 Found 5306 hits with Last Name = 'burns' and Initial = 'm'
Found 5306 hits with Last Name = 'burns' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM220118
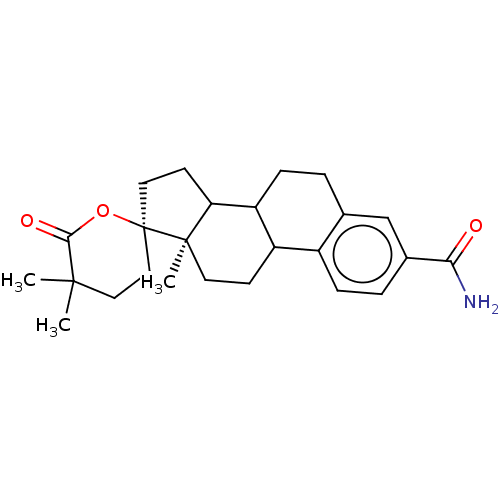
(US9271961, EM1404)Show SMILES C[C@@]12CCC3C(CCc4cc(ccc34)C(N)=O)C1CC[C@@]21CCC(C)(C)C(=O)O1 |r| Show InChI InChI=1S/C25H33NO3/c1-23(2)12-13-25(29-22(23)28)11-9-20-19-7-4-15-14-16(21(26)27)5-6-17(15)18(19)8-10-24(20,25)3/h5-6,14,18-20H,4,7-13H2,1-3H3,(H2,26,27)/t18?,19?,20?,24-,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM220117
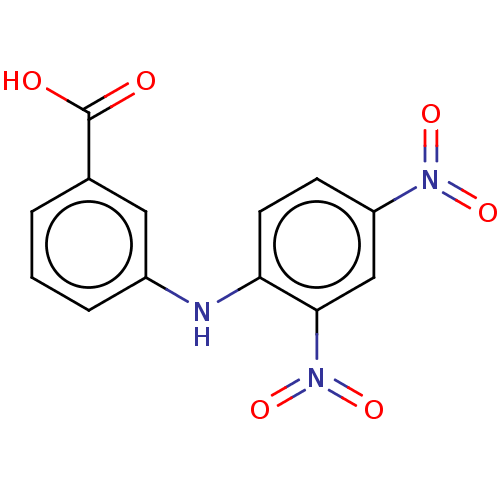
(US9271961, 4-Carboxy-2',4'-dinitrodiphenyl...)Show InChI InChI=1S/C13H9N3O6/c17-13(18)8-2-1-3-9(6-8)14-11-5-4-10(15(19)20)7-12(11)16(21)22/h1-7,14H,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| US Patent
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM220117

(US9271961, 4-Carboxy-2',4'-dinitrodiphenyl...)Show InChI InChI=1S/C13H9N3O6/c17-13(18)8-2-1-3-9(6-8)14-11-5-4-10(15(19)20)7-12(11)16(21)22/h1-7,14H,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| US Patent
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM220121
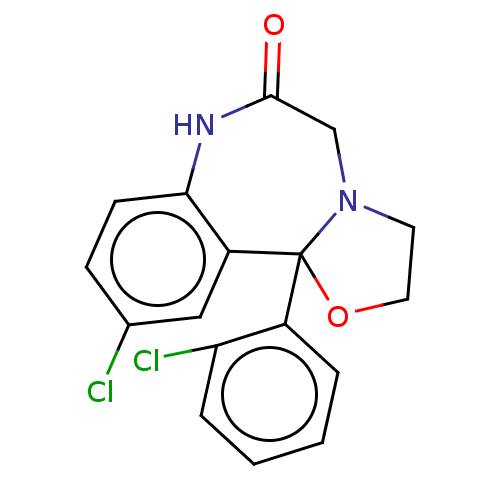
(US9271961, Cloxazolam)Show InChI InChI=1S/C17H14Cl2N2O2/c18-11-5-6-15-13(9-11)17(12-3-1-2-4-14(12)19)21(7-8-23-17)10-16(22)20-15/h1-6,9H,7-8,10H2,(H,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
| US Patent
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM220117

