 Found 27 hits with Last Name = 'burke' and Initial = 'pj'
Found 27 hits with Last Name = 'burke' and Initial = 'pj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carboxypeptidase G2
(Pseudomonas aeruginosa) | BDBM50074672
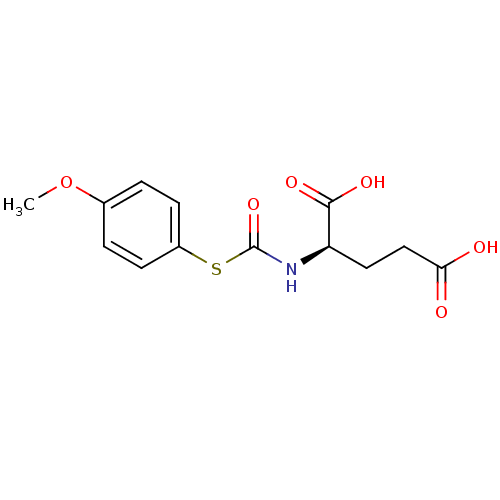
((R)-2-(4-Methoxy-phenylsulfanylcarbonylamino)-pent...)Show InChI InChI=1S/C13H15NO6S/c1-20-8-2-4-9(5-3-8)21-13(19)14-10(12(17)18)6-7-11(15)16/h2-5,10H,6-7H2,1H3,(H,14,19)(H,15,16)(H,17,18)/t10-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College of Science
Curated by ChEMBL
| Assay Description
Inhibitory activity against bacterial carboxypeptidase G2 by Dixon plot assay |
J Med Chem 42: 951-6 (1999)
Article DOI: 10.1021/jm990004i
BindingDB Entry DOI: 10.7270/Q2J965KN |
More data for this
Ligand-Target Pair | |
Carboxypeptidase G2
(Pseudomonas aeruginosa) | BDBM50074680
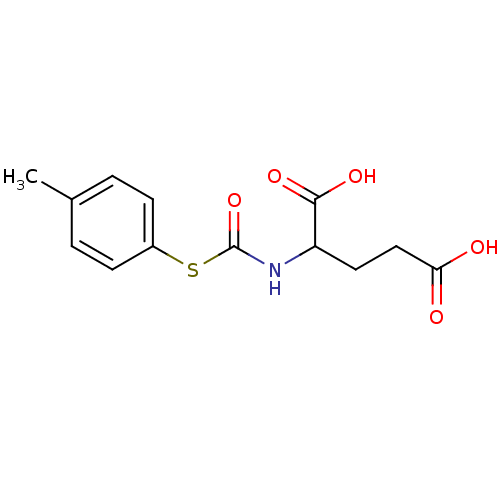
(2-p-Tolylsulfanylcarbonylamino-pentanedioic acid |...)Show InChI InChI=1S/C13H15NO5S/c1-8-2-4-9(5-3-8)20-13(19)14-10(12(17)18)6-7-11(15)16/h2-5,10H,6-7H2,1H3,(H,14,19)(H,15,16)(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College of Science
Curated by ChEMBL
| Assay Description
Inhibitory activity against bacterial carboxypeptidase G2 by Dixon plot assay |
J Med Chem 42: 951-6 (1999)
Article DOI: 10.1021/jm990004i
BindingDB Entry DOI: 10.7270/Q2J965KN |
More data for this
Ligand-Target Pair | |
Carboxypeptidase G2
(Pseudomonas aeruginosa) | BDBM50074674

