

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Tested in vitro for inhibition of human Coagulation factor X | J Med Chem 46: 5298-315 (2003) Article DOI: 10.1021/jm030212h BindingDB Entry DOI: 10.7270/Q2ZW1MP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12751 (1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | -61.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12754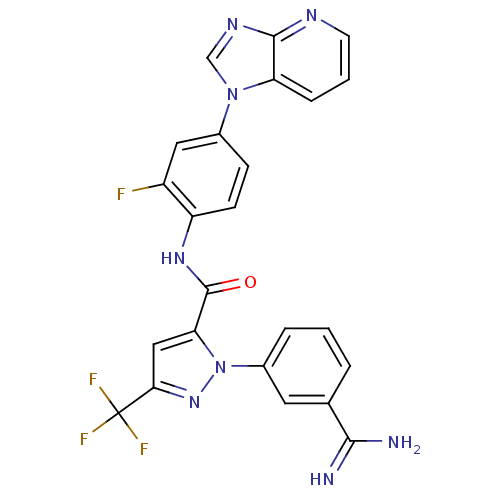 (1-(3-carbamimidoylphenyl)-N-(2-fluoro-4-{1H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0240 | -60.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12757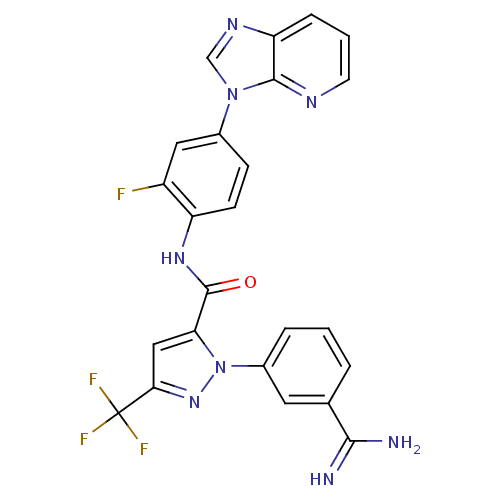 (1-(3-carbamimidoylphenyl)-N-(2-fluoro-4-{3H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0280 | -59.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12755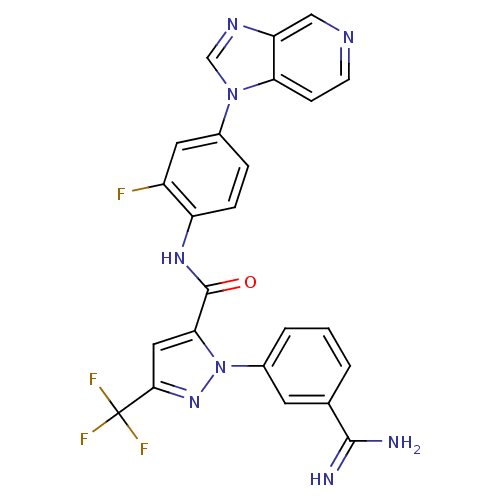 (1-(3-carbamimidoylphenyl)-N-(2-fluoro-4-{1H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0360 | -59.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458771 (CHEMBL4214046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12756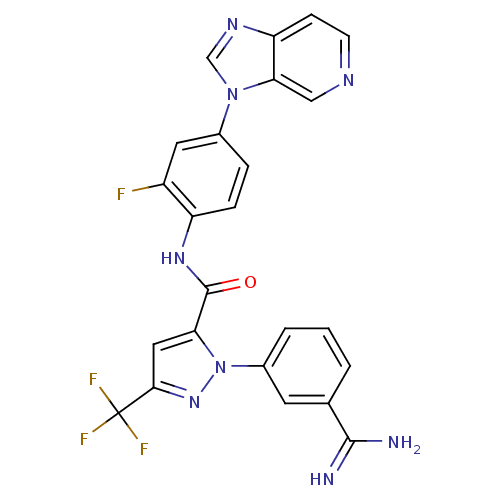 (1-(3-carbamimidoylphenyl)-N-(2-fluoro-4-{3H-imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0440 | -58.