(US9271961, 4-Carboxy-2',4'-dinitrodiphenyl...)Show InChI InChI=1S/C13H9N3O6/c17-13(18)8-2-1-3-9(6-8)14-11-5-4-10(15(19)20)7-12(11)16(21)22/h1-7,14H,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| US Patent
| 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM220116

(US9271961, CBM)Show SMILES COc1ccc2n(cc(CNC(C)=O)c2c1)C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C19H17ClN2O3/c1-12(23)21-10-14-11-22(18-8-7-16(25-2)9-17(14)18)19(24)13-3-5-15(20)6-4-13/h3-9,11H,10H2,1-2H3,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM220121

(US9271961, Cloxazolam)Show InChI InChI=1S/C17H14Cl2N2O2/c18-11-5-6-15-13(9-11)17(12-3-1-2-4-14(12)19)21(7-8-23-17)10-16(22)20-15/h1-6,9H,7-8,10H2,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
| US Patent
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM220121

(US9271961, Cloxazolam)Show InChI InChI=1S/C17H14Cl2N2O2/c18-11-5-6-15-13(9-11)17(12-3-1-2-4-14(12)19)21(7-8-23-17)10-16(22)20-15/h1-6,9H,7-8,10H2,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
| US Patent
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50396691

(CHEMBL449572 | US9271961, Jasmonic acid)Show InChI InChI=1S/C12H18O3/c1-2-3-4-5-10-9(8-12(14)15)6-7-11(10)13/h3-4,9-10H,2,5-8H2,1H3,(H,14,15)/b4-3-/t9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| US Patent
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396691

(CHEMBL449572 | US9271961, Jasmonic acid)Show InChI InChI=1S/C12H18O3/c1-2-3-4-5-10-9(8-12(14)15)6-7-11(10)13/h3-4,9-10H,2,5-8H2,1H3,(H,14,15)/b4-3-/t9-,10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| US Patent
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM220116

(US9271961, CBM)Show SMILES COc1ccc2n(cc(CNC(C)=O)c2c1)C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C19H17ClN2O3/c1-12(23)21-10-14-11-22(18-8-7-16(25-2)9-17(14)18)19(24)13-3-5-15(20)6-4-13/h3-9,11H,10H2,1-2H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM220116

(US9271961, CBM)Show SMILES COc1ccc2n(cc(CNC(C)=O)c2c1)C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C19H17ClN2O3/c1-12(23)21-10-14-11-22(18-8-7-16(25-2)9-17(14)18)19(24)13-3-5-15(20)6-4-13/h3-9,11H,10H2,1-2H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50396691

(CHEMBL449572 | US9271961, Jasmonic acid)Show InChI InChI=1S/C12H18O3/c1-2-3-4-5-10-9(8-12(14)15)6-7-11(10)13/h3-4,9-10H,2,5-8H2,1H3,(H,14,15)/b4-3-/t9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| US Patent
| 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania
US Patent
| Assay Description
Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... |
US Patent US9271961 (2016)
BindingDB Entry DOI: 10.7270/Q27W6B22 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153906
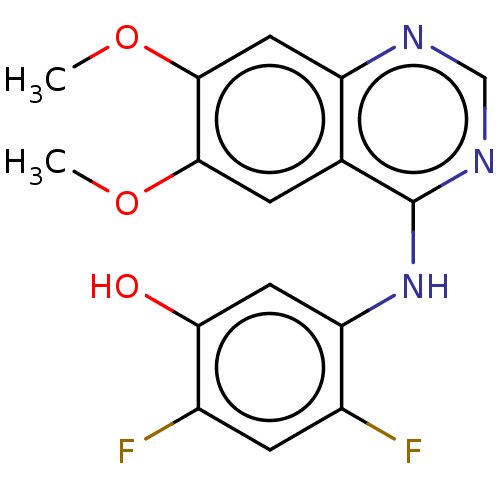
(CHEMBL3775169)Show InChI InChI=1S/C16H13F2N3O3/c1-23-14-3-8-11(6-15(14)24-2)19-7-20-16(8)21-12-5-13(22)10(18)4-9(12)17/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595147

