

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.800 | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM178102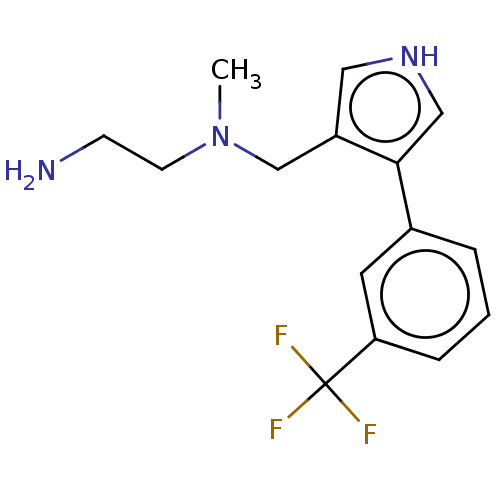 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 11 | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 23 | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM178103 (MS023 (Compound 3) | N1-((4-(4-isopropoxyphenyl)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 55 | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM178101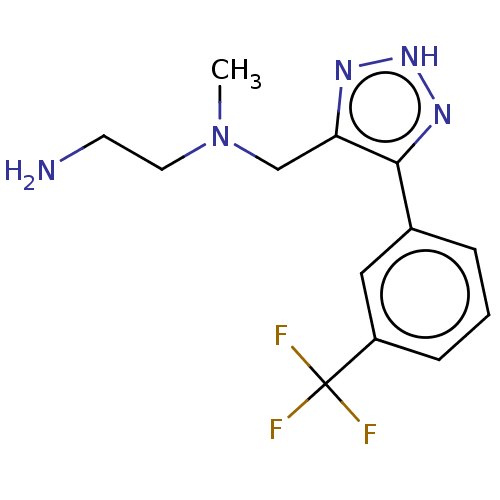 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM178102 (N1-Methyl-N1-((4-(3-(trifluoromethyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 550 | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-S-isoprenylcysteine O-methyltransferase (Homo sapiens (Human)) | BDBM50183409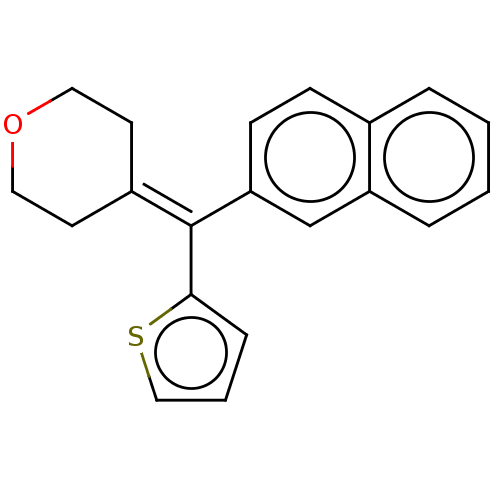 (CHEMBL3822531) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Competitive inhibition of human isoprenylcysteine carboxyl methyltransferase expressed in yeast His10myc3N-Icmt membranes using increasing AFC substr... | Medchemcomm 7: 1016-1021 (2016) BindingDB Entry DOI: 10.7270/Q2C82C8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM178101 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+3 | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-S-isoprenylcysteine O-methyltransferase (Homo sapiens (Human)) | BDBM50183409 (CHEMBL3822531) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Non-competitive inhibition of human isoprenylcysteine carboxyl methyltransferase expressed in yeast His10myc3N-Icmt membranes with increasing [14C]-S... | Medchemcomm 7: 1016-1021 (2016) BindingDB Entry DOI: 10.7270/Q2C82C8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM178101 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM178101 