

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50405683 (CHEMBL174909) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Apparent inhibition constant (Ki) for cytochrome P450 19A1 with androstenedione | J Med Chem 31: 971-6 (1988) BindingDB Entry DOI: 10.7270/Q2154J7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50011767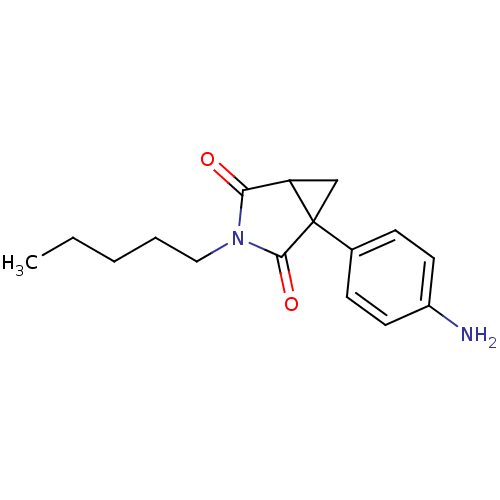 (1-(4-Amino-phenyl)-3-pentyl-3-aza-bicyclo[3.1.0]he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 androstenedione | J Med Chem 31: 971-6 (1988) BindingDB Entry DOI: 10.7270/Q2154J7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50405688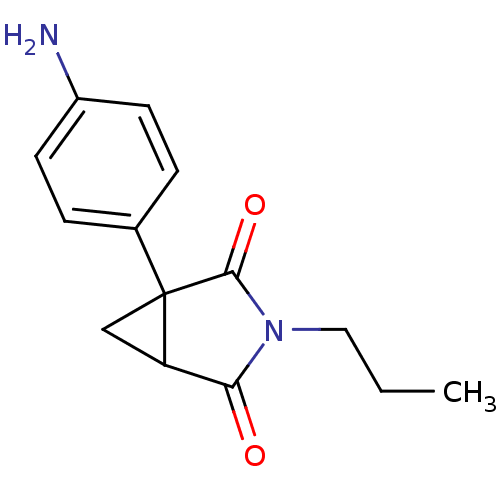 (CHEMBL174407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 androstenedione | J Med Chem 31: 971-6 (1988) BindingDB Entry DOI: 10.7270/Q2154J7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 uM (Km=0.13 uM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025155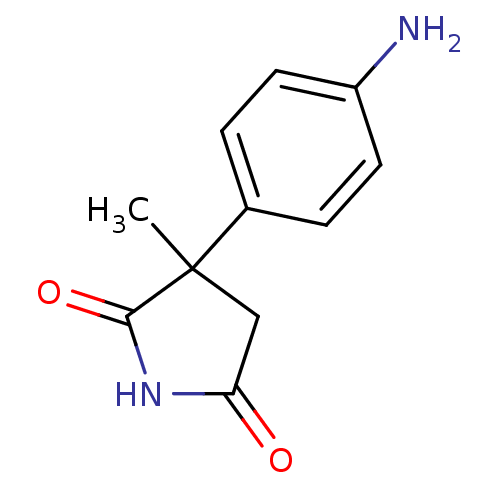 (3-(4-Amino-phenyl)-3-methyl-pyrrolidine-2,5-dione ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against Androstenedione at 0.25 uM (Km=55 nM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025152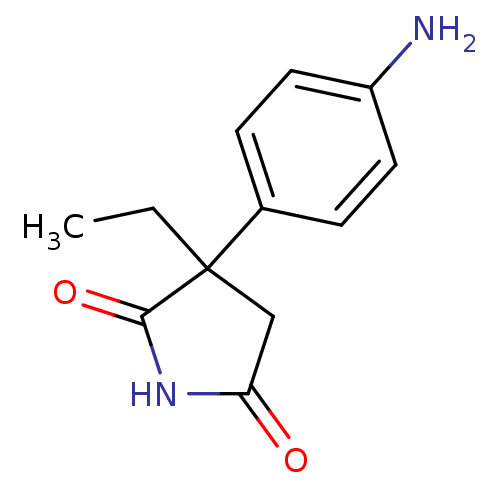 (3-(4-Amino-phenyl)-3-ethyl-pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 uM (Km=0.13 uM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50011760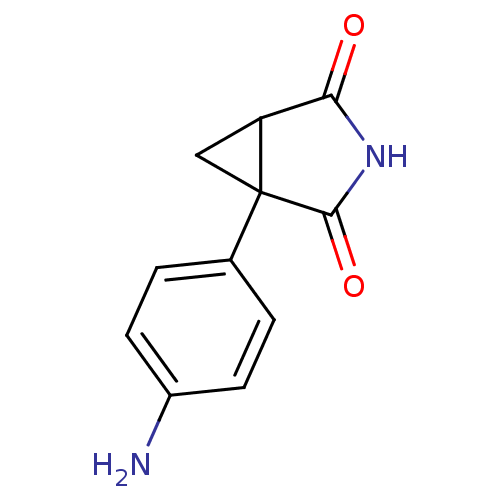 (1-(4-Amino-phenyl)-3-aza-bicyclo[3.1.0]hexane-2,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 androstenedione | J Med Chem 31: 971-6 (1988) BindingDB Entry DOI: 10.7270/Q2154J7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025153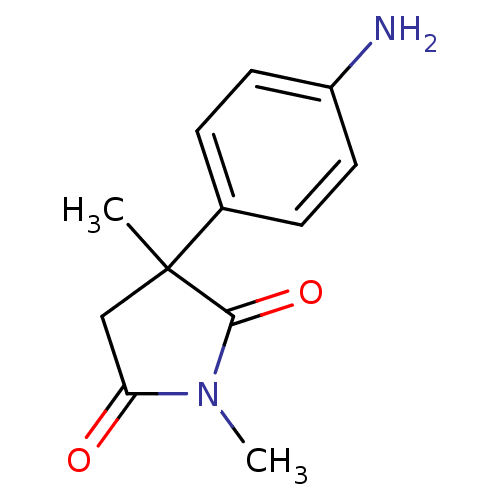 (3-(4-Amino-phenyl)-1,3-dimethyl-pyrrolidine-2,5-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 microM (Km=0.13 uM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025151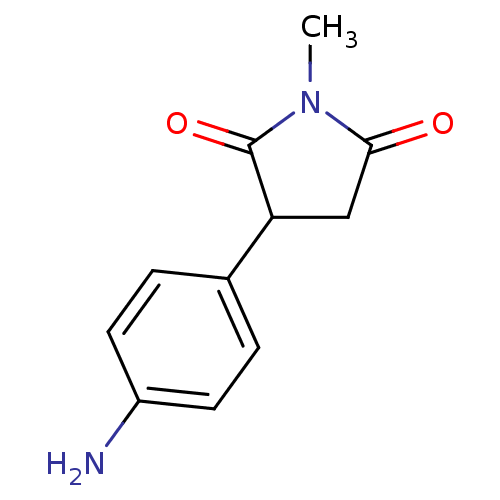 (3-(4-Amino-phenyl)-1-methyl-pyrrolidine-2,5-dione ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 microM (Km=0.13 uM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 with testosterone | J Med Chem 31: 971-6 (1988) BindingDB Entry DOI: 10.7270/Q2154J7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 6.