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312809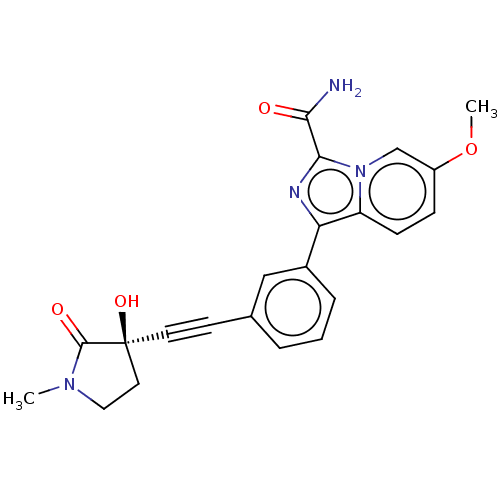 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12766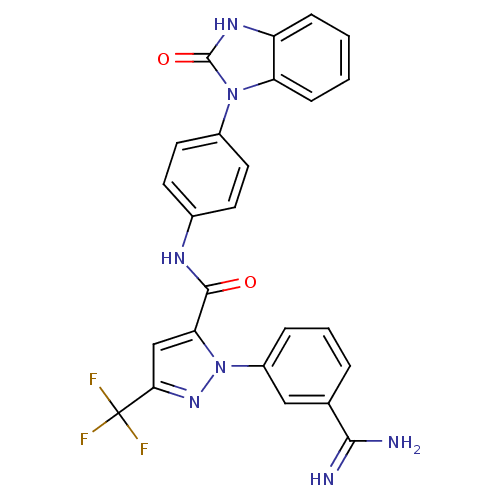 (1-(3-carbamimidoylphenyl)-N-[4-(2-oxo-2,7a-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0580 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM50457820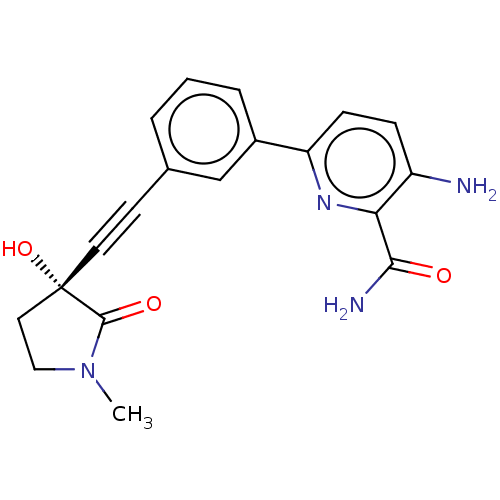 (CHEMBL4216289) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM50457816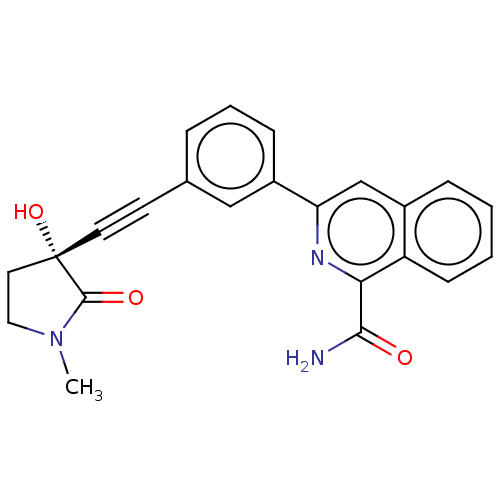 (CHEMBL4215425) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312763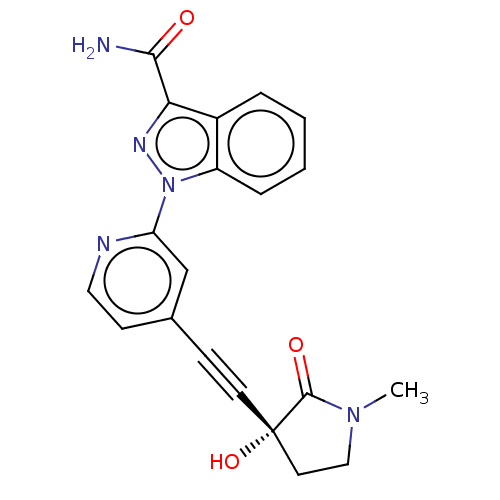 (1-[4-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12763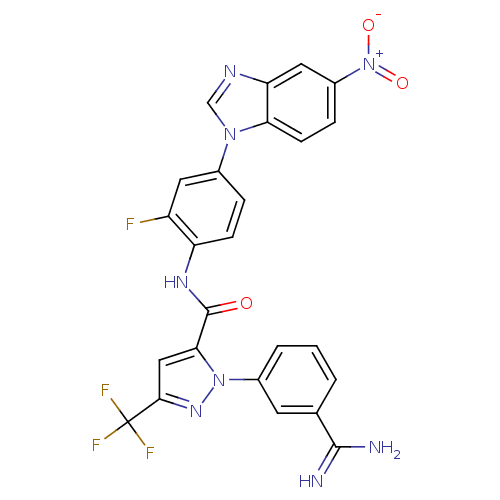 (1-(3-carbamimidoylphenyl)-N-[2-fluoro-4-(5-nitro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086681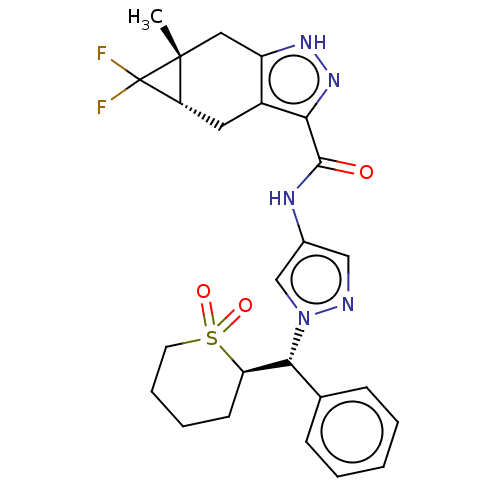 (CHEMBL3426309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12767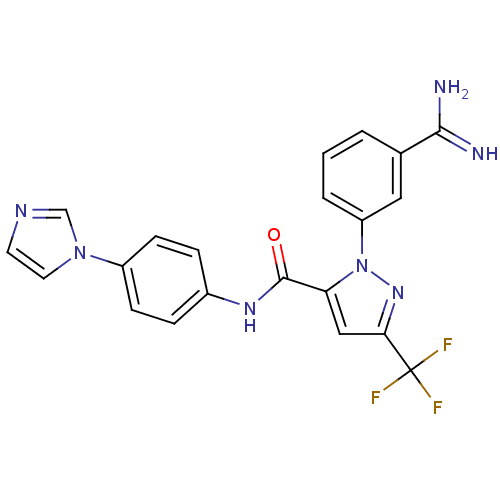 (1-(3-carbamimidoylphenyl)-N-[4-(1H-imidazol-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0910 | -56.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12752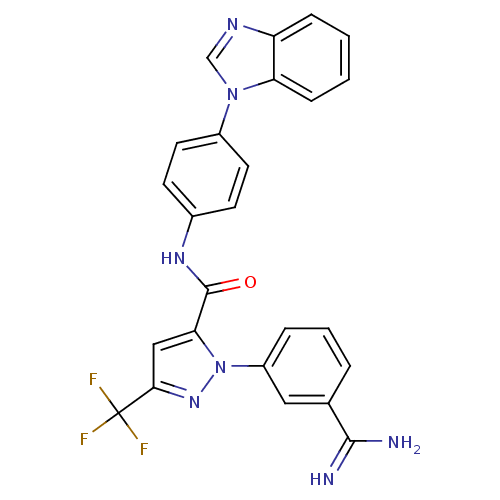 (N-[4-(1H-1,3-benzodiazol-1-yl)phenyl]-1-(3-carbami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0940 | -56.