(2-(3-Methoxy-phenylsulfanylcarbonylamino)-pentaned...)Show InChI InChI=1S/C13H15NO6S/c1-20-8-3-2-4-9(7-8)21-13(19)14-10(12(17)18)5-6-11(15)16/h2-4,7,10H,5-6H2,1H3,(H,14,19)(H,15,16)(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College of Science
Curated by ChEMBL
| Assay Description
Inhibitory activity against bacterial carboxypeptidase G2 by Dixon plot assay |
J Med Chem 42: 951-6 (1999)
Article DOI: 10.1021/jm990004i
BindingDB Entry DOI: 10.7270/Q2J965KN |
More data for this
Ligand-Target Pair | |
Carboxypeptidase G2
(Pseudomonas aeruginosa) | BDBM50074673
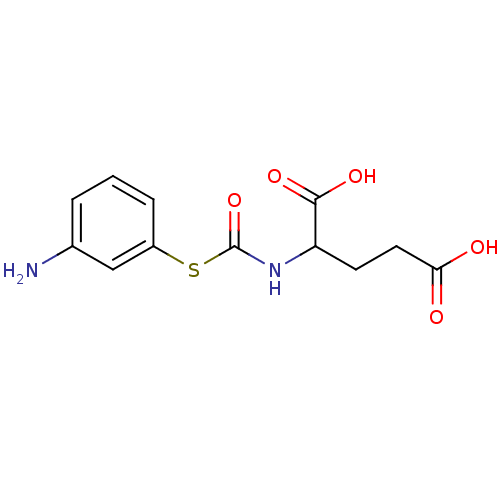
(2-(3-Amino-phenylsulfanylcarbonylamino)-pentanedio...)Show InChI InChI=1S/C12H14N2O5S/c13-7-2-1-3-8(6-7)20-12(19)14-9(11(17)18)4-5-10(15)16/h1-3,6,9H,4-5,13H2,(H,14,19)(H,15,16)(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College of Science
Curated by ChEMBL
| Assay Description
Inhibitory activity against bacterial carboxypeptidase G2 by Dixon plot assay |
J Med Chem 42: 951-6 (1999)
Article DOI: 10.1021/jm990004i
BindingDB Entry DOI: 10.7270/Q2J965KN |
More data for this
Ligand-Target Pair | |
Carboxypeptidase G2
(Pseudomonas aeruginosa) | BDBM50074679

(2-(4-Amino-phenylsulfanylcarbonylamino)-pentanedio...)Show InChI InChI=1S/C12H14N2O5S/c13-7-1-3-8(4-2-7)20-12(19)14-9(11(17)18)5-6-10(15)16/h1-4,9H,5-6,13H2,(H,14,19)(H,15,16)(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College of Science
Curated by ChEMBL
| Assay Description
Inhibitory activity against bacterial carboxypeptidase G2 by Dixon plot assay |
J Med Chem 42: 951-6 (1999)
Article DOI: 10.1021/jm990004i
BindingDB Entry DOI: 10.7270/Q2J965KN |
More data for this
Ligand-Target Pair | |
Carboxypeptidase G2
(Pseudomonas aeruginosa) | BDBM50074678