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 microM (Km=0.13 uM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against Androstenedione at 0.25 uM (Km=55 nM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025152 (3-(4-Amino-phenyl)-3-ethyl-pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 uM (Km=0.13 uM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025151 (3-(4-Amino-phenyl)-1-methyl-pyrrolidine-2,5-dione ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against Androstenedione at 0.25 uM (Km=55 nM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025155 (3-(4-Amino-phenyl)-3-methyl-pyrrolidine-2,5-dione ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against Androstenedione at 0.25 uM (Km=55 nM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025154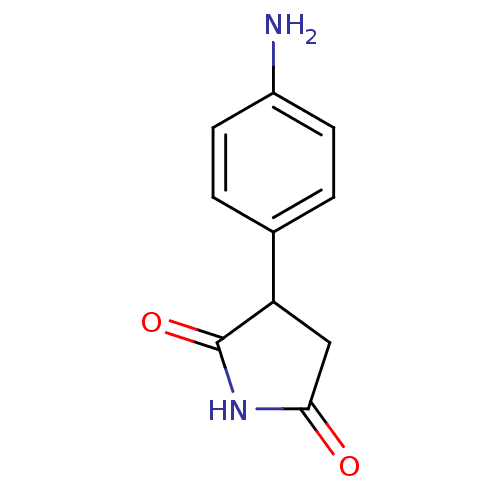 (3-(4-Amino-phenyl)-pyrrolidine-2,5-dione | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against Androstenedione at 0.25 uM (Km=55 nM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025155 (3-(4-Amino-phenyl)-3-methyl-pyrrolidine-2,5-dione ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 uM (Km=0.13 uM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025152 (3-(4-Amino-phenyl)-3-ethyl-pyrrolidine-2,5-dione |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 uM (Km=0.13 uM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025153 (3-(4-Amino-phenyl)-1,3-dimethyl-pyrrolidine-2,5-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 uM (Km=0.13 uM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025153 (3-(4-Amino-phenyl)-1,3-dimethyl-pyrrolidine-2,5-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 microM (Km=0.13 uM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025151 (3-(4-Amino-phenyl)-1-methyl-pyrrolidine-2,5-dione ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 microM (Km=0.13 uM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50025154 (3-(4-Amino-phenyl)-pyrrolidine-2,5-dione | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Cytochrome P450 19A1 against Androstenedione at 0.25 uM (Km=55 nM) | J Med Chem 29: 520-3 (1986) BindingDB Entry DOI: 10.7270/Q28051MG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||