

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50412728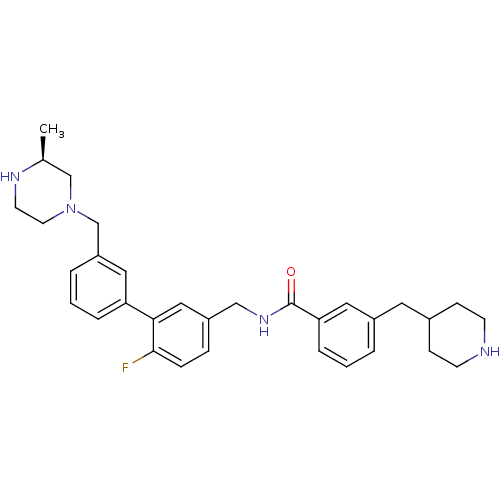 (CHEMBL521523) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human cloned muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 51: 5915-8 (2008) Article DOI: 10.1021/jm800935u BindingDB Entry DOI: 10.7270/Q21G0NHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50345693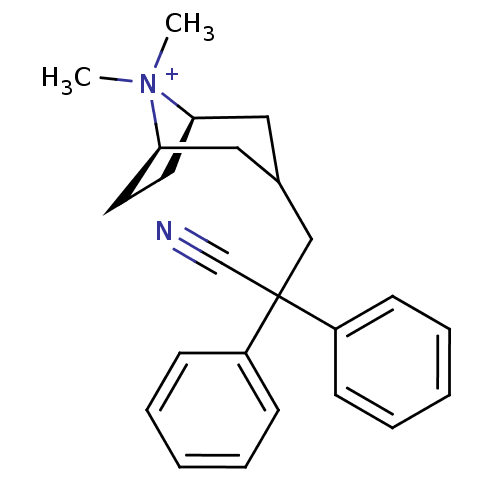 ((3-endo)-3-(2-Cyano-2,2-diphenylethyl)-8,8-dimethy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic M1 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50345693 ((3-endo)-3-(2-Cyano-2,2-diphenylethyl)-8,8-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50267614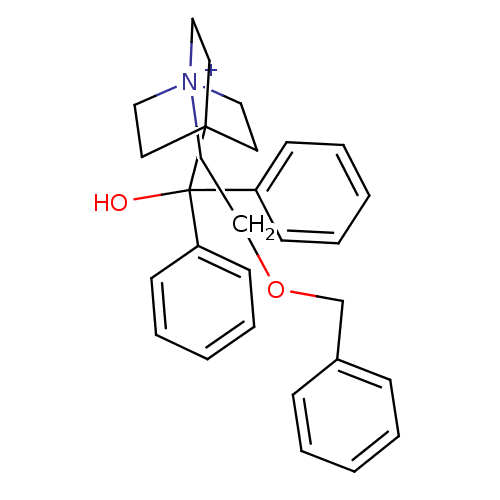 (4-[Hydroxy(diphenyl)methyl]-1-{2-[(phenylmethyl)ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl scopalamine from human cloned muscarinic M3 receptor expressed in CHO cells coexpressing Gqi5 by scintillation proximity... | J Med Chem 52: 2493-505 (2009) Article DOI: 10.1021/jm801601v BindingDB Entry DOI: 10.7270/Q2H131X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50381654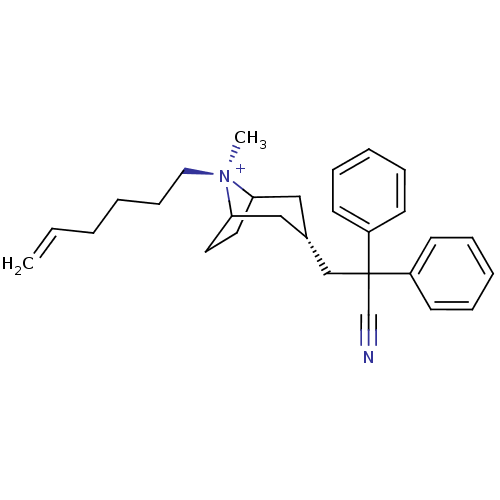 (CHEMBL2023764) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M2 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50381654 (CHEMBL2023764) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50267573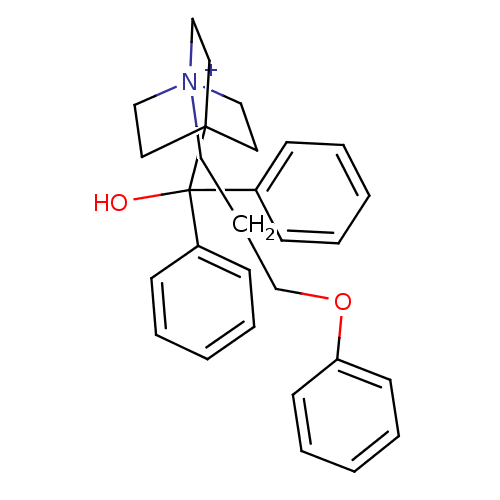 (4-[Hydroxy(diphenyl)methyl]-1-[3-(phenyloxy)propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl scopalamine from human cloned muscarinic M3 receptor expressed in CHO cells coexpressing Gqi5 by scintillation proximity... | J Med Chem 52: 2493-505 (2009) Article DOI: 10.1021/jm801601v BindingDB Entry DOI: 