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12753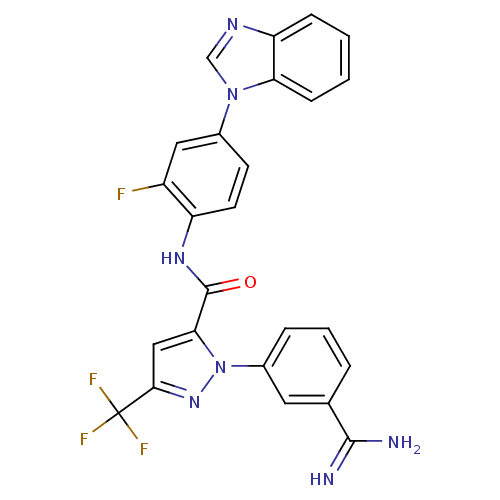 (N-[4-(1H-1,3-benzodiazol-1-yl)-2-fluorophenyl]-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening | J Med Chem 57: 10176-91 (2014) Article DOI: 10.1021/jm501578n BindingDB Entry DOI: 10.7270/Q2XK8H5B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening | J Med Chem 57: 10176-91 (2014) Article DOI: 10.1021/jm501578n BindingDB Entry DOI: 10.7270/Q2XK8H5B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged EGFR catalytic domain (669 to 1210 residues) expressed in baculovirus expression system by mass... | ACS Med Chem Lett 7: 100-4 (2016) Article DOI: 10.1021/acsmedchemlett.5b00428 BindingDB Entry DOI: 10.7270/Q25T3NCC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of wild-type EGFR (unknown origin) by high-throughput biochemical screening | J Med Chem 57: 10176-91 (2014) Article DOI: 10.1021/jm501578n BindingDB Entry DOI: 10.7270/Q2XK8H5B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015266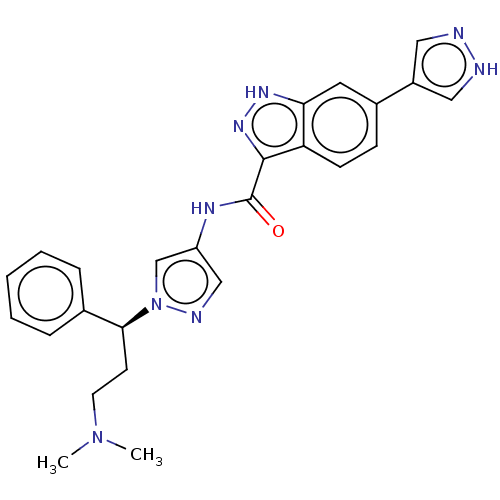 (CHEMBL3263053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50015266 (CHEMBL3263053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of full-length ITK (unknown origin) using biotin-EQEDEPEGIYGVLF-NH2 as substrate by plate reader analysis | Bioorg Med Chem Lett 24: 2448-52 (2014) Article DOI: 10.1016/j.bmcl.2014.04.023 BindingDB Entry DOI: 10.7270/Q2W097G8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of wild-type EGFR (unknown origin) by high-throughput biochemical screening | J Med Chem 57: 10176-91 (2014) Article DOI: 10.1021/jm501578n BindingDB Entry DOI: 10.7270/Q2XK8H5B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335985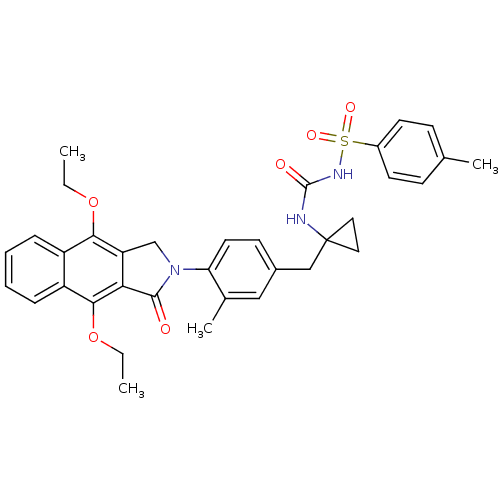 (CHEMBL1669013 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50372064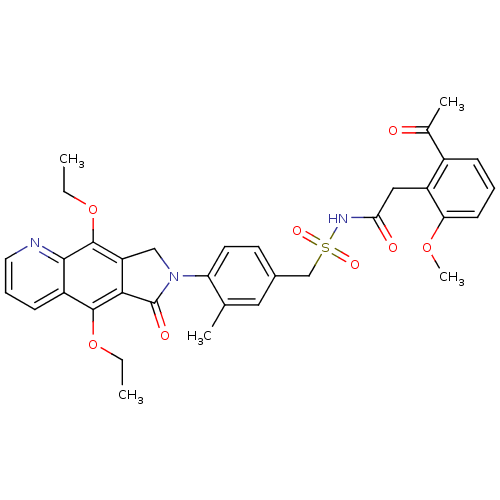 (CHEMBL256873) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Binding affinity to human EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 2048-54 (2008) Article DOI: 10.1016/j.bmcl.2008.01.103 BindingDB Entry DOI: 10.7270/Q2J96770 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12762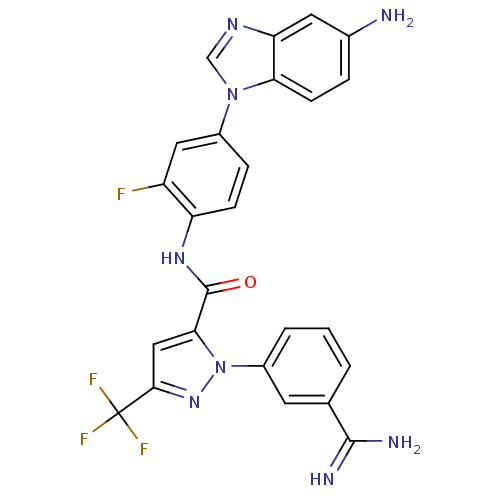 (N-[4-(5-amino-1H-1,3-benzodiazol-1-yl)-2-fluorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -56.