 Found 254 hits with Last Name = 'buist' and Initial = 'n'
Found 254 hits with Last Name = 'buist' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548
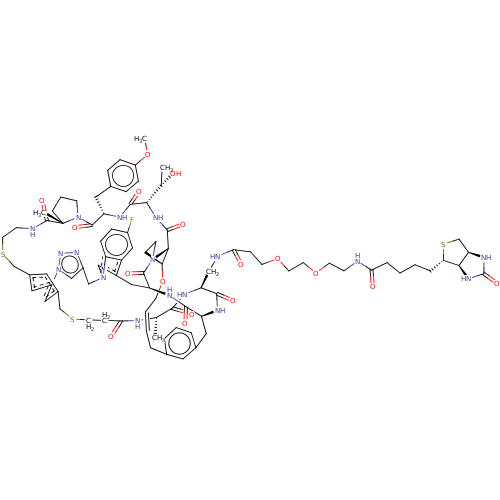
(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547
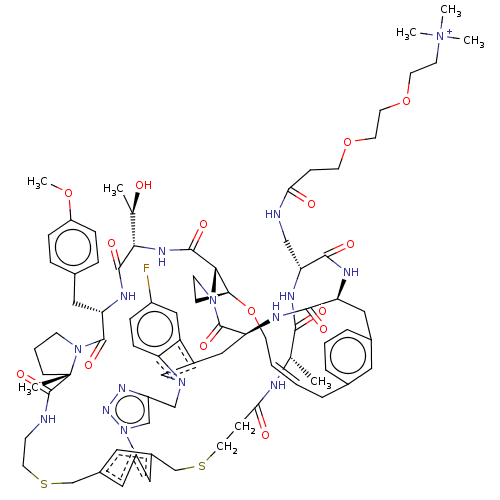
(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546
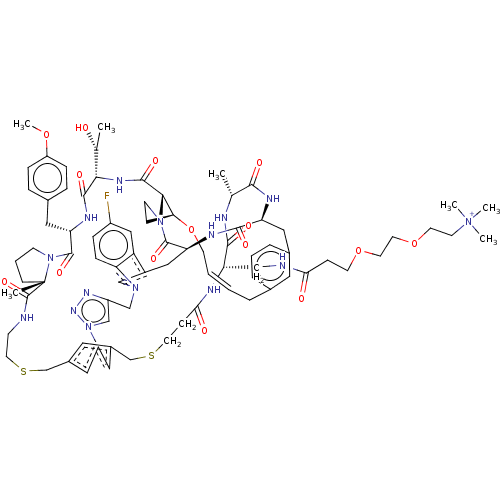
(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547

(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00736 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581545
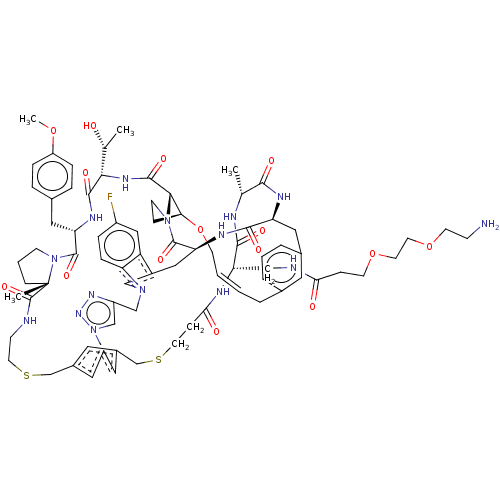
(CHEMBL5084902)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCCN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:94| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544
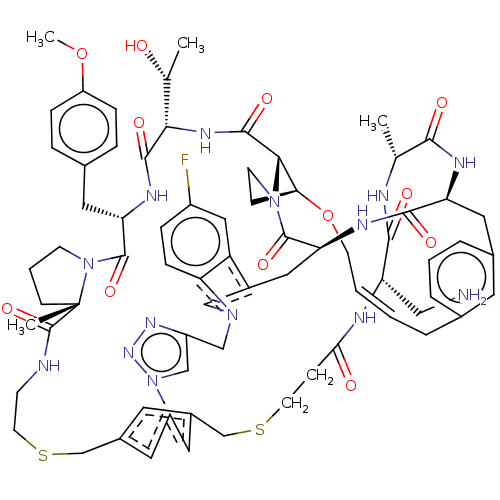
(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00826 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544

(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548

(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546

(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581549
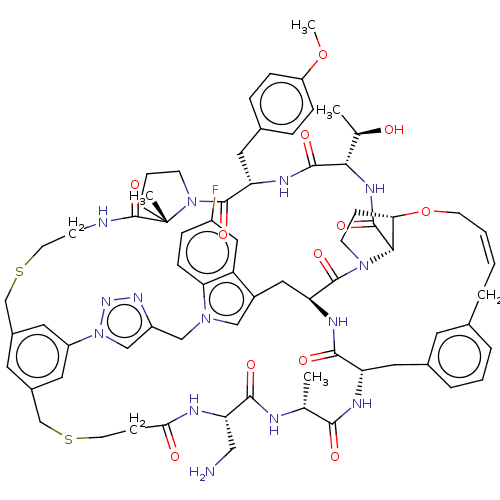
(CHEMBL5082483)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C/CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,c:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581548

