 Found 349 hits with Last Name = 'brus' and Initial = 'b'
Found 349 hits with Last Name = 'brus' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM50247011

(CHEMBL4080419)Show SMILES Cl.CN(C)CCN(CC1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C30H37N3O.ClH/c1-31(2)16-17-33(30(34)28-14-13-24-9-3-4-10-25(24)18-28)22-23-8-7-15-32(21-23)29-19-26-11-5-6-12-27(26)20-29;/h3-6,9-14,18,23,29H,7-8,15-17,19-22H2,1-2H3;1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate at pH 8 by stopped flow assay |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50247011

(CHEMBL4080419)Show SMILES Cl.CN(C)CCN(CC1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C30H37N3O.ClH/c1-31(2)16-17-33(30(34)28-14-13-24-9-3-4-10-25(24)18-28)22-23-8-7-15-32(21-23)29-19-26-11-5-6-12-27(26)20-29;/h3-6,9-14,18,23,29H,7-8,15-17,19-22H2,1-2H3;1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate at pH 8 by stopped flow assay |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8963

(CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...)Show SMILES C(CCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H40N4/c1(2-12-22-34-32-24-14-4-8-18-28(24)36-29-19-9-5-15-25(29)32)3-13-23-35-33-26-16-6-10-20-30(26)37-31-21-11-7-17-27(31)33/h4,6,8,10,14,16,18,20H,1-3,5,7,9,11-13,15,17,19,21-23H2,(H,34,36)(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate at pH 8 by stopped flow assay |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50027375

(CHEMBL3338394)Show SMILES COCCN(C[C@@H]1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C29H34N2O2/c1-33-16-15-31(29(32)27-13-12-23-8-2-3-9-24(23)17-27)21-22-7-6-14-30(20-22)28-18-25-10-4-5-11-26(25)19-28/h2-5,8-13,17,22,28H,6-7,14-16,18-21H2,1H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BChE at 50 nM by stopped flow apparatus method |
J Med Chem 57: 8167-79 (2014)
Article DOI: 10.1021/jm501195e
BindingDB Entry DOI: 10.7270/Q22V2HQ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50027377
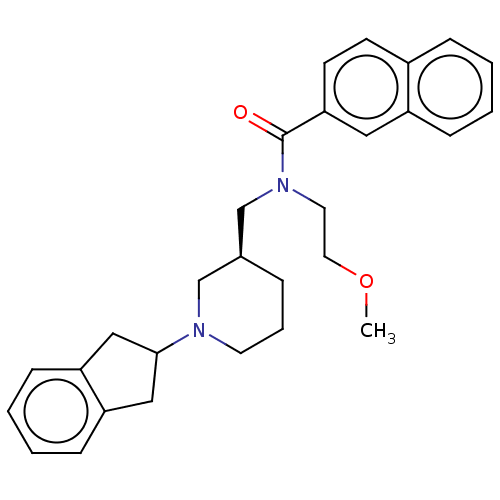
(CHEMBL3338395)Show SMILES COCCN(C[C@H]1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C29H34N2O2/c1-33-16-15-31(29(32)27-13-12-23-8-2-3-9-24(23)17-27)21-22-7-6-14-30(20-22)28-18-25-10-4-5-11-26(25)19-28/h2-5,8-13,17,22,28H,6-7,14-16,18-21H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BChE at 50 nM by stopped flow apparatus method |
J Med Chem 57: 8167-79 (2014)
Article DOI: 10.1021/jm501195e
BindingDB Entry DOI: 10.7270/Q22V2HQ7 |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 412 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 5A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1E
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 188: 244-51 (2006)
Article DOI: 10.1007/s00213-006-0490-4
BindingDB Entry DOI: 10.7270/Q2HX1B8P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50247022