(CHEMBL5205840)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM4627
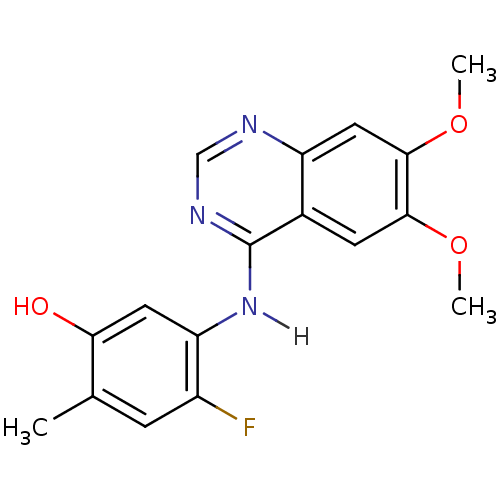
(5-[(6,7-dimethoxyquinazolin-4-yl)amino]-4-fluoro-2...)Show InChI InChI=1S/C17H16FN3O3/c1-9-4-11(18)13(6-14(9)22)21-17-10-5-15(23-2)16(24-3)7-12(10)19-8-20-17/h4-8,22H,1-3H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595148

(CHEMBL5202600)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1cnccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153979
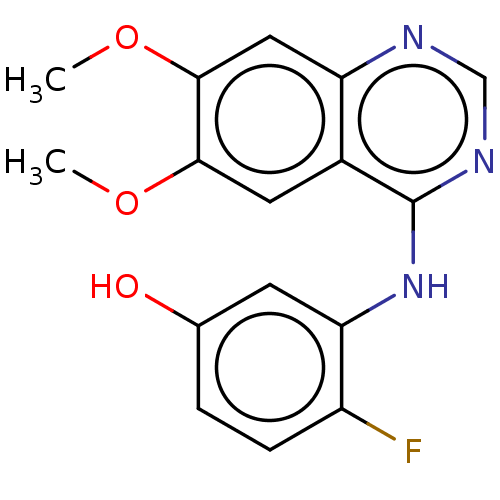
(CHEMBL3774904)Show InChI InChI=1S/C16H14FN3O3/c1-22-14-6-10-12(7-15(14)23-2)18-8-19-16(10)20-13-5-9(21)3-4-11(13)17/h3-8,21H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595161

(CHEMBL5172072)Show SMILES CNC(=O)c1ccc(cn1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595158
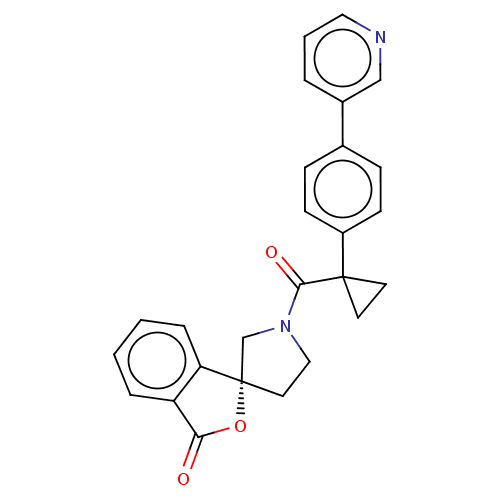
(CHEMBL5203601)Show SMILES O=C(N1CC[C@@]2(C1)OC(=O)c1ccccc21)C1(CC1)c1ccc(cc1)-c1cccnc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11868

(CHEMBL256157 | N-Hydroxy-4-{[(4-phenoxyphenyl)sulf...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccccc3)cc2)CCN(CC#C)CC1 Show InChI InChI=1S/C22H24N2O5S/c1-2-14-24-15-12-22(13-16-24,21(25)23-26)17-30(27,28)20-10-8-19(9-11-20)29-18-6-4-3-5-7-18/h1,3-11,26H,12-17H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 560-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.086
BindingDB Entry DOI: 10.7270/Q2CN73MJ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11868

(CHEMBL256157 | N-Hydroxy-4-{[(4-phenoxyphenyl)sulf...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccccc3)cc2)CCN(CC#C)CC1 Show InChI InChI=1S/C22H24N2O5S/c1-2-14-24-15-12-22(13-16-24,21(25)23-26)17-30(27,28)20-10-8-19(9-11-20)29-18-6-4-3-5-7-18/h1,3-11,26H,12-17H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 560-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.086
BindingDB Entry DOI: 10.7270/Q2CN73MJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM11868