(2-(1-Oxy-pyridin-4-ylsulfanylcarbonylamino)-pentan...)Show InChI InChI=1S/C11H12N2O6S/c14-9(15)2-1-8(10(16)17)12-11(18)20-7-3-5-13(19)6-4-7/h3-6,8H,1-2H2,(H,12,18)(H,14,15)(H,16,17) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College of Science
Curated by ChEMBL
| Assay Description
Inhibitory activity against bacterial carboxypeptidase G2 by Dixon plot assay |
J Med Chem 42: 951-6 (1999)
Article DOI: 10.1021/jm990004i
BindingDB Entry DOI: 10.7270/Q2J965KN |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibition of [3H]E2 binding to estrogen receptor alpha in MCF-7 cell lysate |
J Med Chem 47: 1193-206 (2004)
Article DOI: 10.1021/jm030352r
BindingDB Entry DOI: 10.7270/Q2M61P0X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474781
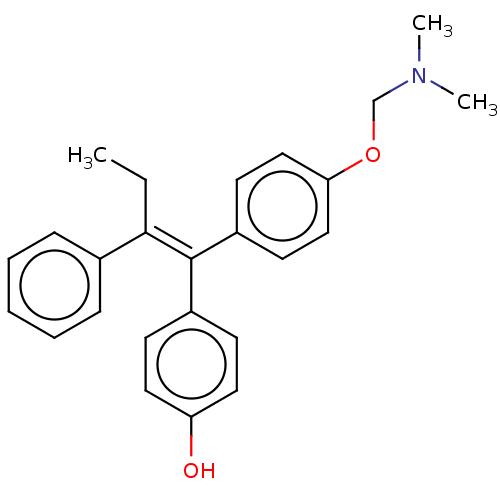
(CHEMBL19195)Show SMILES CC\C(=C(\c1ccc(O)cc1)c1ccc(OCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C25H27NO2/c1-4-24(19-8-6-5-7-9-19)25(20-10-14-22(27)15-11-20)21-12-16-23(17-13-21)28-18-26(2)3/h5-17,27H,4,18H2,1-3H3/b25-24+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibition of [3H]E2 binding to estrogen receptor alpha in MCF-7 cell lysate |
J Med Chem 47: 1193-206 (2004)
Article DOI: 10.1021/jm030352r
BindingDB Entry DOI: 10.7270/Q2M61P0X |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50411320
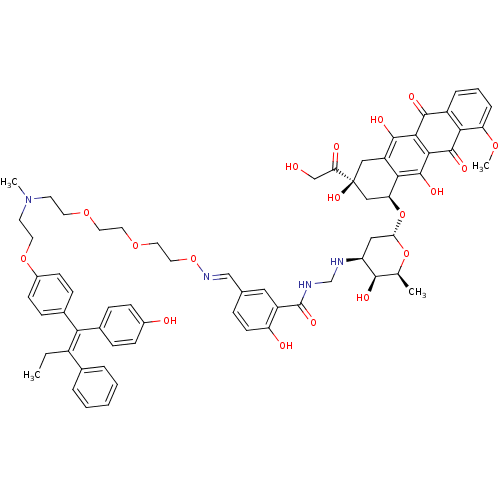
(CHEMBL222694)Show SMILES CC\C(=C(\c1ccc(O)cc1)c1ccc(OCCN(C)CCOCCOCCO\N=C\c2ccc(O)c(c2)C(=O)NCN[C@H]2C[C@H](O[C@H]3C[C@@](O)(Cc4c(O)c5C(=O)c6cccc(OC)c6C(=O)c5c(O)c34)C(=O)CO)O[C@@H](C)[C@H]2O)cc1)c1ccccc1 |r| Show InChI InChI=1S/C67H74N4O18/c1-5-46(41-10-7-6-8-11-41)56(42-15-19-44(73)20-16-42)43-17-21-45(22-18-43)86-27-25-71(3)24-26-84-28-29-85-30-31-87-70-36-40-14-23-51(74)48(32-40)66(81)69-38-68-50-33-55(88-39(2)61(50)76)89-53-35-67(82,54(75)37-72)34-49-58(53)65(80)60-59(63(49)78)62(77)47-12-9-13-52(83-4)57(47)64(60)79/h6-23,32,36,39,50,53,55,61,68,72-74,76,78,80,82H,5,24-31,33-35,37-38H2,1-4H3,(H,69,81)/b56-46+,70-36+/t39-,50-,53-,55-,61+,67-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Growth inhibition of MDA-MB-231 cells containing antiestrogen binding sites |
J Med Chem 47: 6509-18 (2004)
Article DOI: 10.1021/jm049496b
BindingDB Entry DOI: 10.7270/Q23R0V3T |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50411320

