

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168052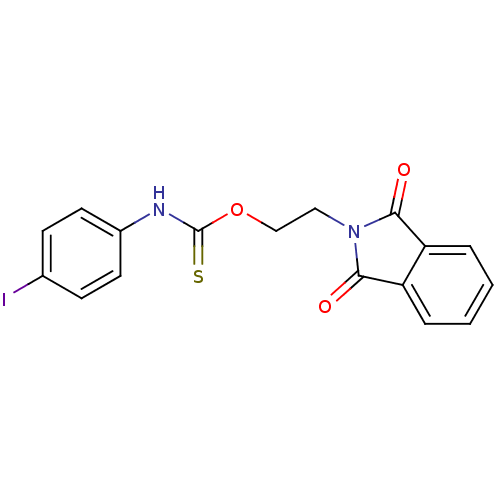 ((4-Iodo-phenyl)-thiocarbamic acid 2-(1,3-dioxo-1,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103N+Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168128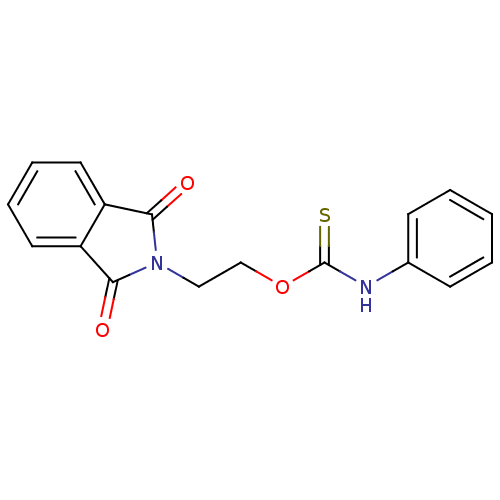 (CHEMBL191655 | O-2-(1,3-dioxoisoindolin-2-yl)ethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 virion reverse transcriptase | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168060 (CHEMBL195108 | N-[2-(4-Iodo-phenylthiocarbamoyloxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493837 (CHEMBL2443298) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493837 (CHEMBL2443298) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103R) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168052 ((4-Iodo-phenyl)-thiocarbamic acid 2-(1,3-dioxo-1,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103R) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493838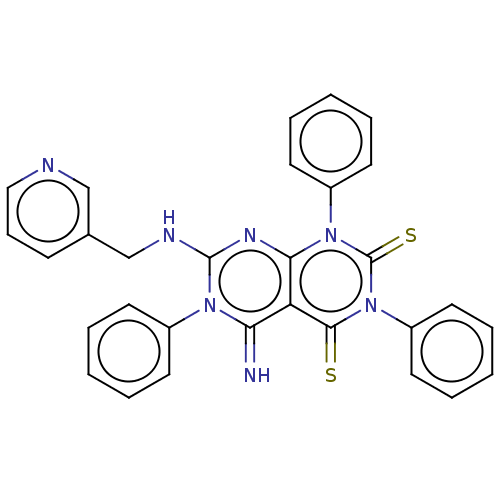 (CHEMBL2443297) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493846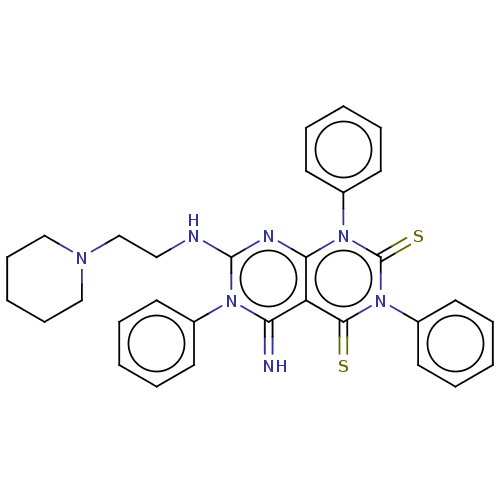 (CHEMBL2443290) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493835 (CHEMBL2443288) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168060 (CHEMBL195108 | N-[2-(4-Iodo-phenylthiocarbamoyloxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103R) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168052 ((4-Iodo-phenyl)-thiocarbamic acid 2-(1,3-dioxo-1,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103N+Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168060 (CHEMBL195108 | N-[2-(4-Iodo-phenylthiocarbamoyloxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103R) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168052 ((4-Iodo-phenyl)-thiocarbamic acid 2-(1,3-dioxo-1,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103N+Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168060 (CHEMBL195108 | N-[2-(4-Iodo-phenylthiocarbamoyloxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103N+Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM241949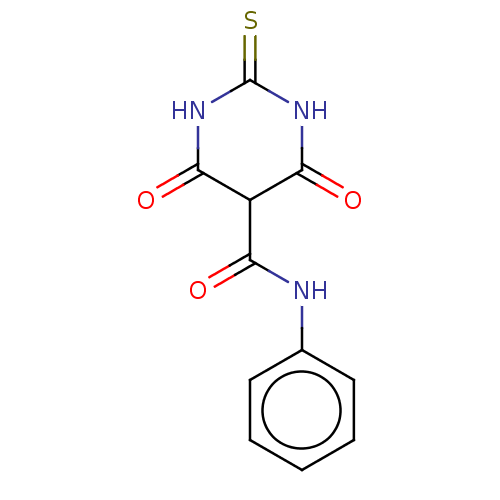 (US9428466, Merbarone) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493842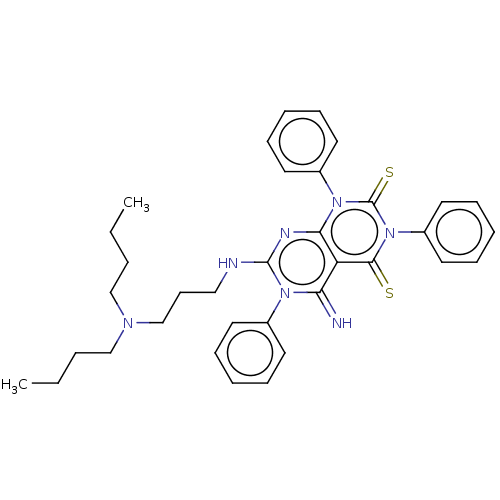 (CHEMBL2443291) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493841 (CHEMBL2443289) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493845 (CHEMBL2443300) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493844 (CHEMBL2440361) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493843 (CHEMBL2443296) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493839 (CHEMBL2443292) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493836 (CHEMBL2443299) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50493840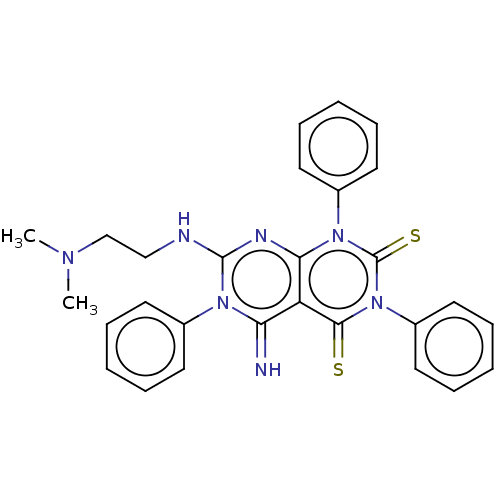 (CHEMBL2440360) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2alpha-mediated relaxation of supercoiled pBR322 after 1 hr by ethidium bromide staining | Bioorg Med Chem 21: 6328-36 (2013) Article DOI: 10.1016/j.bmc.2013.08.056 BindingDB Entry DOI: 10.7270/Q2B56NPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||