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312785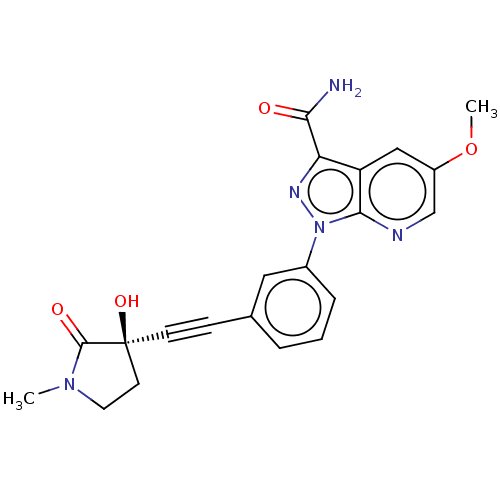 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12657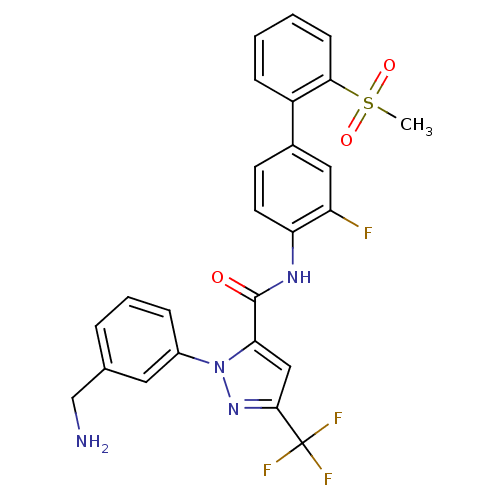 (1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Tested in vitro for inhibition of human Coagulation factor X | J Med Chem 46: 5298-315 (2003) Article DOI: 10.1021/jm030212h BindingDB Entry DOI: 10.7270/Q2ZW1MP2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335986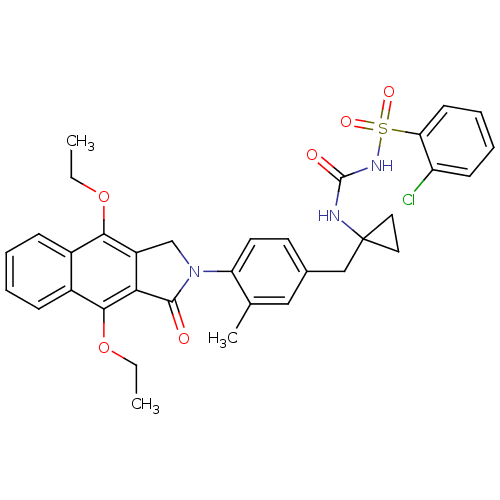 (2-chloro-N-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[f]is...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335980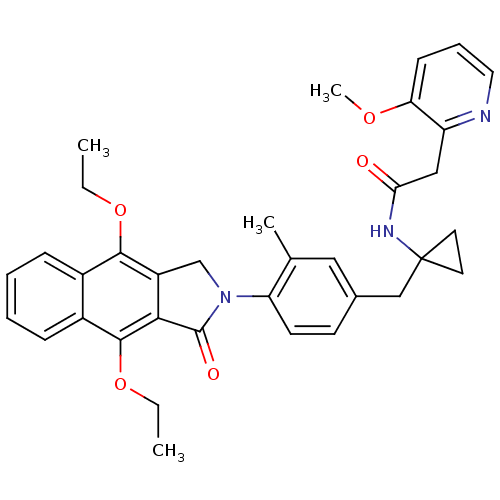 (CHEMBL1669017 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50037076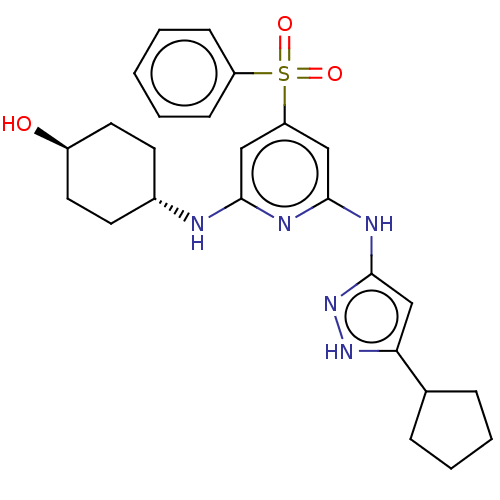 (CHEMBL3355737) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis | Bioorg Med Chem Lett 24: 5818-23 (2014) Article DOI: 10.1016/j.bmcl.2014.10.020 BindingDB Entry DOI: 10.7270/Q2NK3GNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312765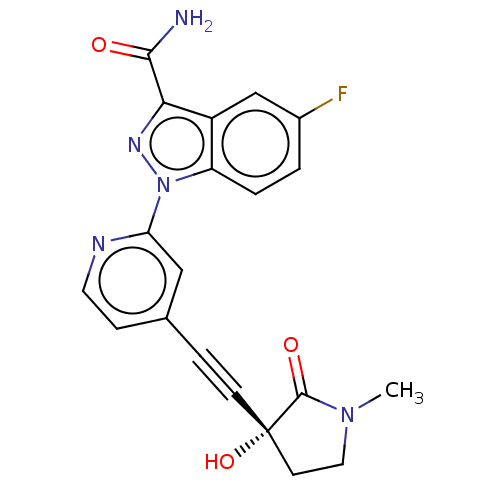 (5-fluoro-1-[4-[2-[(3R)-3-hy- droxy-1-methyl-2-oxo-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50203905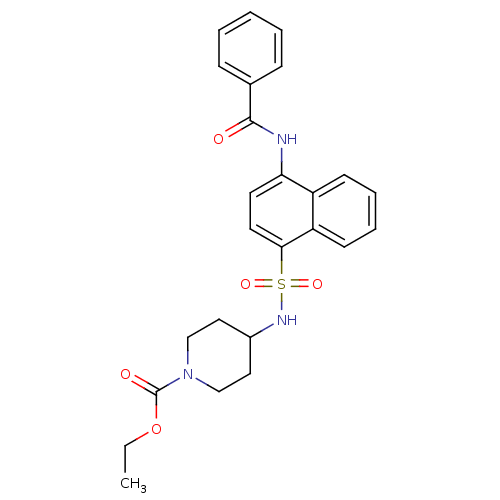 (4-(4-benzoylamino-naphthalene-1-sulfonylamino)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human CCR8 expressed in L1.2 cells by FMAT assay | J Med Chem 50: 566-84 (2007) Article DOI: 10.1021/jm061118e BindingDB Entry DOI: 10.7270/Q24J0DSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335989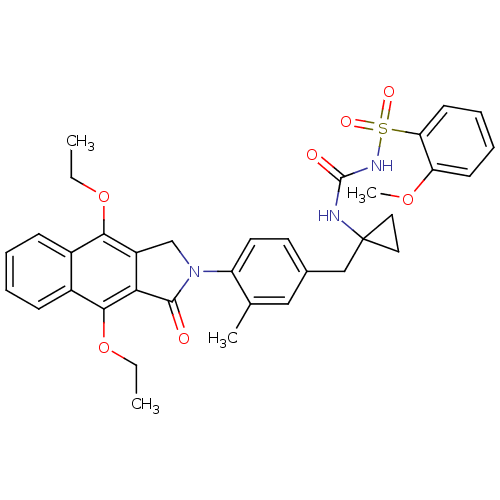 (CHEMBL1669009 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312788 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335981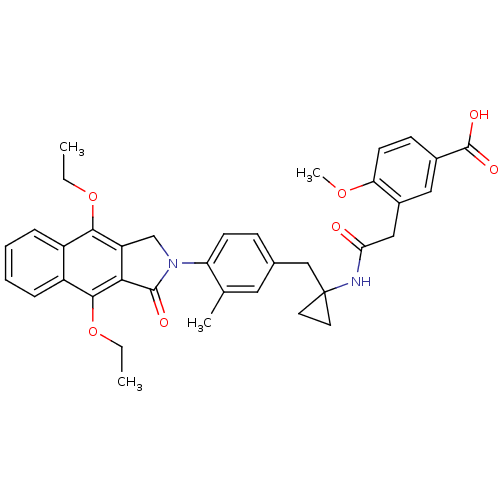 (3-(2-(1-(4-(4,9-diethoxy-1-oxo-1H-benzo[f]isoindol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12769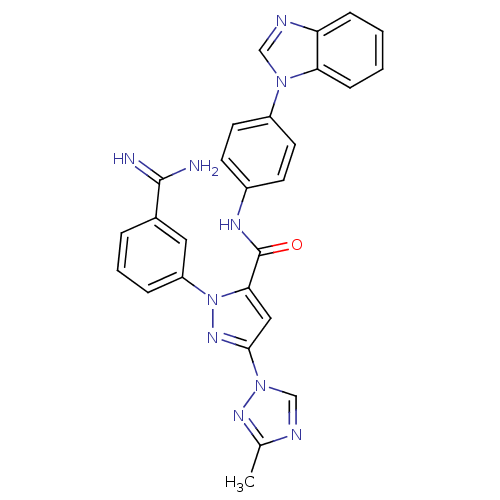 (N-[4-(1H-1,3-benzodiazol-1-yl)phenyl]-1-(3-carbami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12772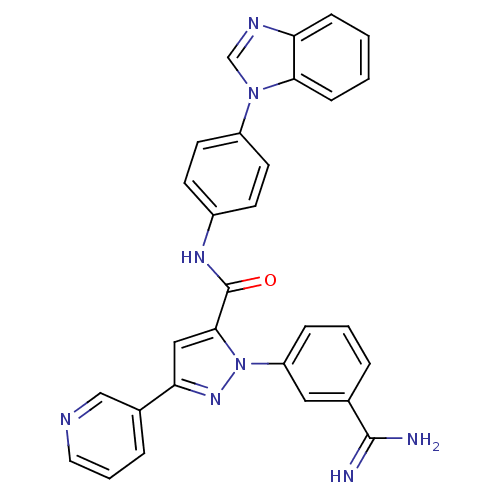 (N-[4-(1H-1,3-benzodiazol-1-yl)phenyl]-1-(3-carbami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... | Bioorg Med Chem Lett 14: 5263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.034 BindingDB Entry DOI: 10.7270/Q2TH8JX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50086604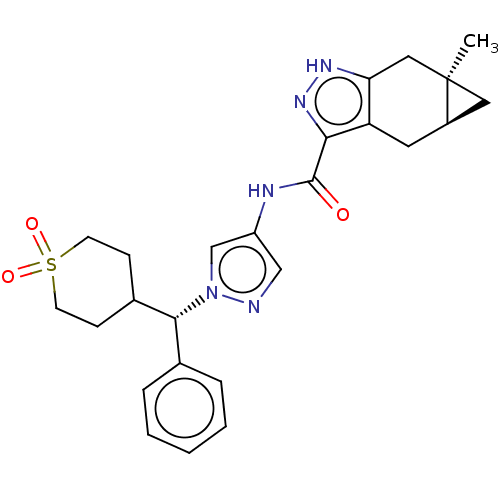 (CHEMBL3426308) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis | J Med Chem 58: 3806-16 (2015) Article DOI: 10.1021/jm501998m BindingDB Entry DOI: 10.7270/Q28K7BTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM50022940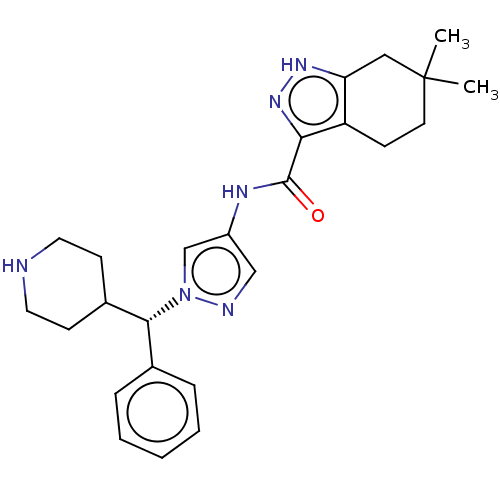 (CHEMBL3298373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of GST-tagged full length ITK (unknown origin) using BLK peptide as substrate after 35 mins | J Med Chem 57: 5714-27 (2014) Article DOI: 10.1021/jm500550e BindingDB Entry DOI: 10.7270/Q2ZK5J7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50335984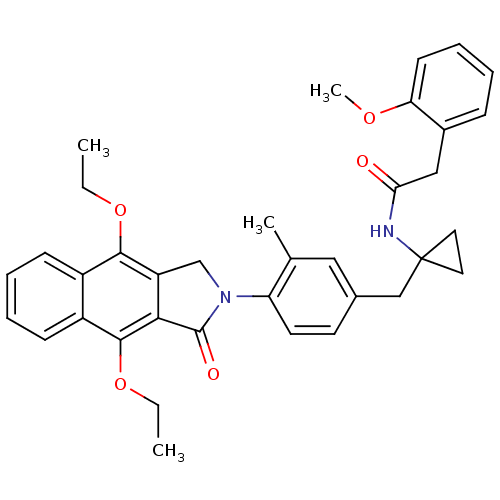 (CHEMBL1669018 | N-(1-(4-(4,9-diethoxy-1-oxo-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at human EP4 receptor expressed in HEK293 cells assessed as PGE2-induced cAMP accumulation by scintillation proximity assay | Bioorg Med Chem Lett 21: 1041-6 (2011) Article DOI: 10.1016/j.bmcl.2010.12.014 BindingDB Entry DOI: 10.7270/Q2VH5P4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50224406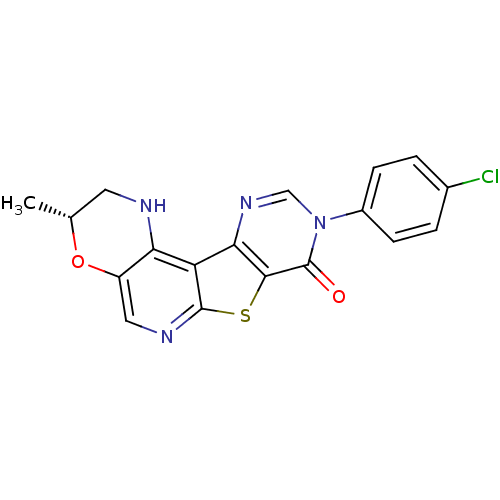 (9-(4-chlorophenyl)-2,3-dihydro-3(R)-methyl-1H-pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]9-Dimethylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4-one from mGluR1 in rat cerebellum membrane | J Med Chem 50: 5550-3 (2007) Article DOI: 