(CHEMBL222694)Show SMILES CC\C(=C(\c1ccc(O)cc1)c1ccc(OCCN(C)CCOCCOCCO\N=C\c2ccc(O)c(c2)C(=O)NCN[C@H]2C[C@H](O[C@H]3C[C@@](O)(Cc4c(O)c5C(=O)c6cccc(OC)c6C(=O)c5c(O)c34)C(=O)CO)O[C@@H](C)[C@H]2O)cc1)c1ccccc1 |r| Show InChI InChI=1S/C67H74N4O18/c1-5-46(41-10-7-6-8-11-41)56(42-15-19-44(73)20-16-42)43-17-21-45(22-18-43)86-27-25-71(3)24-26-84-28-29-85-30-31-87-70-36-40-14-23-51(74)48(32-40)66(81)69-38-68-50-33-55(88-39(2)61(50)76)89-53-35-67(82,54(75)37-72)34-49-58(53)65(80)60-59(63(49)78)62(77)47-12-9-13-52(83-4)57(47)64(60)79/h6-23,32,36,39,50,53,55,61,68,72-74,76,78,80,82H,5,24-31,33-35,37-38H2,1-4H3,(H,69,81)/b56-46+,70-36+/t39-,50-,53-,55-,61+,67-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Growth inhibition of MCF7 cells expressing ER and antiestrogen binding sites |
J Med Chem 47: 6509-18 (2004)
Article DOI: 10.1021/jm049496b
BindingDB Entry DOI: 10.7270/Q23R0V3T |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50411320

(CHEMBL222694)Show SMILES CC\C(=C(\c1ccc(O)cc1)c1ccc(OCCN(C)CCOCCOCCO\N=C\c2ccc(O)c(c2)C(=O)NCN[C@H]2C[C@H](O[C@H]3C[C@@](O)(Cc4c(O)c5C(=O)c6cccc(OC)c6C(=O)c5c(O)c34)C(=O)CO)O[C@@H](C)[C@H]2O)cc1)c1ccccc1 |r| Show InChI InChI=1S/C67H74N4O18/c1-5-46(41-10-7-6-8-11-41)56(42-15-19-44(73)20-16-42)43-17-21-45(22-18-43)86-27-25-71(3)24-26-84-28-29-85-30-31-87-70-36-40-14-23-51(74)48(32-40)66(81)69-38-68-50-33-55(88-39(2)61(50)76)89-53-35-67(82,54(75)37-72)34-49-58(53)65(80)60-59(63(49)78)62(77)47-12-9-13-52(83-4)57(47)64(60)79/h6-23,32,36,39,50,53,55,61,68,72-74,76,78,80,82H,5,24-31,33-35,37-38H2,1-4H3,(H,69,81)/b56-46+,70-36+/t39-,50-,53-,55-,61+,67-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Growth inhibition of MDA-MB-435 cells containing antiestrogen binding sites |
J Med Chem 47: 6509-18 (2004)
Article DOI: 10.1021/jm049496b
BindingDB Entry DOI: 10.7270/Q23R0V3T |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50411319
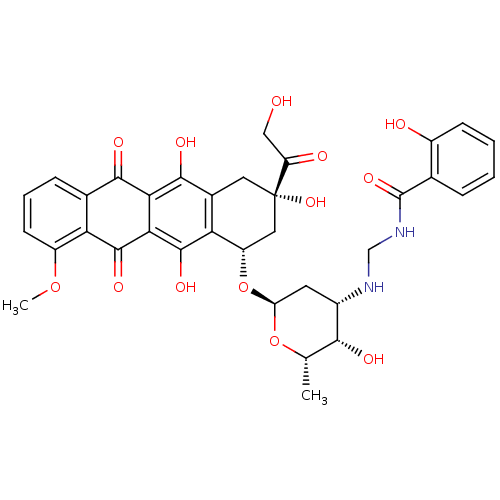
(CHEMBL266892)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](NCNC(=O)c6ccccc6O)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C35H36N2O13/c1-15-29(41)19(36-14-37-34(46)16-6-3-4-8-20(16)39)10-24(49-15)50-22-12-35(47,23(40)13-38)11-18-26(22)33(45)28-27(31(18)43)30(42)17-7-5-9-21(48-2)25(17)32(28)44/h3-9,15,19,22,24,29,36,38-39,41,43,45,47H,10-14H2,1-2H3,(H,37,46)/t15-,19-,22-,24-,29+,35-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Growth inhibition of MDA-MB-435 cells containing antiestrogen binding sites |
J Med Chem 47: 6509-18 (2004)
Article DOI: 10.1021/jm049496b
BindingDB Entry DOI: 10.7270/Q23R0V3T |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50411320