(CHEMBL4089230)Show SMILES CN(C)CCN(CC1CCCN(C1)C1Cc2ccccc2C1)C(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C30H37N3O/c1-31(2)16-17-33(30(34)28-14-13-24-9-3-4-10-25(24)18-28)22-23-8-7-15-32(21-23)29-19-26-11-5-6-12-27(26)20-29/h3-6,9-14,18,23,29H,7-8,15-17,19-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50557619
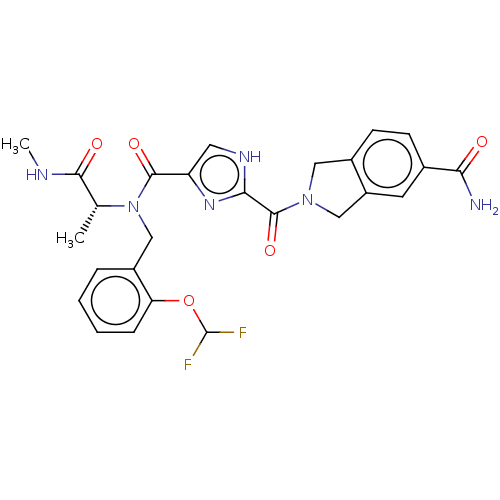
(CHEMBL4796239)Show SMILES CNC(=O)[C@@H](C)N(Cc1ccccc1OC(F)F)C(=O)c1c[nH]c(n1)C(=O)N1Cc2ccc(cc2C1)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length His-tagged TAK1-TAB1 fusion protein (437 to 504 residues) expressed in baculovirus expression system usin... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00547
BindingDB Entry DOI: 10.7270/Q20005S0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50068350
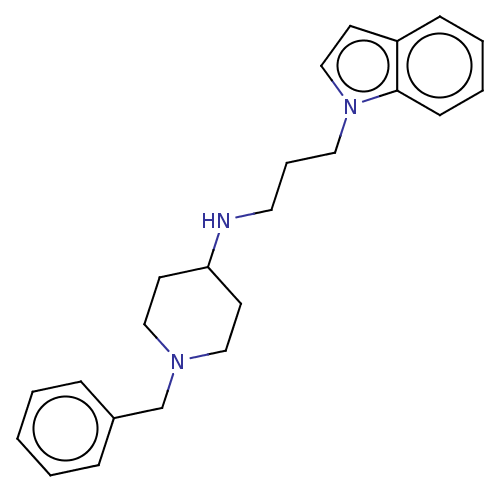
(CHEMBL3402303)Show InChI InChI=1S/C23H29N3/c1-2-7-20(8-3-1)19-25-16-12-22(13-17-25)24-14-6-15-26-18-11-21-9-4-5-10-23(21)26/h1-5,7-11,18,22,24H,6,12-17,19H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method |
Bioorg Med Chem 23: 2445-57 (2015)
Article DOI: 10.1016/j.bmc.2015.03.051
BindingDB Entry DOI: 10.7270/Q2CZ38VB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in horse serum pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's assay |
Bioorg Med Chem 23: 1629-37 (2015)
Article DOI: 10.1016/j.bmc.2015.01.045
BindingDB Entry DOI: 10.7270/Q2PN979Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE after 5 mins by Ellman's method |
Eur J Med Chem 92: 738-49 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.027
BindingDB Entry DOI: 10.7270/Q23T9JXZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant microsomal MAO-A expressed in baculovirus-infected insect cells using p-tyramine as substrate preincubated for 15 min... |
Bioorg Med Chem 25: 633-645 (2017)
Article DOI: 10.1016/j.bmc.2016.11.032
BindingDB Entry DOI: 10.7270/Q2HH6N2G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50246979
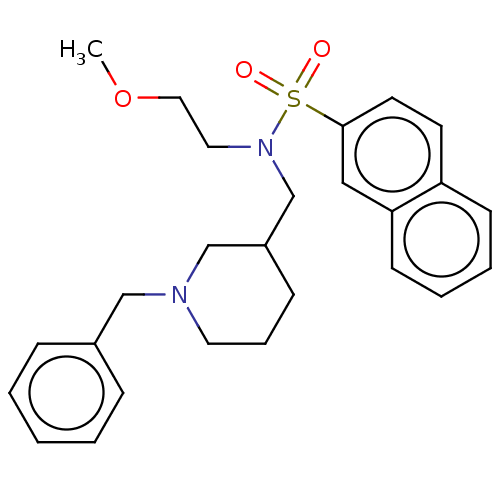
(CHEMBL4105611 | US20230331674, Table 1.1)Show SMILES COCCN(CC1CCCN(Cc2ccccc2)C1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C26H32N2O3S/c1-31-17-16-28(32(29,30)26-14-13-24-11-5-6-12-25(24)18-26)21-23-10-7-15-27(20-23)19-22-8-3-2-4-9-22/h2-6,8-9,11-14,18,23H,7,10,15-17,19-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BChE using butyrylthiocholine iodide as substrate preincubated for 300 secs followed by substrate addition measured f... |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50557620
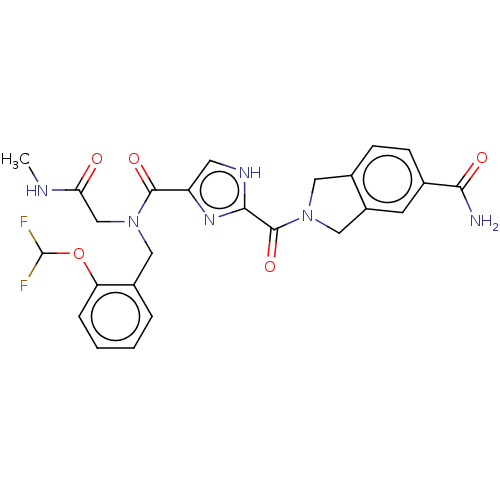
(CHEMBL4752978)Show SMILES CNC(=O)CN(Cc1ccccc1OC(F)F)C(=O)c1c[nH]c(n1)C(=O)N1Cc2ccc(cc2C1)C(N)=O | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length His-tagged TAK1-TAB1 fusion protein (437 to 504 residues) expressed in baculovirus expression system usin... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00547
BindingDB Entry DOI: 10.7270/Q20005S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Inhibition of AChE in mouse brain homogenate using acetylthiocholine as substrate preincubated for 300 secs followed by substrate addition by Ellman'... |
J Med Chem 61: 119-139 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01086
BindingDB Entry DOI: 10.7270/Q2S75JRJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE pre-incubated for 5 mins before addition of acetylthiocholine iodide substrate by Ellman's assay |
Bioorg Med Chem 23: 1629-37 (2015)
Article DOI: 10.1016/j.bmc.2015.01.045
BindingDB Entry DOI: 10.7270/Q2PN979Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE after 5 mins by Ellman's method |
Eur J Med Chem 92: 738-49 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.027
BindingDB Entry DOI: 10.7270/Q23T9JXZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE after 5 mins by Ellman's method |
Eur J Med Chem 92: 738-49 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.027
BindingDB Entry DOI: 10.7270/Q23T9JXZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50068302