10.1021/jm070590c BindingDB Entry DOI: 10.7270/Q2D50MQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306314 ((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | Bioorg Med Chem Lett 20: 836-40 (2010) Article DOI: 10.1016/j.bmcl.2009.12.100 BindingDB Entry DOI: 10.7270/Q23N23HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50306528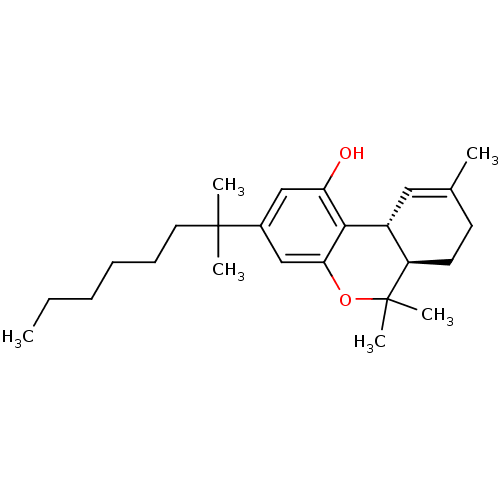 ((6aR,10aR)-6,6,9-trimethyl-3-(2-methyloctan-2-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from CB2 receptor | Bioorg Med Chem Lett 20: 1424-6 (2010) Article DOI: 10.1016/j.bmcl.2009.12.092 BindingDB Entry DOI: 10.7270/Q2PC32GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306440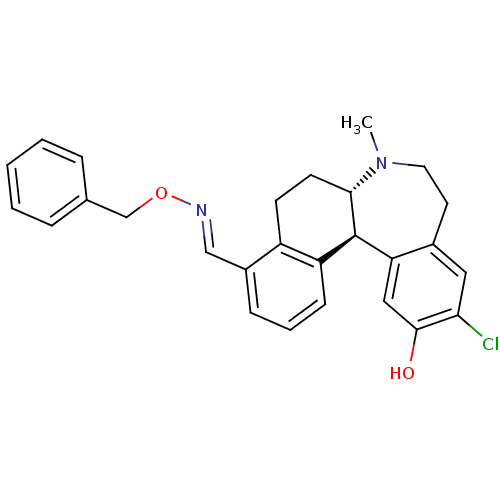 (11-chloro-12-hydroxy-7-methyl-6,6a,7,8,9,13b-hexah...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50306442 ((6aS,13bS)-11-chloro-7-methyl-4-phenyl-6,6a,7,8,9,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCh23390 from dopamine D1 receptor expressed in mouse LTK cells by scintillation counting | Bioorg Med Chem Lett 20: 832-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.094 BindingDB Entry DOI: 10.7270/Q2FX79JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 19293 total ) | Next | Last >> |