(CHEMBL222694)Show SMILES CC\C(=C(\c1ccc(O)cc1)c1ccc(OCCN(C)CCOCCOCCO\N=C\c2ccc(O)c(c2)C(=O)NCN[C@H]2C[C@H](O[C@H]3C[C@@](O)(Cc4c(O)c5C(=O)c6cccc(OC)c6C(=O)c5c(O)c34)C(=O)CO)O[C@@H](C)[C@H]2O)cc1)c1ccccc1 |r| Show InChI InChI=1S/C67H74N4O18/c1-5-46(41-10-7-6-8-11-41)56(42-15-19-44(73)20-16-42)43-17-21-45(22-18-43)86-27-25-71(3)24-26-84-28-29-85-30-31-87-70-36-40-14-23-51(74)48(32-40)66(81)69-38-68-50-33-55(88-39(2)61(50)76)89-53-35-67(82,54(75)37-72)34-49-58(53)65(80)60-59(63(49)78)62(77)47-12-9-13-52(83-4)57(47)64(60)79/h6-23,32,36,39,50,53,55,61,68,72-74,76,78,80,82H,5,24-31,33-35,37-38H2,1-4H3,(H,69,81)/b56-46+,70-36+/t39-,50-,53-,55-,61+,67-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Growth inhibition of multidrug resistant MCF7/Adr cells containing antiestrogen binding sites |
J Med Chem 47: 6509-18 (2004)
Article DOI: 10.1021/jm049496b
BindingDB Entry DOI: 10.7270/Q23R0V3T |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50411319

(CHEMBL266892)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](NCNC(=O)c6ccccc6O)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C35H36N2O13/c1-15-29(41)19(36-14-37-34(46)16-6-3-4-8-20(16)39)10-24(49-15)50-22-12-35(47,23(40)13-38)11-18-26(22)33(45)28-27(31(18)43)30(42)17-7-5-9-21(48-2)25(17)32(28)44/h3-9,15,19,22,24,29,36,38-39,41,43,45,47H,10-14H2,1-2H3,(H,37,46)/t15-,19-,22-,24-,29+,35-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Growth inhibition of MCF7 cells expressing ER and antiestrogen binding sites |
J Med Chem 47: 6509-18 (2004)
Article DOI: 10.1021/jm049496b
BindingDB Entry DOI: 10.7270/Q23R0V3T |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50411319

(CHEMBL266892)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](NCNC(=O)c6ccccc6O)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C35H36N2O13/c1-15-29(41)19(36-14-37-34(46)16-6-3-4-8-20(16)39)10-24(49-15)50-22-12-35(47,23(40)13-38)11-18-26(22)33(45)28-27(31(18)43)30(42)17-7-5-9-21(48-2)25(17)32(28)44/h3-9,15,19,22,24,29,36,38-39,41,43,45,47H,10-14H2,1-2H3,(H,37,46)/t15-,19-,22-,24-,29+,35-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Growth inhibition of MDA-MB-231 cells containing antiestrogen binding sites |
J Med Chem 47: 6509-18 (2004)
Article DOI: 10.1021/jm049496b
BindingDB Entry DOI: 10.7270/Q23R0V3T |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474779
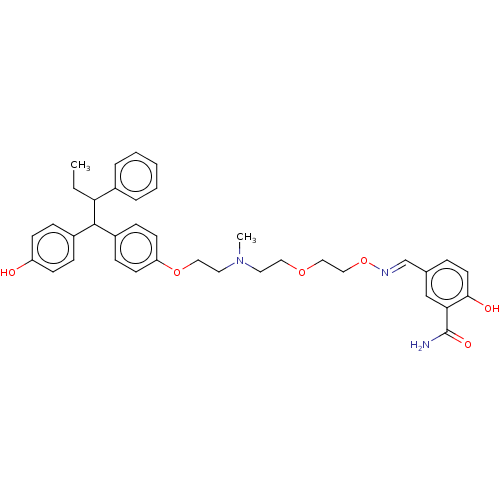
(CHEMBL19481)Show SMILES CCC(C(c1ccc(O)cc1)c1ccc(OCCN(C)CCOCCO\N=C\c2ccc(O)c(c2)C(N)=O)cc1)c1ccccc1 Show InChI InChI=1S/C37H43N3O6/c1-3-33(28-7-5-4-6-8-28)36(29-10-14-31(41)15-11-29)30-12-16-32(17-13-30)45-22-20-40(2)19-21-44-23-24-46-39-26-27-9-18-35(42)34(25-27)37(38)43/h4-18,25-26,33,36,41-42H,3,19-24H2,1-2H3,(H2,38,43)/b39-26+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibition of [3H]E2 binding to estrogen receptor alpha in MCF-7 cell lysate |
J Med Chem 47: 1193-206 (2004)
Article DOI: 10.1021/jm030352r
BindingDB Entry DOI: 10.7270/Q2M61P0X |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474783