10.7270/Q2H131X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50267614 (4-[Hydroxy(diphenyl)methyl]-1-{2-[(phenylmethyl)ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl scopalamine from human cloned muscarinic M2 receptor expressed in CHO cells coexpressing Gqi5 by scintillation proximity... | J Med Chem 52: 2493-505 (2009) Article DOI: 10.1021/jm801601v BindingDB Entry DOI: 10.7270/Q2H131X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50381654 (CHEMBL2023764) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M3 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50267614 (4-[Hydroxy(diphenyl)methyl]-1-{2-[(phenylmethyl)ox...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl scopalamine from human cloned muscarinic M1 receptor expressed in CHO cells coexpressing Gqi5 by scintillation proximity... | J Med Chem 52: 2493-505 (2009) Article DOI: 10.1021/jm801601v BindingDB Entry DOI: 10.7270/Q2H131X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50345693 ((3-endo)-3-(2-Cyano-2,2-diphenylethyl)-8,8-dimethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic M2 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50412340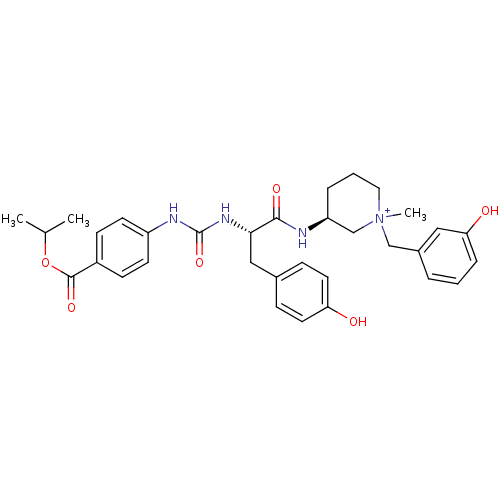 (CHEMBL540359) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M1 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50345692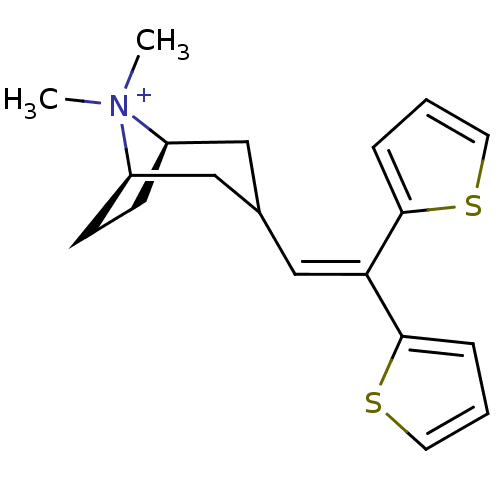 ((3-endo)-3-(2,2-Di-2-thienylethenyl)-8,8-dimethyl-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic M1 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50345692 ((3-endo)-3-(2,2-Di-2-thienylethenyl)-8,8-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50267573 (4-[Hydroxy(diphenyl)methyl]-1-[3-(phenyloxy)propyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl scopalamine from human cloned muscarinic M1 