRET assay |
J Med Chem 56: 6054-68 (2013)
Article DOI: 10.1021/jm4006719
BindingDB Entry DOI: 10.7270/Q2VT1TG7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50299972
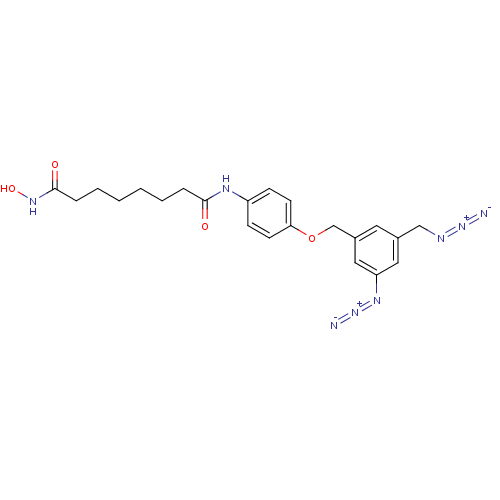
(CHEMBL585164 | Octanedioic Acid [4-(3-Azido-5-azid...)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(OCc2cc(CN=[N+]=[N-])cc(c2)N=[N+]=[N-])cc1 Show InChI InChI=1S/C22H26N8O4/c23-29-25-14-16-11-17(13-19(12-16)27-30-24)15-34-20-9-7-18(8-10-20)26-21(31)5-3-1-2-4-6-22(32)28-33/h7-13,33H,1-6,14-15H2,(H,26,31)(H,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using fluorescent acetylated substrate |
J Med Chem 52: 7003-13 (2009)
Article DOI: 10.1021/jm9005077
BindingDB Entry DOI: 10.7270/Q2ST7PWM |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50437949

(CHEMBL2408918 | US9403843, 34)Show SMILES OC(=O)[C@H]1O[C@@H]1C(=O)N[C@@H](Cc1cscn1)C(=O)Nc1nc(cs1)-c1ccc(cc1)C#C |r| Show InChI InChI=1S/C21H16N4O5S2/c1-2-11-3-5-12(6-4-11)15-9-32-21(24-15)25-18(26)14(7-13-8-31-10-22-13)23-19(27)16-17(30-16)20(28)29/h1,3-6,8-10,14,16-17H,7H2,(H,23,27)(H,28,29)(H,24,25,26)/t14-,16-,17-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of papain after using SucLLVYAMC as substrate by FRET assay |
J Med Chem 56: 6054-68 (2013)
Article DOI: 10.1021/jm4006719
BindingDB Entry DOI: 10.7270/Q2VT1TG7 |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50437948