(CHEMBL3402305)Show InChI InChI=1S/C26H35N3/c1(2-9-18-29-21-14-24-12-6-7-13-26(24)29)8-17-27-25-15-19-28(20-16-25)22-23-10-4-3-5-11-23/h3-7,10-14,21,25,27H,1-2,8-9,15-20,22H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method |
Bioorg Med Chem 23: 2445-57 (2015)
Article DOI: 10.1016/j.bmc.2015.03.051
BindingDB Entry DOI: 10.7270/Q2CZ38VB |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50438317

(CHEMBL2408611)Show SMILES Nc1ncc(-c2cnn(c2)[C@H]2CC[C@H](O)CC2)c2c(Cl)c(oc12)-c1cccc2nnsc12 |r,wU:13.14,wD:10.10,(28,-9.67,;29.47,-9.2,;30.61,-10.23,;32.07,-9.75,;32.39,-8.26,;33.86,-7.78,;34.33,-6.31,;35.87,-6.31,;36.35,-7.78,;35.1,-8.68,;37.81,-8.25,;38.13,-9.77,;39.58,-10.25,;40.73,-9.22,;42.2,-9.7,;40.42,-7.71,;38.95,-7.23,;31.26,-7.23,;31.25,-5.69,;32.5,-4.79,;29.79,-5.22,;28.89,-6.46,;29.79,-7.7,;29.32,-3.75,;30.36,-2.61,;29.89,-1.15,;28.39,-.82,;27.35,-1.96,;25.81,-1.96,;25.33,-3.42,;26.58,-4.33,;27.82,-3.43,)| Show InChI InChI=1S/C22H19ClN6O2S/c23-18-17-15(11-8-26-29(10-11)12-4-6-13(30)7-5-12)9-25-22(24)20(17)31-19(18)14-2-1-3-16-21(14)32-28-27-16/h1-3,8-10,12-13,30H,4-7H2,(H2,24,25)/t12-,13- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length His-tagged TAK1-TAB1 fusion protein (437 to 504 residues) expressed in baculovirus expression system usin... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00547
BindingDB Entry DOI: 10.7270/Q20005S0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data