(CHEMBL5085124)Show SMILES [H][C@@]12CS[C@@H](CCCCC(=O)NCCOCCOCCC(=O)NC[C@@H]3NC(=O)[C@H](C)NC(=O)CCSCc4cc5CSCCNC(=O)[C@]6(C)CCCN6C(=O)[C@H](Cc6ccc(OC)cc6)NC(=O)[C@@H](NC(=O)[C@@H]6[C@@H]7CCN6C(=O)[C@H](Cc6cn(Cc8cn(nn8)-c(c5)c4)c4ccc(F)cc64)NC(=O)[C@H](Cc4cccc(C\C=C\CO7)c4)NC3=O)[C@H](C)O)[C@]1([H])NC(=O)N2 |r,t:119| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581547

(CHEMBL5081349)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581546

(CHEMBL5084416)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCC[N+](C)(C)C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:97| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581545

(CHEMBL5084902)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CNC(=O)CCOCCOCCN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:94| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581544

(CHEMBL5086475)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@@H]3Cc5cn(Cc6cn(nn6)-c6cc(CSCCNC(=O)[C@]7(C)CCCN7C2=O)cc(CSCCC(=O)N[C@@H](CN)C(=O)N[C@H](C)C(=O)N[C@@H](Cc2cccc(C\C=C\CO4)c2)C(=O)N3)c6)c2ccc(F)cc52)[C@@H](C)O)cc1 |r,t:83| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581542
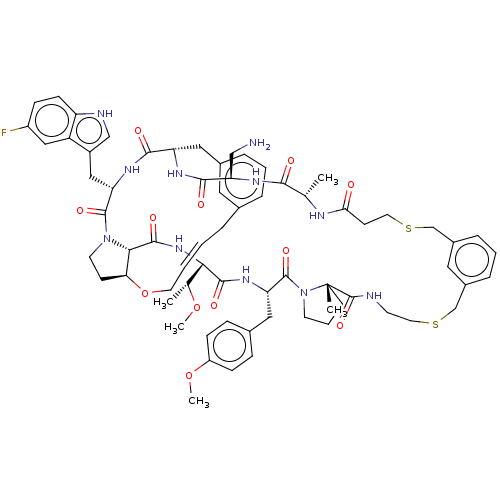
(CHEMBL5081587)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@@H](CN)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581542

(CHEMBL5081587)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@@H](CN)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581540
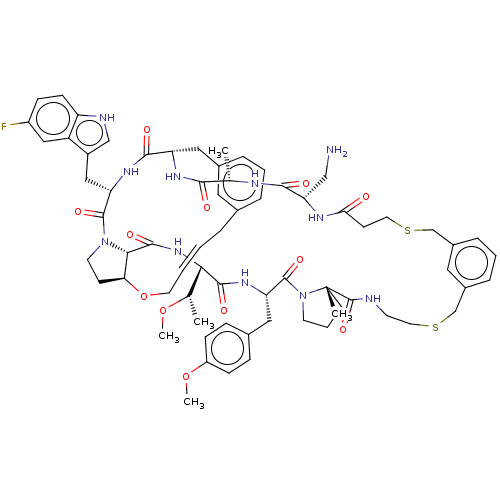
(CHEMBL5087487)Show SMILES CO[C@@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@H](C)NC(=O)[C@H](CN)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581537
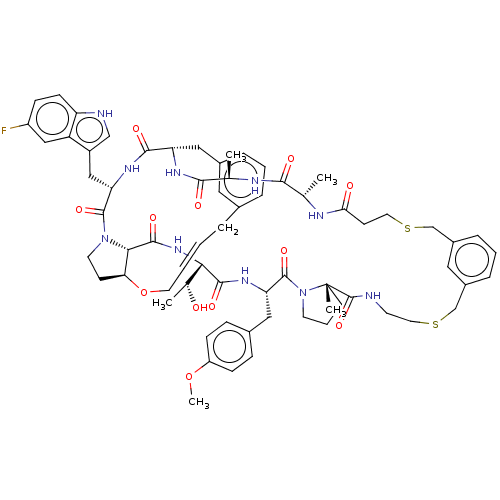
(CHEMBL5086286)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@H](Cc3c[nH]c5ccc(F)cc35)NC(=O)[C@H](Cc3cccc(C\C=C\CO4)c3)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc3cccc(CSCCNC(=O)[C@]4(C)CCCN4C2=O)c3)[C@@H](C)O)cc1 |r,t:48| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581535
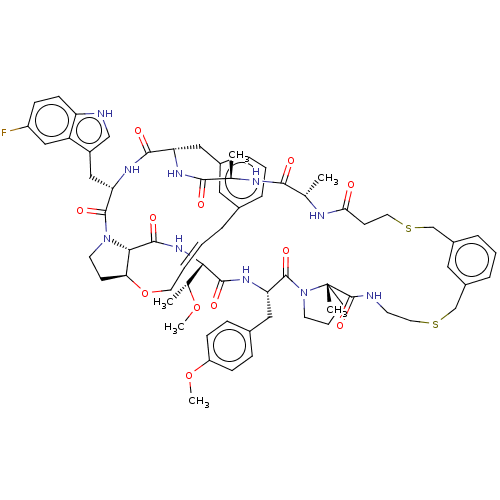
(CHEMBL5091040)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581537