(CHEMBL278308)Show SMILES CCC(C(c1ccc(O)cc1)c1ccc(OCCN(C)CCOCCOCCO\N=C\c2ccc(O)c(c2)C(N)=O)cc1)c1ccccc1 Show InChI InChI=1S/C39H47N3O7/c1-3-35(30-7-5-4-6-8-30)38(31-10-14-33(43)15-11-31)32-12-16-34(17-13-32)48-22-20-42(2)19-21-46-23-24-47-25-26-49-41-28-29-9-18-37(44)36(27-29)39(40)45/h4-18,27-28,35,38,43-44H,3,19-26H2,1-2H3,(H2,40,45)/b41-28+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibition of [3H]E2 binding to estrogen receptor alpha in MCF-7 cell lysate |
J Med Chem 47: 1193-206 (2004)
Article DOI: 10.1021/jm030352r
BindingDB Entry DOI: 10.7270/Q2M61P0X |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM22984

((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Growth inhibition of MDA-MB-435 cells containing antiestrogen binding sites |
J Med Chem 47: 6509-18 (2004)
Article DOI: 10.1021/jm049496b
BindingDB Entry DOI: 10.7270/Q23R0V3T |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM22984

((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Growth inhibition of MCF7 cells expressing ER and antiestrogen binding sites |
J Med Chem 47: 6509-18 (2004)
Article DOI: 10.1021/jm049496b
BindingDB Entry DOI: 10.7270/Q23R0V3T |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474784
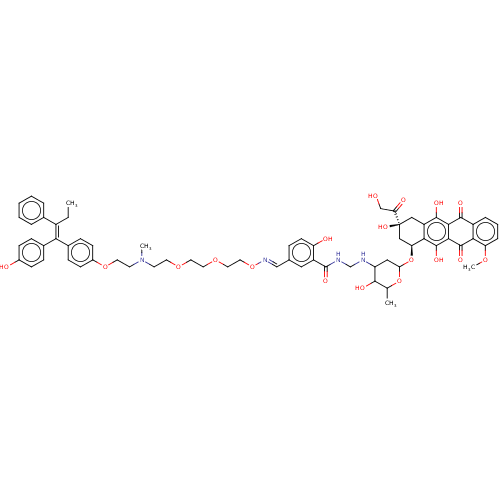
(CHEMBL410193)Show SMILES CC\C(=C(\c1ccc(O)cc1)c1ccc(OCCN(C)CCOCCOCCO\N=C\c2ccc(O)c(c2)C(=O)NCNC2CC(O[C@H]3C[C@@](O)(Cc4c(O)c5C(=O)c6cccc(OC)c6C(=O)c5c(O)c34)C(=O)CO)OC(C)C2O)cc1)c1ccccc1 Show InChI InChI=1S/C67H74N4O18/c1-5-46(41-10-7-6-8-11-41)56(42-15-19-44(73)20-16-42)43-17-21-45(22-18-43)86-27-25-71(3)24-26-84-28-29-85-30-31-87-70-36-40-14-23-51(74)48(32-40)66(81)69-38-68-50-33-55(88-39(2)61(50)76)89-53-35-67(82,54(75)37-72)34-49-58(53)65(80)60-59(63(49)78)62(77)47-12-9-13-52(83-4)57(47)64(60)79/h6-23,32,36,39,50,53,55,61,68,72-74,76,78,80,82H,5,24-31,33-35,37-38H2,1-4H3,(H,69,81)/b56-46+,70-36+/t39?,50?,53-,55?,61?,67-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibition of [3H]E2 binding to estrogen receptor alpha in MCF-7 cell lysate |
J Med Chem 47: 1193-206 (2004)
Article DOI: 10.1021/jm030352r
BindingDB Entry DOI: 10.7270/Q2M61P0X |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM22984