(CHEMBL2408899 | US9403843, 50)Show SMILES OC(=O)[C@H]1O[C@@H]1C(=O)N[C@@H](Cc1cscn1)C(=O)NCc1cn(nn1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C19H17FN6O5S/c20-10-1-3-13(4-2-10)26-7-12(24-25-26)6-21-17(27)14(5-11-8-32-9-22-11)23-18(28)15-16(31-15)19(29)30/h1-4,7-9,14-16H,5-6H2,(H,21,27)(H,23,28)(H,29,30)/t14-,15-,16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of papain after using SucLLVYAMC as substrate by FRET assay |
J Med Chem 56: 6054-68 (2013)
Article DOI: 10.1021/jm4006719
BindingDB Entry DOI: 10.7270/Q2VT1TG7 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC5 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC11 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50356635
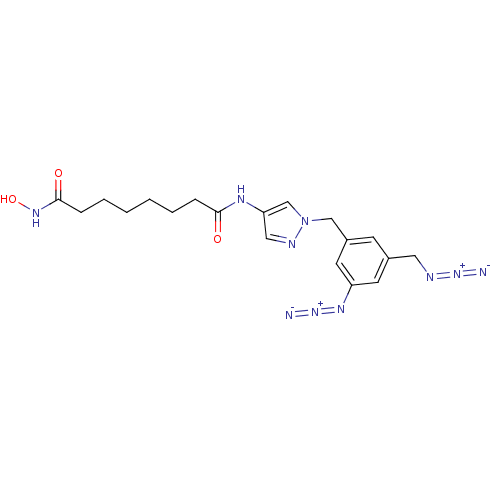
(CHEMBL1914708)Show SMILES ONC(=O)CCCCCCC(=O)Nc1cnn(Cc2cc(CN=[N+]=[N-])cc(c2)N=[N+]=[N-])c1 Show InChI InChI=1S/C19H24N10O3/c20-27-22-10-14-7-15(9-16(8-14)25-28-21)12-29-13-17(11-23-29)24-18(30)5-3-1-2-4-6-19(31)26-32/h7-9,11,13,32H,1-6,10,12H2,(H,24,30)(H,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using Fluor de Lys as substrate |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50356639
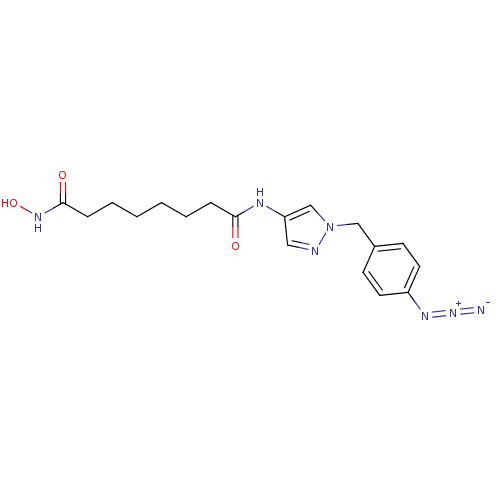
(CHEMBL1914705)Show SMILES ONC(=O)CCCCCCC(=O)Nc1cnn(Cc2ccc(cc2)N=[N+]=[N-])c1 Show InChI InChI=1S/C18H23N7O3/c19-24-22-15-9-7-14(8-10-15)12-25-13-16(11-20-25)21-17(26)5-3-1-2-4-6-18(27)23-28/h7-11,13,28H,1-6,12H2,(H,21,26)(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3/NCoR2 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3/NCoR2 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50356639

(CHEMBL1914705)Show SMILES ONC(=O)CCCCCCC(=O)Nc1cnn(Cc2ccc(cc2)N=[N+]=[N-])c1 Show InChI InChI=1S/C18H23N7O3/c19-24-22-15-9-7-14(8-10-15)12-25-13-16(11-20-25)21-17(26)5-3-1-2-4-6-18(27)23-28/h7-11,13,28H,1-6,12H2,(H,21,26)(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using Fluor de Lys as substrate |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50356634
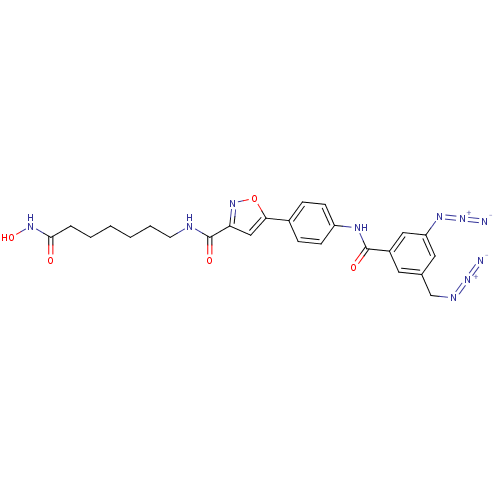
(CHEMBL1914702)Show SMILES ONC(=O)CCCCCCNC(=O)c1cc(on1)-c1ccc(NC(=O)c2cc(CN=[N+]=[N-])cc(c2)N=[N+]=[N-])cc1 Show InChI InChI=1S/C25H26N10O5/c26-34-29-15-16-11-18(13-20(12-16)31-35-27)24(37)30-19-8-6-17(7-9-19)22-14-21(33-40-22)25(38)28-10-4-2-1-3-5-23(36)32-39/h6-9,11-14,39H,1-5,10,15H2,(H,28,38)(H,30,37)(H,32,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50356637
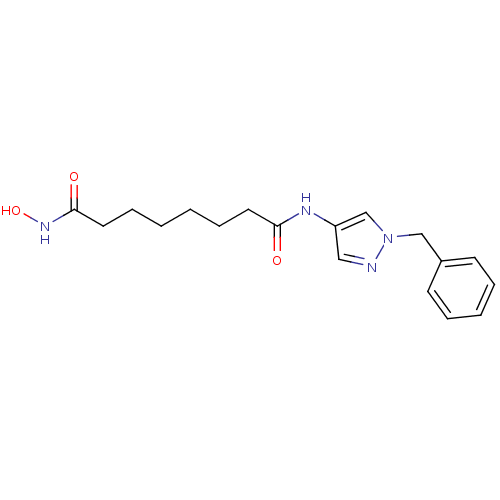
(CHEMBL1914703)Show InChI InChI=1S/C18H24N4O3/c23-17(10-6-1-2-7-11-18(24)21-25)20-16-12-19-22(14-16)13-15-8-4-3-5-9-15/h3-5,8-9,12,14,25H,1-2,6-7,10-11,13H2,(H,20,23)(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3/NCoR2 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50356634