(CHEMBL256157 | N-Hydroxy-4-{[(4-phenoxyphenyl)sulf...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccccc3)cc2)CCN(CC#C)CC1 Show InChI InChI=1S/C22H24N2O5S/c1-2-14-24-15-12-22(13-16-24,21(25)23-26)17-30(27,28)20-10-8-19(9-11-20)29-18-6-4-3-5-7-18/h1,3-11,26H,12-17H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 18: 560-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.086
BindingDB Entry DOI: 10.7270/Q2CN73MJ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153902
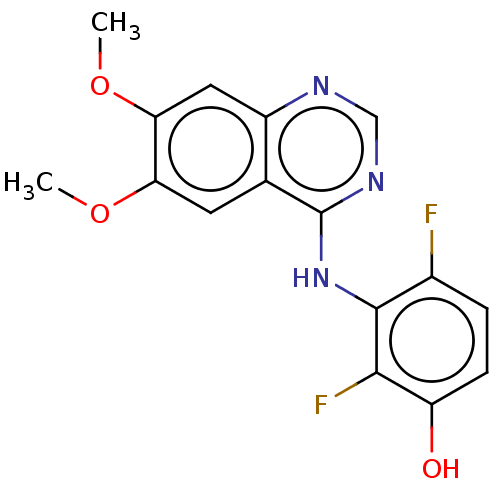
(CHEMBL3775557)Show InChI InChI=1S/C16H13F2N3O3/c1-23-12-5-8-10(6-13(12)24-2)19-7-20-16(8)21-15-9(17)3-4-11(22)14(15)18/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50589332
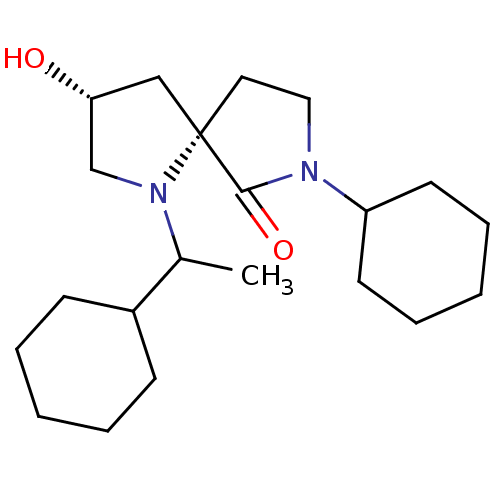
(CHEMBL5208100)Show SMILES CC(C1CCCCC1)N1C[C@H](O)C[C@]11CCN(C2CCCCC2)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128884
BindingDB Entry DOI: 10.7270/Q22J6GTK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153903
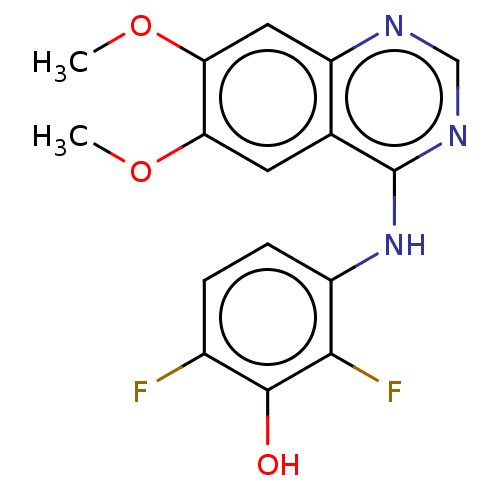
(CHEMBL3775336)Show InChI InChI=1S/C16H13F2N3O3/c1-23-12-5-8-11(6-13(12)24-2)19-7-20-16(8)21-10-4-3-9(17)15(22)14(10)18/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50589325

(CHEMBL5186299)Show SMILES CNC(=O)c1ccc(cn1)-c1ccc(CN2C[C@@H](F)C[C@]22CCN(C3CCCCC3)C2=O)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128884
BindingDB Entry DOI: 10.7270/Q22J6GTK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50589324