((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Growth inhibition of MDA-MB-231 cells containing antiestrogen binding sites |
J Med Chem 47: 6509-18 (2004)
Article DOI: 10.1021/jm049496b
BindingDB Entry DOI: 10.7270/Q23R0V3T |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474780

(CHEMBL266703)Show SMILES CC\C(=C(\c1ccc(O)cc1)c1ccc(OCCN(C)CCOCCO\N=C\c2ccc(O)c(c2)C(=O)NCNC2CC(O[C@H]3C[C@@](O)(Cc4c(O)c5C(=O)c6cccc(OC)c6C(=O)c5c(O)c34)C(=O)CO)OC(C)C2O)cc1)c1ccccc1 Show InChI InChI=1S/C65H70N4O17/c1-5-44(39-10-7-6-8-11-39)54(40-15-19-42(71)20-16-40)41-17-21-43(22-18-41)83-27-25-69(3)24-26-82-28-29-84-68-34-38-14-23-49(72)46(30-38)64(79)67-36-66-48-31-53(85-37(2)59(48)74)86-51-33-65(80,52(73)35-70)32-47-56(51)63(78)58-57(61(47)76)60(75)45-12-9-13-50(81-4)55(45)62(58)77/h6-23,30,34,37,48,51,53,59,66,70-72,74,76,78,80H,5,24-29,31-33,35-36H2,1-4H3,(H,67,79)/b54-44+,68-34+/t37?,48?,51-,53?,59?,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibition of [3H]E2 binding to estrogen receptor alpha in MCF-7 cell lysate |
J Med Chem 47: 1193-206 (2004)
Article DOI: 10.1021/jm030352r
BindingDB Entry DOI: 10.7270/Q2M61P0X |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474785
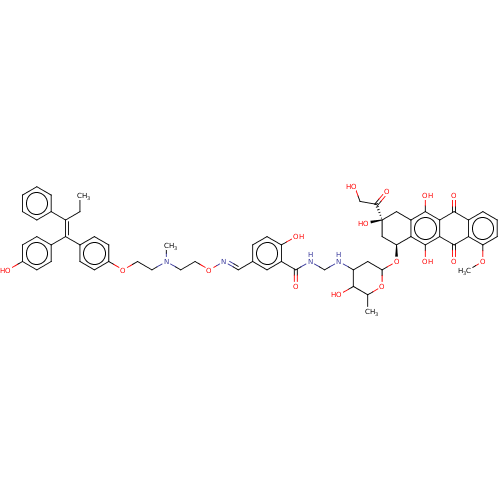
(CHEMBL266185)Show SMILES CC\C(=C(\c1ccc(O)cc1)c1ccc(OCCN(C)CCO\N=C\c2ccc(O)c(c2)C(=O)NCNC2CC(O[C@H]3C[C@@](O)(Cc4c(O)c5C(=O)c6cccc(OC)c6C(=O)c5c(O)c34)C(=O)CO)OC(C)C2O)cc1)c1ccccc1 Show InChI InChI=1S/C63H66N4O16/c1-5-42(37-10-7-6-8-11-37)52(38-15-19-40(69)20-16-38)39-17-21-41(22-18-39)80-26-24-67(3)25-27-81-66-32-36-14-23-47(70)44(28-36)62(77)65-34-64-46-29-51(82-35(2)57(46)72)83-49-31-63(78,50(71)33-68)30-45-54(49)61(76)56-55(59(45)74)58(73)43-12-9-13-48(79-4)53(43)60(56)75/h6-23,28,32,35,46,49,51,57,64,68-70,72,74,76,78H,5,24-27,29-31,33-34H2,1-4H3,(H,65,77)/b52-42+,66-32+/t35?,46?,49-,51?,57?,63-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibition of [3H]E2 binding to estrogen receptor alpha in MCF-7 cell lysate |
J Med Chem 47: 1193-206 (2004)
Article DOI: 10.1021/jm030352r
BindingDB Entry DOI: 10.7270/Q2M61P0X |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474782