receptor expressed in CHO cells coexpressing Gqi5 by scintillation proximity... | J Med Chem 52: 2493-505 (2009) Article DOI: 10.1021/jm801601v BindingDB Entry DOI: 10.7270/Q2H131X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50345692 ((3-endo)-3-(2,2-Di-2-thienylethenyl)-8,8-dimethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic M2 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50267573 (4-[Hydroxy(diphenyl)methyl]-1-[3-(phenyloxy)propyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl scopalamine from human cloned muscarinic M2 receptor expressed in CHO cells coexpressing Gqi5 by scintillation proximity... | J Med Chem 52: 2493-505 (2009) Article DOI: 10.1021/jm801601v BindingDB Entry DOI: 10.7270/Q2H131X8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50412340 (CHEMBL540359) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M2 receptor expressed in CHO cells coexpressed with Gqi5 by scintillatio... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50412340 (CHEMBL540359) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M3 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193985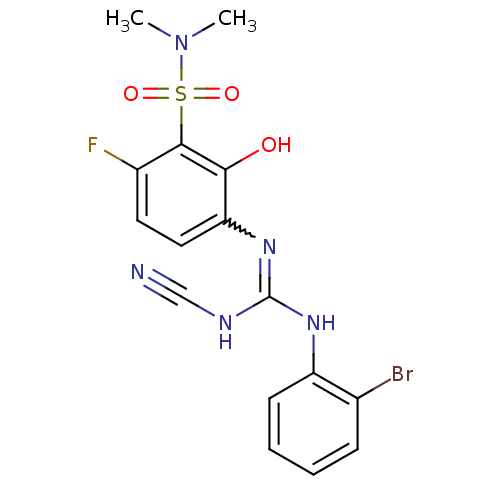 (3-{[[(2-bromophenyl)amino](cyanoimino)methyl]amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50472190 (CHEMBL167638) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4C (Rattus norvegicus) | BDBM50042058 ((-)-rolipram | (4R)-4-[3-(cyclopentyloxy)-4-methox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Competition of [3H]rolipram binding sites in the central nervous system (HPDE4) in rat brain cytosol | J Med Chem 41: 821-35 (1998) Article DOI: 10.1021/jm970090r BindingDB Entry DOI: 10.7270/Q2WD439M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193979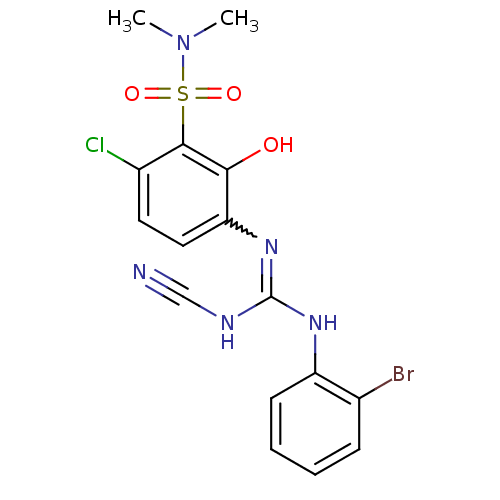 (3-{[[(2-bromophenyl)amino](cyanoimino)methyl]amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193972 (3-(2-bromophenyl)-2-cyano-1-(2-hydroxy-4-methyl-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193971 (3-{[[(2-bromophenyl)amino](cyanoimino)methyl]amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50152761 (3-[3-(2-Bromo-phenyl)-ureido]-6-chloro-2-hydroxy-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit [125I]IL-8 binding towards C-X-C chemokine receptor type 2 of human expressed in CHO cells | Bioorg Med Chem Lett 14: 4375-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.097 BindingDB Entry DOI: 10.7270/Q2SQ914M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193980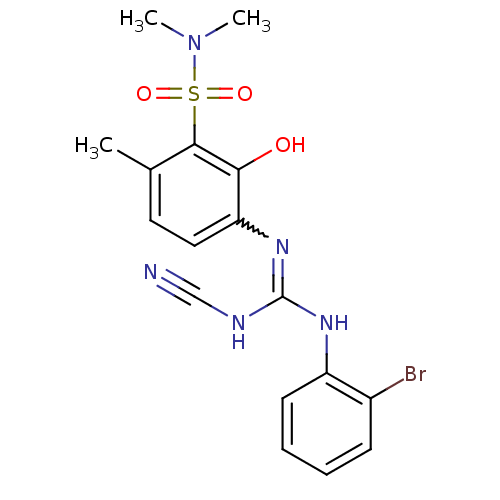 (3-{[[(2-bromophenyl)amino](cyanoimino)methyl]amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50203030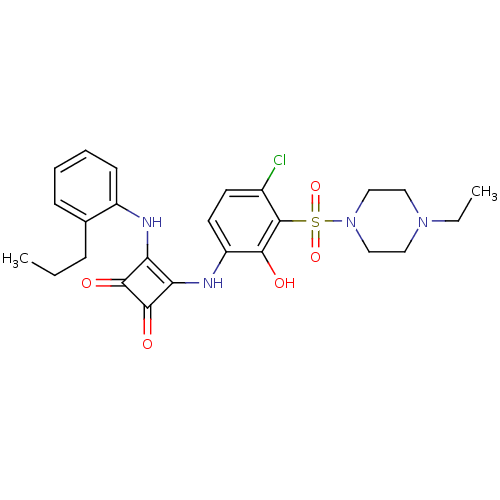 (3-[4-chloro-3-(4-ethyl-piperazine-1-sulfonyl)-2-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human recombinant CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 17: 1713-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.067 BindingDB Entry DOI: 10.7270/Q2Z320GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50203016 (6-chloro-3-(3,4-dioxo-2-(phenylamino)cyclobut-1-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human recombinant CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 17: 1713-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.067 BindingDB Entry DOI: 10.7270/Q2Z320GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193970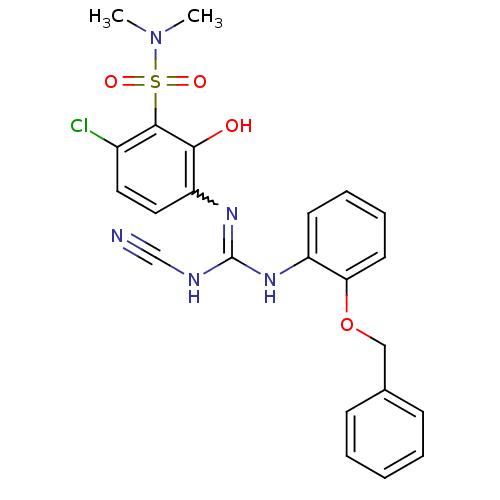 (3-[2-(benzyloxy)phenyl]-1-[4-chloro-3-(dimethylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193974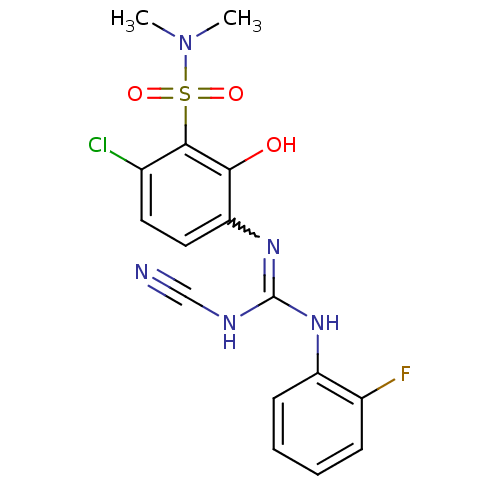 (6-chloro-3-({(cyanoimino)[(2-fluorophenyl)amino]me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193978 (3-(2-bromophenyl)-1-[4-chloro-3-({3-[(2R,6S)-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50152759 (3-[3-(2-Bromo-phenyl)-ureido]-6-chloro-2-hydroxy-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit [125I]IL-8 binding towards C-X-C chemokine receptor type 2 of human expressed in CHO cells | Bioorg Med Chem Lett 14: 4375-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.097 BindingDB Entry DOI: 10.7270/Q2SQ914M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50203007 (3-(2-bromophenylamino)-4-(4-chloro-2-hydroxy-3-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human recombinant CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 17: 1713-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.067 BindingDB Entry DOI: 10.7270/Q2Z320GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50345679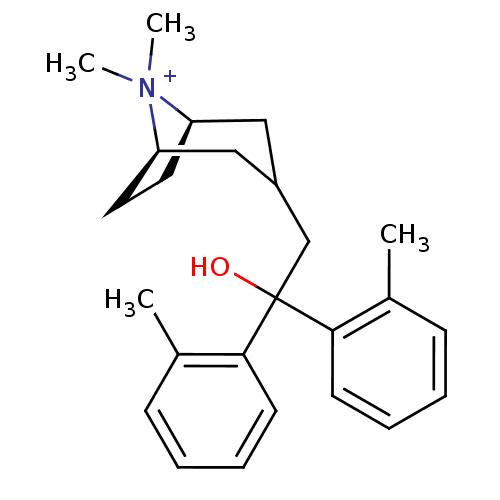 ((3-endo)-3-[2-Hydroxy-2,2-bis(2-methylphenyl)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human M3 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by FLIPR | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50345681 ((3-endo)-1,1-Bis-(3-fluorophenyl)-2-(8,8-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against human M3 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobilization by FLIPR | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193982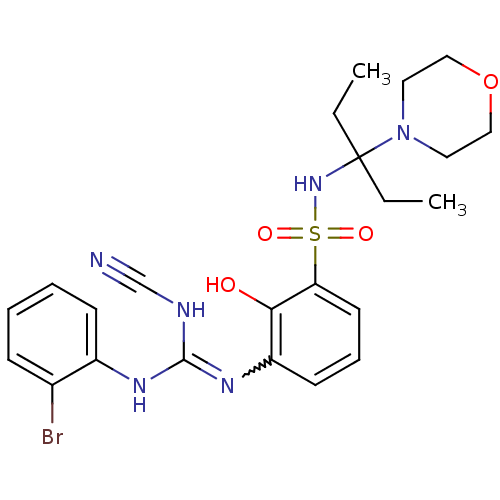 (3-(2-bromophenyl)-2-cyano-1-(2-hydroxy-3-{[3-(morp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193981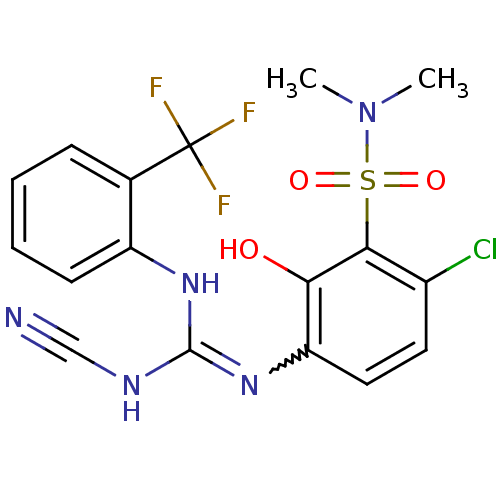 (6-chloro-3-[((cyanoimino){[2-(trifluoromethyl)phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50152760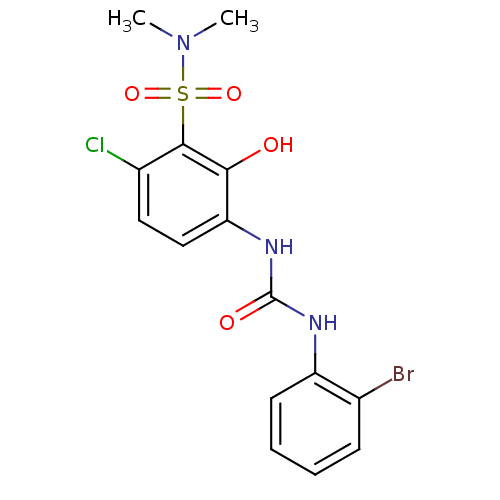 (3-[3-(2-Bromo-phenyl)-ureido]-6-chloro-2-hydroxy-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Concentration required to inhibit [125I]IL-8 binding towards C-X-C chemokine receptor type 2 of human expressed in CHO cells | Bioorg Med Chem Lett 14: 4375-8 (2004) Article DOI: 10.1016/j.bmcl.2004.06.097 BindingDB Entry DOI: 10.7270/Q2SQ914M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193977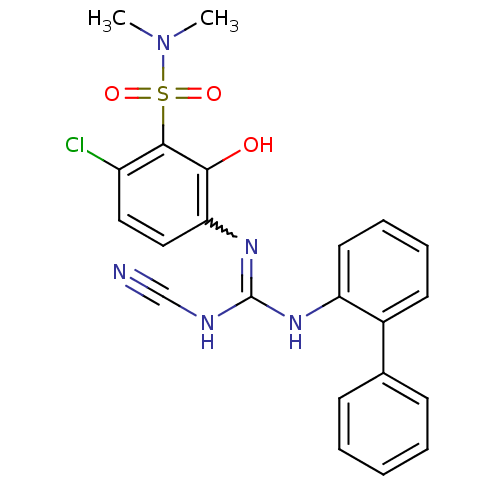 (1-[4-chloro-3-(dimethylsulfamoyl)-2-hydroxyphenyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50203035 (3-(2-bromo-phenylamino)-4-[4-fluoro-2-hydroxy-3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human recombinant CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 17: 1713-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.067 BindingDB Entry DOI: 10.7270/Q2Z320GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50203036 (6-chloro-3-(3,4-dioxo-2-(phenylamino)cyclobut-1-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human recombinant CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 17: 1713-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.067 BindingDB Entry DOI: 10.7270/Q2Z320GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50203028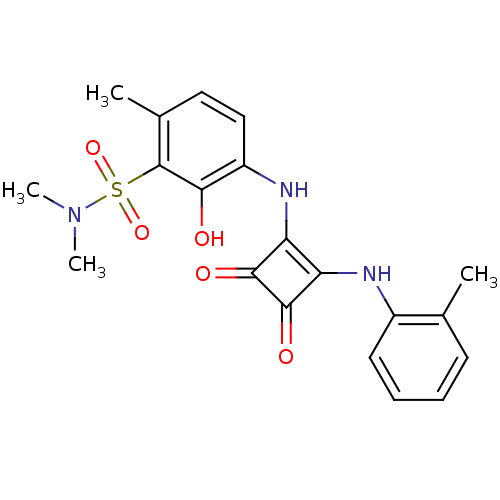 (3-(2-(o-toluidino)-3,4-dioxocyclobut-1-enylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human recombinant CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 17: 1713-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.067 BindingDB Entry DOI: 10.7270/Q2Z320GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50203003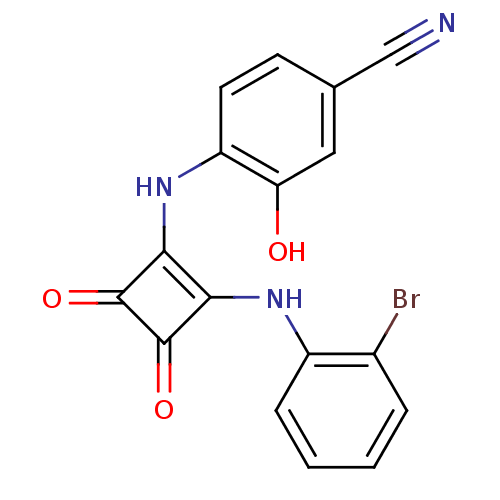 (4-(2-(2-bromophenylamino)-3,4-dioxocyclobut-1-enyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human