(CHEMBL5182702)Show SMILES F[C@@H]1CN(Cc2ccc(Cl)cc2)[C@@]2(CCN(C3CCCCC3)C2=O)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128884
BindingDB Entry DOI: 10.7270/Q22J6GTK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595152

(CHEMBL5187458)Show SMILES O=C(N1CC[C@@]2(C1)OC(=O)c1ccccc21)C1(CC1)c1ccc(nc1)-c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4627

(5-[(6,7-dimethoxyquinazolin-4-yl)amino]-4-fluoro-2...)Show InChI InChI=1S/C17H16FN3O3/c1-9-4-11(18)13(6-14(9)22)21-17-10-5-15(23-2)16(24-3)7-12(10)19-8-20-17/h4-8,22H,1-3H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human KDR expressed in insect Sf21 cells preincubated for 15 mins followed by substrate addition measured after ... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595147

(CHEMBL5205840)Show SMILES Clc1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153908
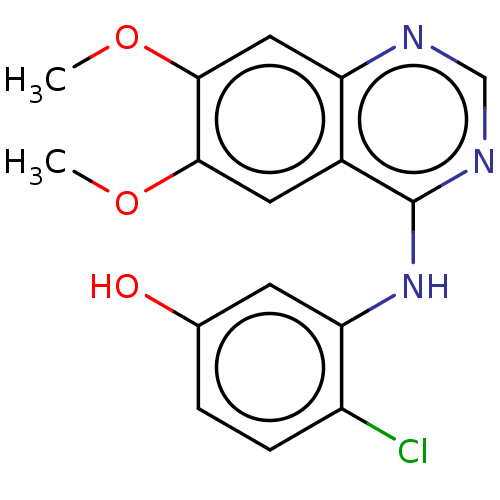
(CHEMBL3774489)Show InChI InChI=1S/C16H14ClN3O3/c1-22-14-6-10-12(7-15(14)23-2)18-8-19-16(10)20-13-5-9(21)3-4-11(13)17/h3-8,21H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50154001
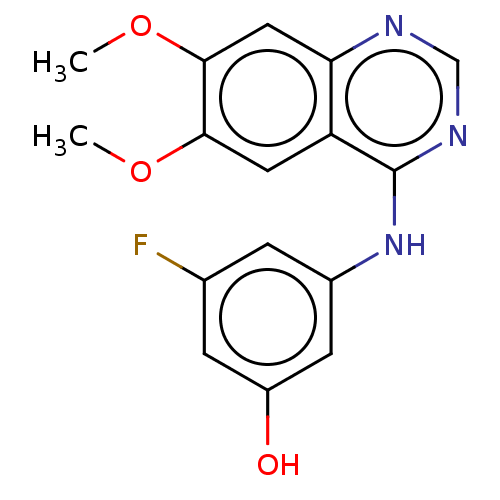
(CHEMBL3775934)Show InChI InChI=1S/C16H14FN3O3/c1-22-14-6-12-13(7-15(14)23-2)18-8-19-16(12)20-10-3-9(17)4-11(21)5-10/h3-8,21H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595159

(CHEMBL5175978)Show SMILES CNC(=O)c1ccc(nc1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595157

(CHEMBL5188613)Show SMILES O=C(N1CC[C@@]2(C1)OC(=O)c1ccccc21)C1(CC1)c1ccc(cc1)-c1ccccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50589323

(CHEMBL5189335)Show SMILES CNC(=O)c1ccc(cn1)-c1ccc(CN2C[C@H](O)C[C@]22CCN(C3CCCCC3)C2=O)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128884
BindingDB Entry DOI: 10.7270/Q22J6GTK |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595161

(CHEMBL5172072)Show SMILES CNC(=O)c1ccc(cn1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50154249
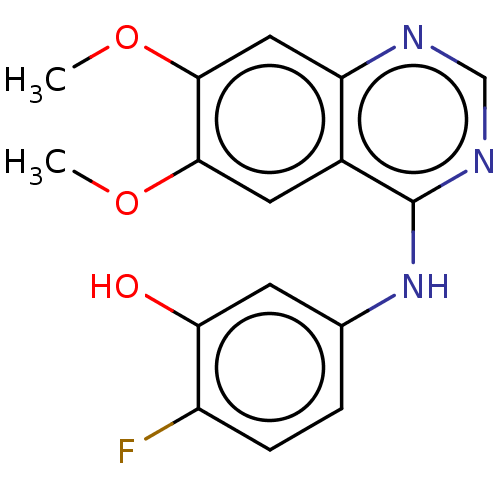
(CHEMBL3775879)Show InChI InChI=1S/C16H14FN3O3/c1-22-14-6-10-12(7-15(14)23-2)18-8-19-16(10)20-9-3-4-11(17)13(21)5-9/h3-8,21H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595159