(CHEMBL19535)Show SMILES CCC(C(c1ccc(O)cc1)c1ccc(OCCN(C)CCO\N=C\c2ccc(O)c(c2)C(N)=O)cc1)c1ccccc1 Show InChI InChI=1S/C35H39N3O5/c1-3-31(26-7-5-4-6-8-26)34(27-10-14-29(39)15-11-27)28-12-16-30(17-13-28)42-21-19-38(2)20-22-43-37-24-25-9-18-33(40)32(23-25)35(36)41/h4-18,23-24,31,34,39-40H,3,19-22H2,1-2H3,(H2,36,41)/b37-24+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibition of [3H]E2 binding to estrogen receptor alpha in MCF-7 cell lysate |
J Med Chem 47: 1193-206 (2004)
Article DOI: 10.1021/jm030352r
BindingDB Entry DOI: 10.7270/Q2M61P0X |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50411319

(CHEMBL266892)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](NCNC(=O)c6ccccc6O)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C35H36N2O13/c1-15-29(41)19(36-14-37-34(46)16-6-3-4-8-20(16)39)10-24(49-15)50-22-12-35(47,23(40)13-38)11-18-26(22)33(45)28-27(31(18)43)30(42)17-7-5-9-21(48-2)25(17)32(28)44/h3-9,15,19,22,24,29,36,38-39,41,43,45,47H,10-14H2,1-2H3,(H,37,46)/t15-,19-,22-,24-,29+,35-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Growth inhibition of multidrug resistant MCF7/Adr cells containing antiestrogen binding sites |
J Med Chem 47: 6509-18 (2004)
Article DOI: 10.1021/jm049496b
BindingDB Entry DOI: 10.7270/Q23R0V3T |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM20607
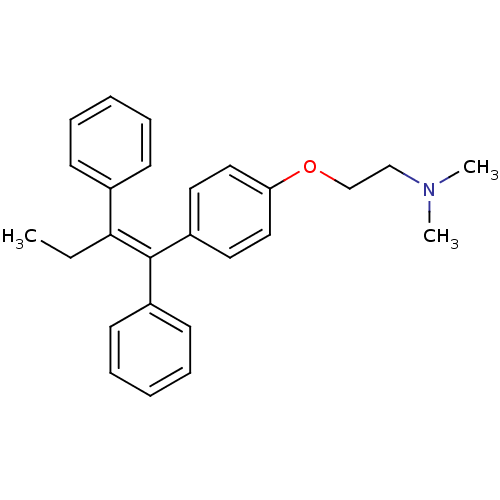
((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO/c1-4-25(21-11-7-5-8-12-21)26(22-13-9-6-10-14-22)23-15-17-24(18-16-23)28-20-19-27(2)3/h5-18H,4,19-20H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibition of [3H]E2 binding to estrogen receptor alpha in MCF-7 cell lysate |
J Med Chem 47: 1193-206 (2004)
Article DOI: 10.1021/jm030352r
BindingDB Entry DOI: 10.7270/Q2M61P0X |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM22984

((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Growth inhibition of multidrug resistant MCF7/Adr cells containing antiestrogen binding sites |
J Med Chem 47: 6509-18 (2004)
Article DOI: 10.1021/jm049496b
BindingDB Entry DOI: 10.7270/Q23R0V3T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data