(CHEMBL5086286)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@H](Cc3c[nH]c5ccc(F)cc35)NC(=O)[C@H](Cc3cccc(C\C=C\CO4)c3)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc3cccc(CSCCNC(=O)[C@]4(C)CCCN4C2=O)c3)[C@@H](C)O)cc1 |r,t:48| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581540

(CHEMBL5087487)Show SMILES CO[C@@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@H](C)NC(=O)[C@H](CN)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581535

(CHEMBL5091040)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C\CO3)c2)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,t:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581543
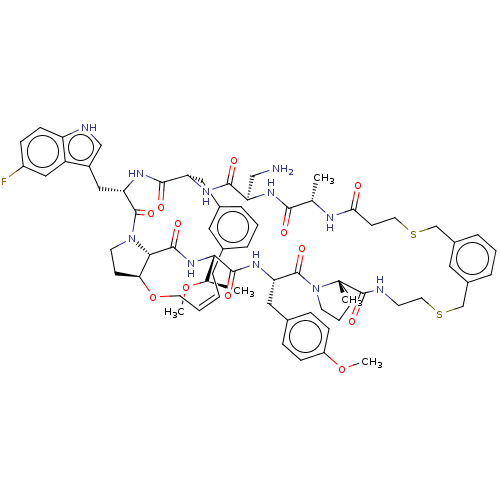
(CHEMBL5094648)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C/CO3)c2)NC(=O)[C@@H](CN)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,c:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50554754
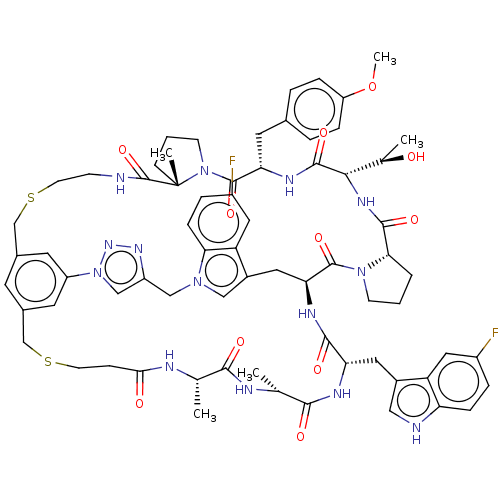
(CHEMBL4790764)Show SMILES [H][C@]1(NC(=O)[C@@H]2CCCN2C(=O)[C@@H]2Cc3cn(Cc4cn(nn4)-c4cc(CSCCNC(=O)[C@]5(C)CCCN5C(=O)[C@H](Cc5ccc(OC)cc5)NC1=O)cc(CSCCC(=O)N[C@@H](C)C(=O)N[C@H](C)C(=O)N[C@@H](Cc1c[nH]c5ccc(F)cc15)C(=O)N2)c4)c1ccc(F)cc31)[C@@H](C)O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581539
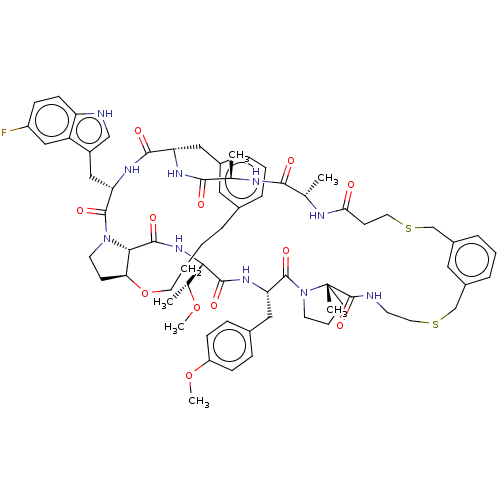
(CHEMBL5084586)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(CCCCO3)c2)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581543