(CHEMBL5175978)Show SMILES CNC(=O)c1ccc(nc1)-c1ccc(cc1)C1(CC1)C(=O)N1CC[C@@]2(C1)OC(=O)c1ccccc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50589333

(CHEMBL5183283)Show SMILES CC(C1CCC1)N1C[C@H](O)C[C@]11CCN(C2CCCCC2)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128884
BindingDB Entry DOI: 10.7270/Q22J6GTK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50154246
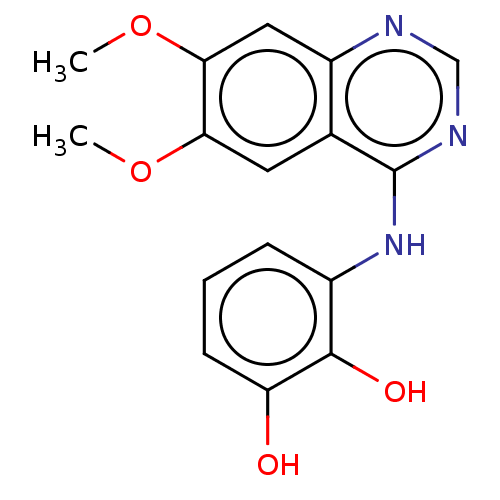
(CHEMBL3774580)Show InChI InChI=1S/C16H15N3O4/c1-22-13-6-9-11(7-14(13)23-2)17-8-18-16(9)19-10-4-3-5-12(20)15(10)21/h3-8,20-21H,1-2H3,(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50153895
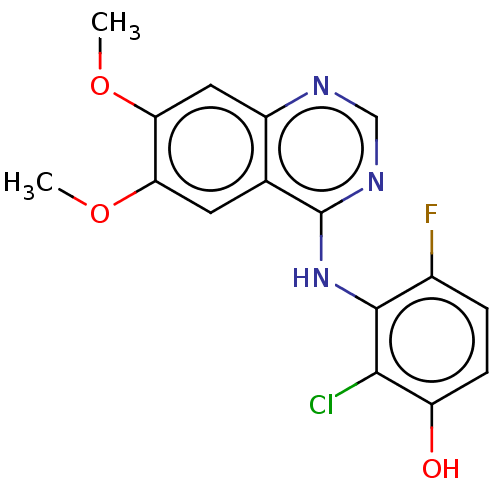
(CHEMBL3774953)Show InChI InChI=1S/C16H13ClFN3O3/c1-23-12-5-8-10(6-13(12)24-2)19-7-20-16(8)21-15-9(18)3-4-11(22)14(15)17/h3-7,22H,1-2H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50229639
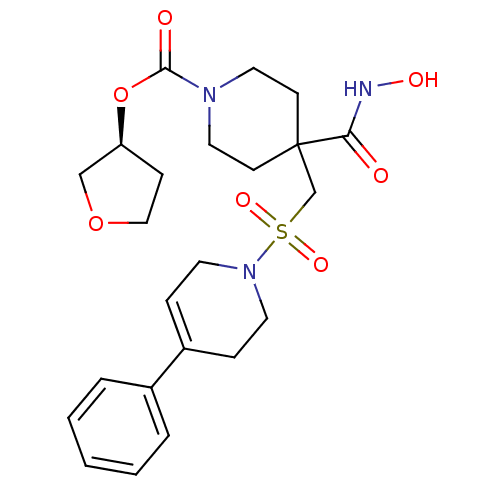
((S)-tetrahydrofuran-3-yl 4-(hydroxycarbamoyl)-4-((...)Show SMILES ONC(=O)C1(CS(=O)(=O)N2CCC(=CC2)c2ccccc2)CCN(CC1)C(=O)O[C@H]1CCOC1 |c:12| Show InChI InChI=1S/C23H31N3O7S/c27-21(24-29)23(9-13-25(14-10-23)22(28)33-20-8-15-32-16-20)17-34(30,31)26-11-6-19(7-12-26)18-4-2-1-3-5-18/h1-6,20,29H,7-17H2,(H,24,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 560-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.086
BindingDB Entry DOI: 10.7270/Q2CN73MJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595144