(CHEMBL1914702)Show SMILES ONC(=O)CCCCCCNC(=O)c1cc(on1)-c1ccc(NC(=O)c2cc(CN=[N+]=[N-])cc(c2)N=[N+]=[N-])cc1 Show InChI InChI=1S/C25H26N10O5/c26-34-29-15-16-11-18(13-20(12-16)31-35-27)24(37)30-19-8-6-17(7-9-19)22-14-21(33-40-22)25(38)28-10-4-2-1-3-5-23(36)32-39/h6-9,11-14,39H,1-5,10,15H2,(H,28,38)(H,30,37)(H,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3/NCoR2 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50356634

(CHEMBL1914702)Show SMILES ONC(=O)CCCCCCNC(=O)c1cc(on1)-c1ccc(NC(=O)c2cc(CN=[N+]=[N-])cc(c2)N=[N+]=[N-])cc1 Show InChI InChI=1S/C25H26N10O5/c26-34-29-15-16-11-18(13-20(12-16)31-35-27)24(37)30-19-8-6-17(7-9-19)22-14-21(33-40-22)25(38)28-10-4-2-1-3-5-23(36)32-39/h6-9,11-14,39H,1-5,10,15H2,(H,28,38)(H,30,37)(H,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50356638
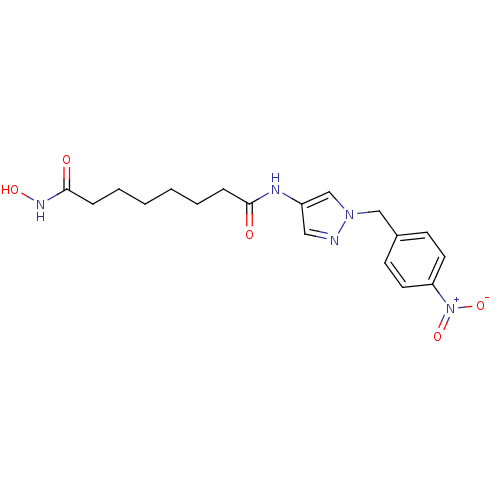
(CHEMBL1914704)Show SMILES ONC(=O)CCCCCCC(=O)Nc1cnn(Cc2ccc(cc2)[N+]([O-])=O)c1 Show InChI InChI=1S/C18H23N5O5/c24-17(5-3-1-2-4-6-18(25)21-26)20-15-11-19-22(13-15)12-14-7-9-16(10-8-14)23(27)28/h7-11,13,26H,1-6,12H2,(H,20,24)(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3/NCoR2 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50356636
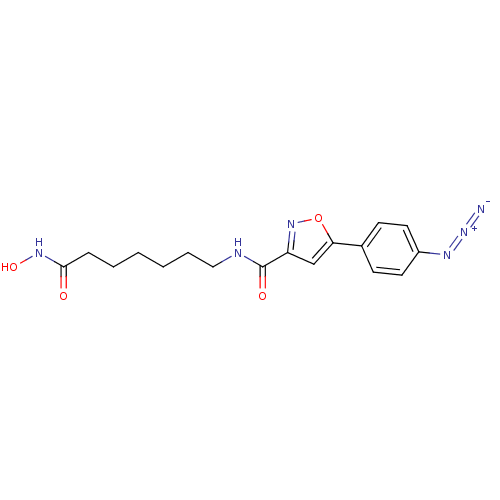
(CHEMBL1914701)Show SMILES ONC(=O)CCCCCCNC(=O)c1cc(on1)-c1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C17H20N6O4/c18-23-20-13-8-6-12(7-9-13)15-11-14(22-27-15)17(25)19-10-4-2-1-3-5-16(24)21-26/h6-9,11,26H,1-5,10H2,(H,19,25)(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3/NCoR2 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50356637