(CHEMBL5094648)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C/CO3)c2)NC(=O)[C@@H](CN)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,c:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET plus assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581541
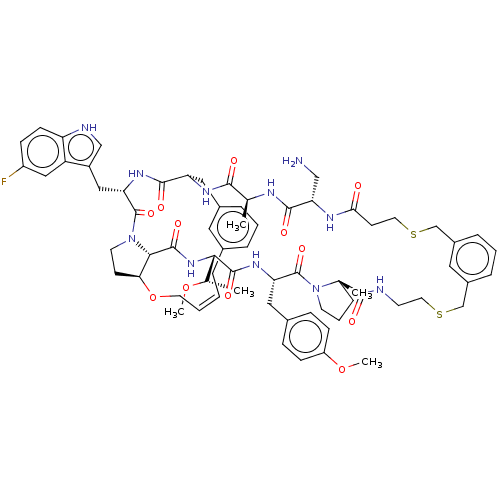
(CHEMBL5078356)Show SMILES CO[C@@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C/CO3)c2)NC(=O)[C@H](C)NC(=O)[C@H](CN)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,c:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581538

(CHEMBL5081995)Show SMILES COc1ccc(C[C@@H]2NC(=O)[C@@H](NC(=O)[C@@H]3[C@@H]4CCN3C(=O)[C@H](Cc3c[nH]c5ccc(F)cc35)NC(=O)[C@H](Cc3cccc(C\C=C/CO4)c3)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc3cccc(CSCCNC(=O)[C@]4(C)CCCN4C2=O)c3)[C@@H](C)O)cc1 |r,c:48| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM50581536
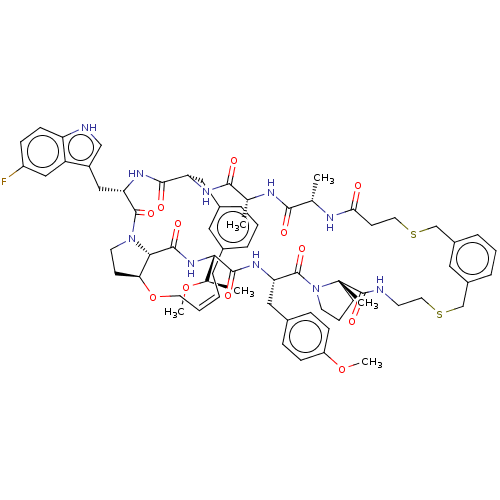
(CHEMBL5081260)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2[C@@H]3CCN2C(=O)[C@H](Cc2c[nH]c4ccc(F)cc24)NC(=O)[C@H](Cc2cccc(C\C=C/CO3)c2)NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc2cccc(CSCCNC(=O)[C@]3(C)CCCN3C(=O)[C@H](Cc3ccc(OC)cc3)NC1=O)c2 |r,c:41| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM586332
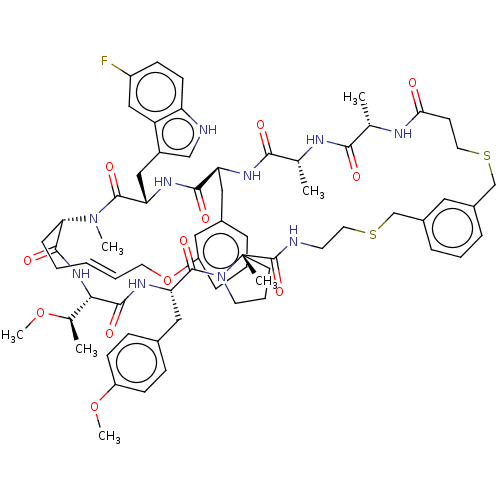
(US11530244, Compound 503)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2CC\C=C\COc3cccc(C[C@H](NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc4cccc(CSCCNC(=O)[C@]5(C)CCCN5C(=O)[C@H](Cc5ccc(OC)cc5)NC1=O)c4)C(=O)N[C@@H](Cc1c[nH]c4ccc(F)cc14)C(=O)N2C)c3 |r,t:11| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 9
(Homo sapiens (Human)) | BDBM586331
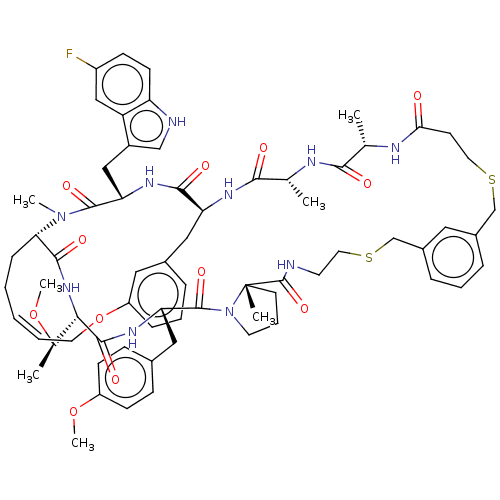
(US11530244, Compound 501)Show SMILES CO[C@H](C)[C@@H]1NC(=O)[C@@H]2CC\C=C/COc3cccc(C[C@H](NC(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)CCSCc4cccc(CSCCNC(=O)[C@]5(C)CCCN5C(=O)[C@H](Cc5ccc(OC)cc5)NC1=O)c4)C(=O)N[C@@H](Cc1c[nH]c4ccc(F)cc14)C(=O)N2C)c3 |r,c:11| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01599
BindingDB Entry DOI: 10.7270/Q2TM7G0Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092190
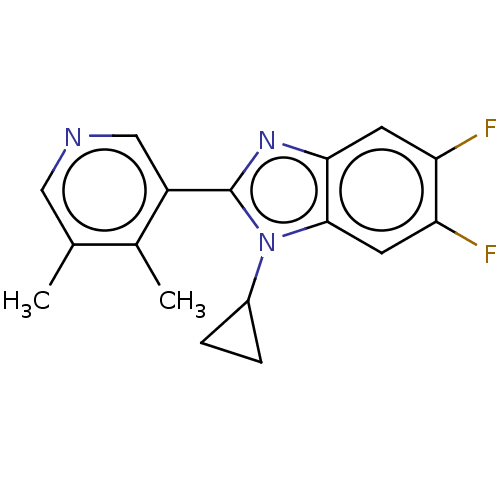
(CHEMBL3582482)Show InChI InChI=1S/C17H15F2N3/c1-9-7-20-8-12(10(9)2)17-21-15-5-13(18)14(19)6-16(15)22(17)11-3-4-11/h5-8,11H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092142