(CHEMBL5183831)Show SMILES Cc1cccc(C)c1C1CCN(C1)C(=O)C1(CC1)c1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50595156
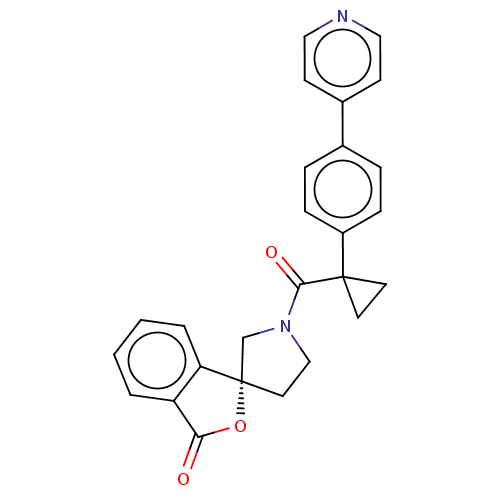
(CHEMBL5187680)Show SMILES O=C(N1CC[C@@]2(C1)OC(=O)c1ccccc21)C1(CC1)c1ccc(cc1)-c1ccncc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128782
BindingDB Entry DOI: 10.7270/Q2FF3XBX |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM4622
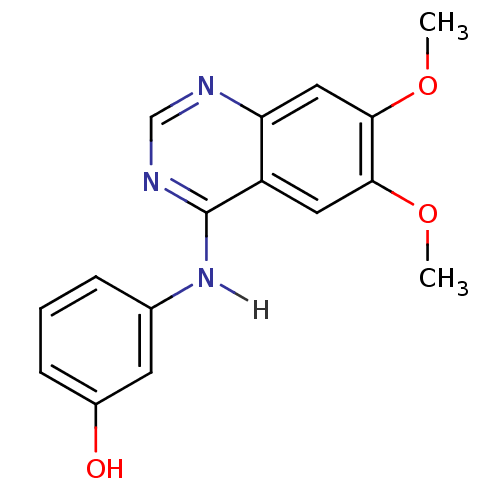
(3-[(6,7-dimethoxyquinazolin-4-yl)amino]phenol | An...)Show InChI InChI=1S/C16H15N3O3/c1-21-14-7-12-13(8-15(14)22-2)17-9-18-16(12)19-10-4-3-5-11(20)6-10/h3-9,20H,1-2H3,(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM26477
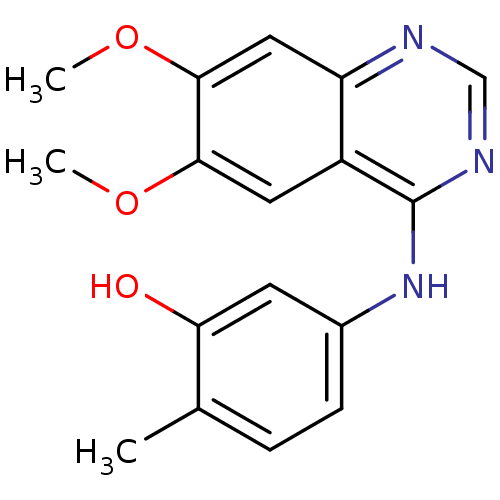
(5-[(6,7-dimethoxyquinazolin-4-yl)amino]-2-methylph...)Show InChI InChI=1S/C17H17N3O3/c1-10-4-5-11(6-14(10)21)20-17-12-7-15(22-2)16(23-3)8-13(12)18-9-19-17/h4-9,21H,1-3H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... |
Eur J Med Chem 112: 20-32 (2016)
Article DOI: 10.1016/j.ejmech.2016.01.039
BindingDB Entry DOI: 10.7270/Q27W6F1V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data