(CHEMBL1914703)Show InChI InChI=1S/C18H24N4O3/c23-17(10-6-1-2-7-11-18(24)21-25)20-16-12-19-22(14-16)13-15-8-4-3-5-9-15/h3-5,8-9,12,14,25H,1-2,6-7,10-11,13H2,(H,20,23)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using Fluor de Lys as substrate |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50356638

(CHEMBL1914704)Show SMILES ONC(=O)CCCCCCC(=O)Nc1cnn(Cc2ccc(cc2)[N+]([O-])=O)c1 Show InChI InChI=1S/C18H23N5O5/c24-17(5-3-1-2-4-6-18(25)21-26)20-15-11-19-22(13-15)12-14-7-9-16(10-8-14)23(27)28/h7-11,13,26H,1-6,12H2,(H,20,24)(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 using Fluor de Lys as substrate |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50356634

(CHEMBL1914702)Show SMILES ONC(=O)CCCCCCNC(=O)c1cc(on1)-c1ccc(NC(=O)c2cc(CN=[N+]=[N-])cc(c2)N=[N+]=[N-])cc1 Show InChI InChI=1S/C25H26N10O5/c26-34-29-15-16-11-18(13-20(12-16)31-35-27)24(37)30-19-8-6-17(7-9-19)22-14-21(33-40-22)25(38)28-10-4-2-1-3-5-23(36)32-39/h6-9,11-14,39H,1-5,10,15H2,(H,28,38)(H,30,37)(H,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Pantothenate synthetase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50373142

(CHEMBL428787)Show SMILES CC(C)(C)C1CCc2onc(C(=O)Nc3cnn(Cc4ccc5ccccc5c4)c3)c2C1 |w:4.3| Show InChI InChI=1S/C26H28N4O2/c1-26(2,3)20-10-11-23-22(13-20)24(29-32-23)25(31)28-21-14-27-30(16-21)15-17-8-9-18-6-4-5-7-19(18)12-17/h4-9,12,14,16,20H,10-11,13,15H2,1-3H3,(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis pantothenate synthetase |
J Med Chem 51: 1999-2002 (2008)
Article DOI: 10.1021/jm701372r
BindingDB Entry DOI: 10.7270/Q2028SD7 |
More data for this
Ligand-Target Pair | |
Pantothenate synthetase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50373146
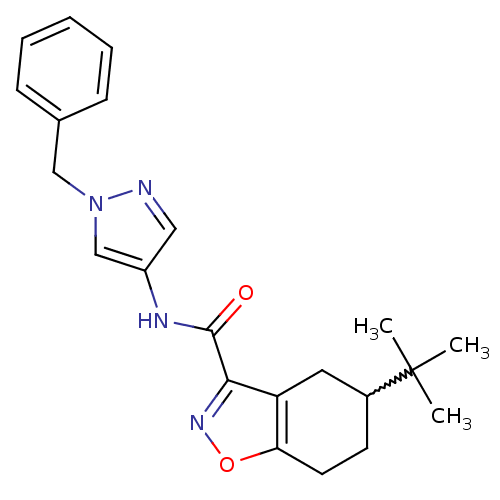
(CHEMBL408197)Show SMILES CC(C)(C)C1CCc2onc(C(=O)Nc3cnn(Cc4ccccc4)c3)c2C1 |w:4.3| Show InChI InChI=1S/C22H26N4O2/c1-22(2,3)16-9-10-19-18(11-16)20(25-28-19)21(27)24-17-12-23-26(14-17)13-15-7-5-4-6-8-15/h4-8,12,14,16H,9-11,13H2,1-3H3,(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis pantothenate synthetase |
J Med Chem 51: 1999-2002 (2008)
Article DOI: 10.1021/jm701372r
BindingDB Entry DOI: 10.7270/Q2028SD7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50356635

(CHEMBL1914708)Show SMILES ONC(=O)CCCCCCC(=O)Nc1cnn(Cc2cc(CN=[N+]=[N-])cc(c2)N=[N+]=[N-])c1 Show InChI InChI=1S/C19H24N10O3/c20-27-22-10-14-7-15(9-16(8-14)25-28-21)12-29-13-17(11-23-29)24-18(30)5-3-1-2-4-6-19(31)26-32/h7-9,11,13,32H,1-6,10,12H2,(H,24,30)(H,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50356634