(CHEMBL3582477)Show SMILES CC(C)(O)c1cncc(c1)-c1nc2cc(F)c(F)cc2n1C1CC1 Show InChI InChI=1S/C11H17NO8/c1-4(14)12-8-5(15)2-7(11(18)19)20-10(8)9(17)6(16)3-13/h2,5-6,8-10,13,15-17H,3H2,1H3,(H,12,14)(H,18,19)/t5-,6-,8+,9-,10+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092185
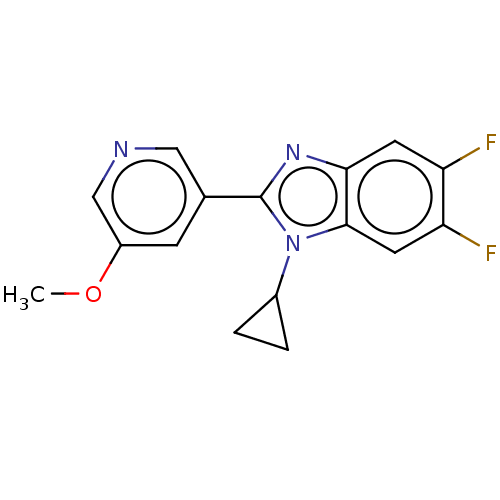
(CHEMBL3582474)Show InChI InChI=1S/C16H13F2N3O/c1-22-11-4-9(7-19-8-11)16-20-14-5-12(17)13(18)6-15(14)21(16)10-2-3-10/h4-8,10H,2-3H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092182
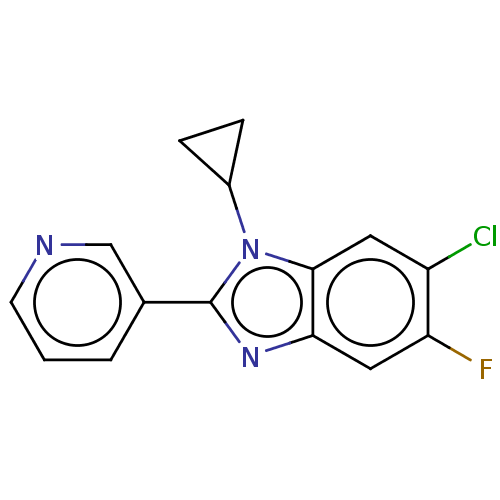
(CHEMBL3582470)Show InChI InChI=1S/C15H11ClFN3/c16-11-6-14-13(7-12(11)17)19-15(20(14)10-3-4-10)9-2-1-5-18-8-9/h1-2,5-8,10H,3-4H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092137
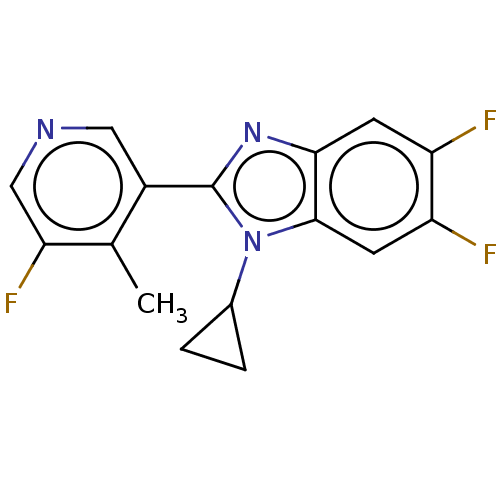
(CHEMBL3582481)Show InChI InChI=1S/C16H12F3N3/c1-8-10(6-20-7-13(8)19)16-21-14-4-11(17)12(18)5-15(14)22(16)9-2-3-9/h4-7,9H,2-3H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092183
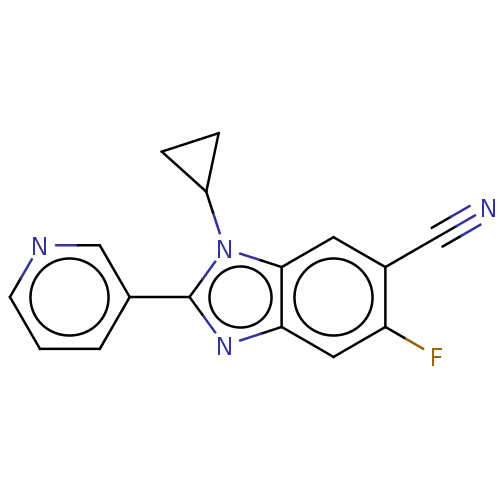