recombinant CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 17: 1713-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.067 BindingDB Entry DOI: 10.7270/Q2Z320GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50187002 (4-(3,4-dioxo-2-(phenylamino)cyclobut-1-enylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human recombinant CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 17: 1713-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.067 BindingDB Entry DOI: 10.7270/Q2Z320GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50203003 (4-(2-(2-bromophenylamino)-3,4-dioxocyclobut-1-enyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from human recombinant CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 17: 1713-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.067 BindingDB Entry DOI: 10.7270/Q2Z320GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193984 (6-chloro-3-({(cyanoimino)[(2-ethylphenyl)amino]met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50412344 (CHEMBL526009) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of acetylcholine-induced calcium mobi... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193973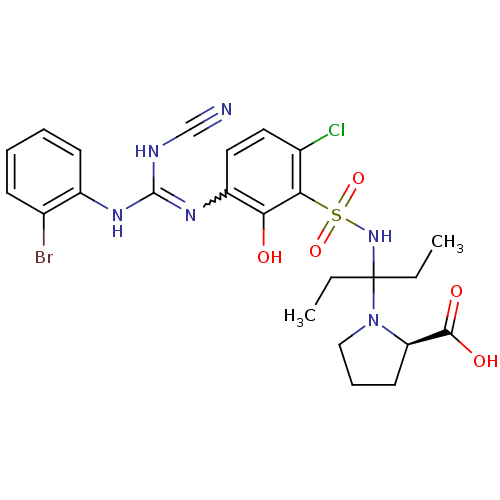 ((R)-1-(3-(3-(3-(2-bromophenyl)-2-cyanoguanidino)-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50193976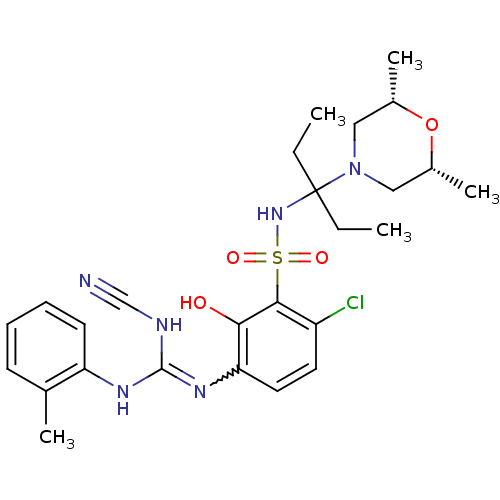 (1-[4-chloro-3-({3-[(2R,6S)-2,6-dimethylmorpholin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [125I]IL8 from CXCR2 expressed in CHO cells | Bioorg Med Chem Lett 16: 5513-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.042 BindingDB Entry DOI: 10.7270/Q2NV9HWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 358 total ) | Next | Last >> |