(CHEMBL1914702)Show SMILES ONC(=O)CCCCCCNC(=O)c1cc(on1)-c1ccc(NC(=O)c2cc(CN=[N+]=[N-])cc(c2)N=[N+]=[N-])cc1 Show InChI InChI=1S/C25H26N10O5/c26-34-29-15-16-11-18(13-20(12-16)31-35-27)24(37)30-19-8-6-17(7-9-19)22-14-21(33-40-22)25(38)28-10-4-2-1-3-5-23(36)32-39/h6-9,11-14,39H,1-5,10,15H2,(H,28,38)(H,30,37)(H,32,36) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC11 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Pantothenate synthetase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50373148
(CHEMBL412059)Show SMILES Cc1ccccc1Cn1cc(NC(=O)c2noc3CCC(Cc23)C(C)(C)C)cn1 |w:20.25| Show InChI InChI=1S/C23H28N4O2/c1-15-7-5-6-8-16(15)13-27-14-18(12-24-27)25-22(28)21-19-11-17(23(2,3)4)9-10-20(19)29-26-21/h5-8,12,14,17H,9-11,13H2,1-4H3,(H,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis pantothenate synthetase |
J Med Chem 51: 1999-2002 (2008)
Article DOI: 10.1021/jm701372r
BindingDB Entry DOI: 10.7270/Q2028SD7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50356635

(CHEMBL1914708)Show SMILES ONC(=O)CCCCCCC(=O)Nc1cnn(Cc2cc(CN=[N+]=[N-])cc(c2)N=[N+]=[N-])c1 Show InChI InChI=1S/C19H24N10O3/c20-27-22-10-14-7-15(9-16(8-14)25-28-21)12-29-13-17(11-23-29)24-18(30)5-3-1-2-4-6-19(31)26-32/h7-9,11,13,32H,1-6,10,12H2,(H,24,30)(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3/NCoR2 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | |
Pantothenate synthetase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50373140

(CHEMBL261486)Show SMILES CC(C)(C)C1CCc2onc(C(=O)Nc3cnn(Cc4ccc(F)cc4F)c3)c2C1 |w:4.3| Show InChI InChI=1S/C22H24F2N4O2/c1-22(2,3)14-5-7-19-17(8-14)20(27-30-19)21(29)26-16-10-25-28(12-16)11-13-4-6-15(23)9-18(13)24/h4,6,9-10,12,14H,5,7-8,11H2,1-3H3,(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis pantothenate synthetase |
J Med Chem 51: 1999-2002 (2008)
Article DOI: 10.1021/jm701372r
BindingDB Entry DOI: 10.7270/Q2028SD7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 mins |
J Med Chem 54: 4350-64 (2011)
Article DOI: 10.1021/jm2001025
BindingDB Entry DOI: 10.7270/Q2RX9CG0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pantothenate synthetase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50373145

(CHEMBL409940)Show SMILES CC(C)(C)C1CCc2onc(C(=O)Nc3cnn(Cc4ccccc4Br)c3)c2C1 |w:4.3| Show InChI InChI=1S/C22H25BrN4O2/c1-22(2,3)15-8-9-19-17(10-15)20(26-29-19)21(28)25-16-11-24-27(13-16)12-14-6-4-5-7-18(14)23/h4-7,11,13,15H,8-10,12H2,1-3H3,(H,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis pantothenate synthetase |
J Med Chem 51: 1999-2002 (2008)
Article DOI: 10.1021/jm701372r
BindingDB Entry DOI: 10.7270/Q2028SD7 |
More data for this
Ligand-Target Pair | |
Pantothenate synthetase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50373136

(CHEMBL438202)Show SMILES CC(C)(C)C1CCc2onc(C(=O)Nc3cnn(Cc4ccc(cc4)C(=O)NCCc4ccccc4)c3)c2C1 |w:4.3| Show InChI InChI=1S/C31H35N5O3/c1-31(2,3)24-13-14-27-26(17-24)28(35-39-27)30(38)34-25-18-33-36(20-25)19-22-9-11-23(12-10-22)29(37)32-16-15-21-7-5-4-6-8-21/h4-12,18,20,24H,13-17,19H2,1-3H3,(H,32,37)(H,34,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis pantothenate synthetase |
J Med Chem 51: 1999-2002 (2008)
Article DOI: 10.1021/jm701372r
BindingDB Entry DOI: 10.7270/Q2028SD7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data