(CHEMBL3582471)Show InChI InChI=1S/C16H11FN4/c17-13-7-14-15(6-11(13)8-18)21(12-3-4-12)16(20-14)10-2-1-5-19-9-10/h1-2,5-7,9,12H,3-4H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092191
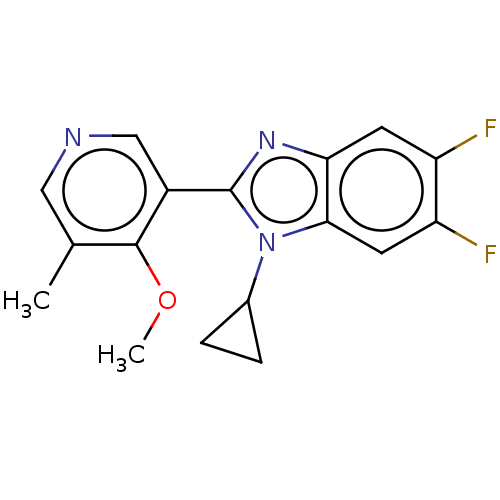
(CHEMBL3582483)Show InChI InChI=1S/C17H15F2N3O/c1-9-7-20-8-11(16(9)23-2)17-21-14-5-12(18)13(19)6-15(14)22(17)10-3-4-10/h5-8,10H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092186
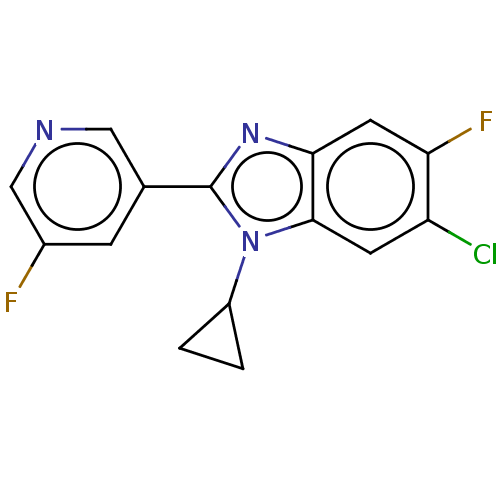
(CHEMBL3582475)Show InChI InChI=1S/C15H10ClF2N3/c16-11-4-14-13(5-12(11)18)20-15(21(14)10-1-2-10)8-3-9(17)7-19-6-8/h3-7,10H,1-2H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092187
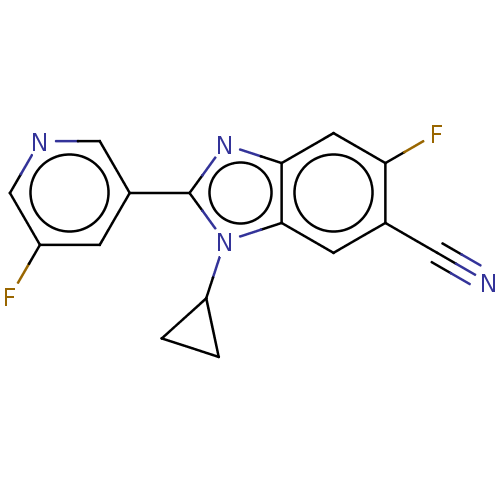
(CHEMBL3582476)Show InChI InChI=1S/C16H10F2N4/c17-11-3-10(7-20-8-11)16-21-14-5-13(18)9(6-19)4-15(14)22(16)12-1-2-12/h3-5,7-8,12H,1-2H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092179
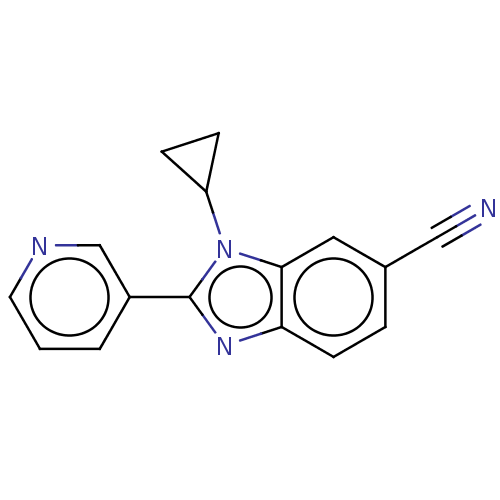
(CHEMBL3582467)Show InChI InChI=1S/C16H12N4/c17-9-11-3-6-14-15(8-11)20(13-4-5-13)16(19-14)12-2-1-7-18-10-12/h1-3,6-8,10,13H,4-5H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092181
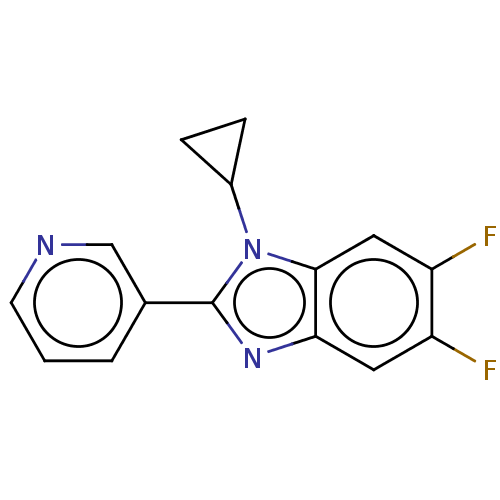
(CHEMBL3582469)Show InChI InChI=1S/C15H11F2N3/c16-11-6-13-14(7-12(11)17)20(10-3-4-10)15(19-13)9-2-1-5-18-8-9/h1-2,5-8,10H,3-4H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092177
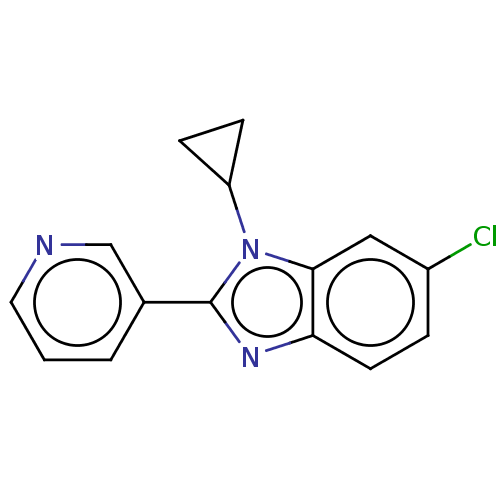
(CHEMBL3582465)Show InChI InChI=1S/C15H12ClN3/c16-11-3-6-13-14(8-11)19(12-4-5-12)15(18-13)10-2-1-7-17-9-10/h1-3,6-9,12H,4-5H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092153
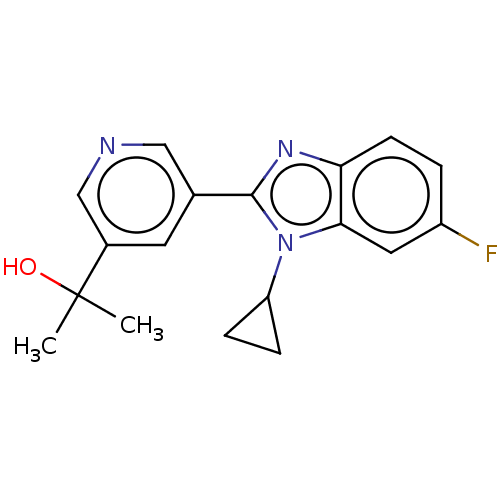
(CHEMBL3582478)Show InChI InChI=1S/C43H54N6O6/c1-5-28(4)39-42(53)44-22-11-23-55-32-19-16-30(17-20-32)25-36(41(52)49-39)45-26-37(50)35(24-29-12-7-6-8-13-29)47-43(54)38(27(2)3)48-40(51)34-21-18-31-14-9-10-15-33(31)46-34/h6-10,12-21,27-28,35-39,45,50H,5,11,22-26H2,1-4H3,(H,44,53)(H,47,54)(H,48,51)(H,49,52)/t28?,35-,36-,37+,38-,39-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092192

(CHEMBL3582451)Show InChI InChI=1S/C15H12FN3/c16-11-3-6-13-14(8-11)19(12-4-5-12)15(18-13)10-2-1-7-17-9-10/h1-3,6-9,12H,4-5H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092139
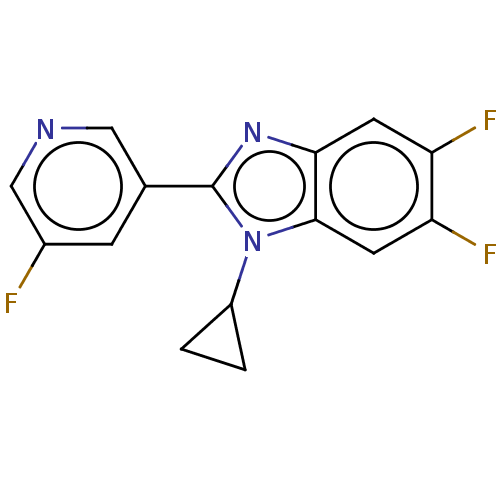
(CHEMBL3582472)Show InChI InChI=1S/C15H10F3N3/c16-9-3-8(6-19-7-9)15-20-13-4-11(17)12(18)5-14(13)21(15)10-1-2-10/h3-7,10H,1-2H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50428337
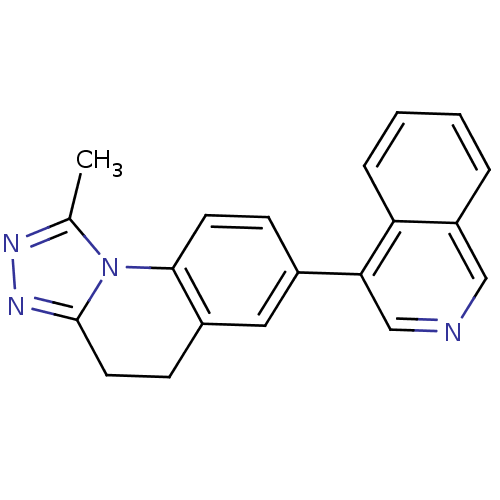
(CHEMBL2331706)Show InChI InChI=1S/C20H16N4/c1-13-22-23-20-9-7-15-10-14(6-8-19(15)24(13)20)18-12-21-11-16-4-2-3-5-17(16)18/h2-6,8,10-12H,7,9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells assessed as aldosterone level after 3 hrs by HTRF assay in presence of 125 nM 11-deoxycorticostero... |
ACS Med Chem Lett 6: 861-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00048
BindingDB Entry DOI: 10.7270/Q2K939BB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092189
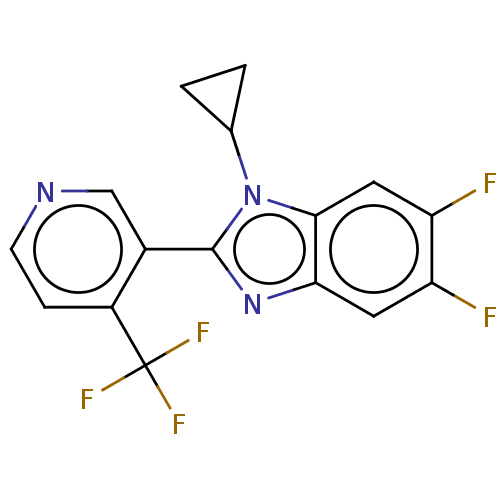
(CHEMBL3582480)Show InChI InChI=1S/C16H10F5N3/c17-11-5-13-14(6-12(11)18)24(8-1-2-8)15(23-13)9-7-22-4-3-10(9)16(19,20)21/h3-8H,1-2H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50092188
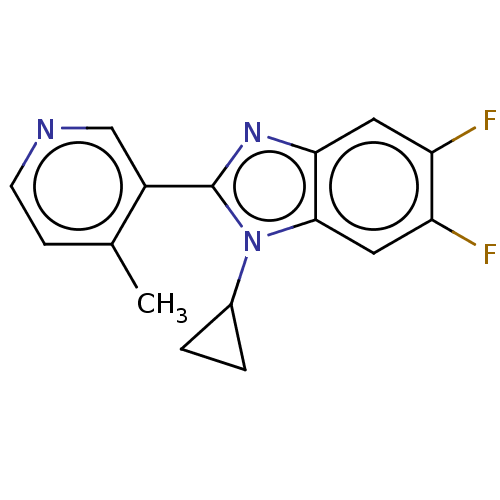
(CHEMBL3582479)Show InChI InChI=1S/C16H13F2N3/c1-9-4-5-19-8-11(9)16-20-14-6-12(17)13(18)7-15(14)21(16)10-2-3-10/h4-8,10H,2-3H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in V79 cells incubated for 1 hr before 11-deoxycorticosterone substrate addition by HTRF-based assay |
ACS Med Chem Lett 6: 573-8 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00054
BindingDB Entry DOI: 10.7270/Q2K64KT8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data