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.75E+4 | n/a | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM178101 (N1-Methyl-N1-((5-(3-(trifluoromethyl)phenyl)-2H-1,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto | Assay Description In brief, the tritiated S-adenosyl-L-methionine (3HSAM, PerkinElmer Life Sciences) was used as the donor of methyl group. The (3H) methylated biotin ... | ACS Chem Biol 11: 772-81 (2016) Article DOI: 10.1021/acschembio.5b00839 BindingDB Entry DOI: 10.7270/Q2765D4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM218187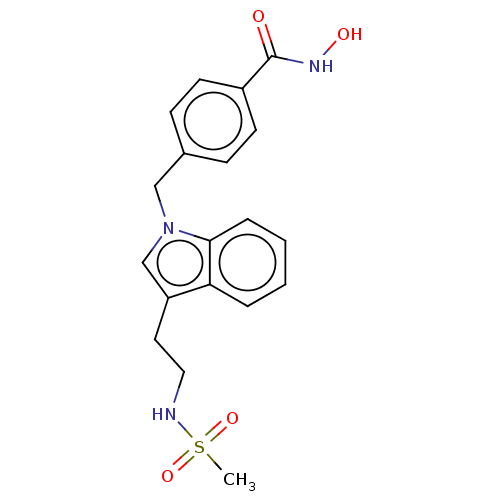 (US9249087, 30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380385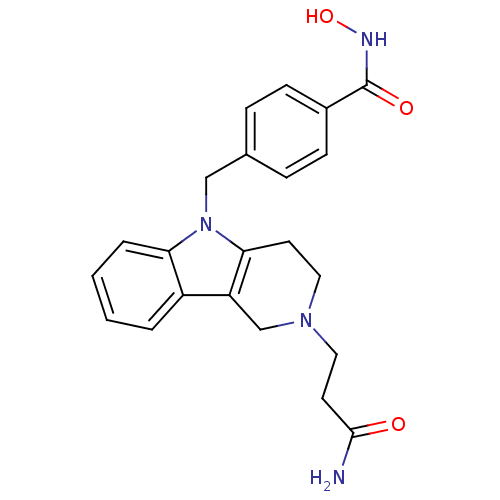 (CHEMBL2018447) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.459 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380391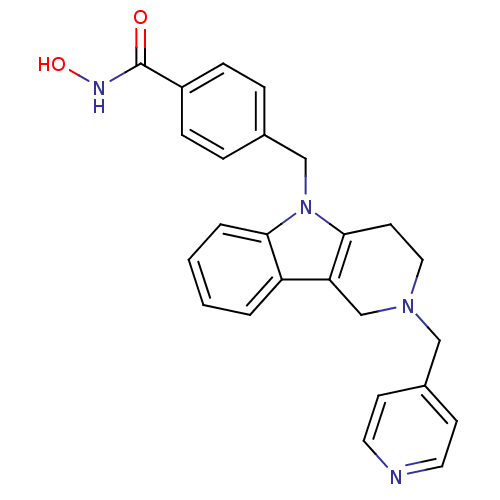 (CHEMBL2018446) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.582 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380400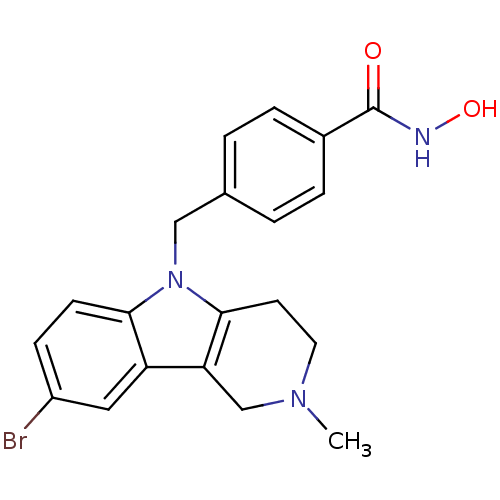 (CHEMBL2018301) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.704 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380390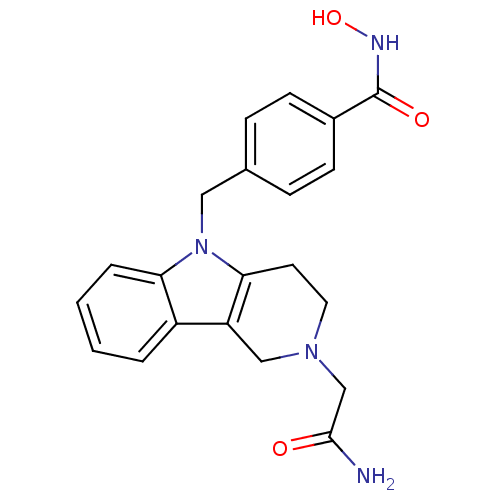 (CHEMBL2018448) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.799 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380386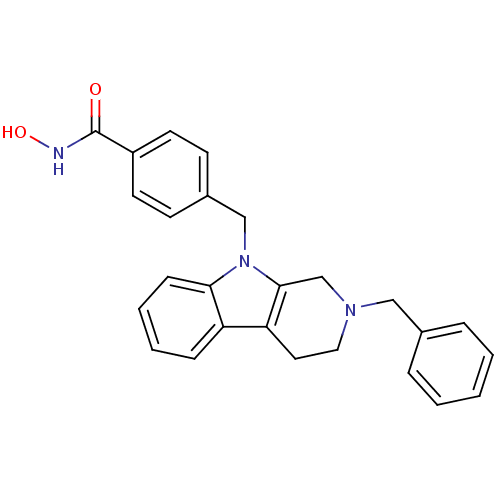 (CHEMBL2018452) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.872 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380384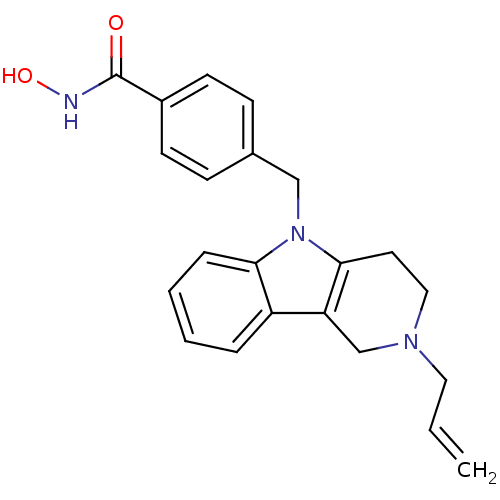 (CHEMBL2018442) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.972 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380401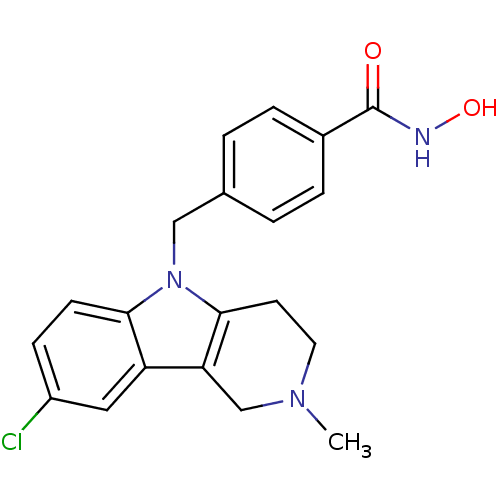 (CHEMBL2018300) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380387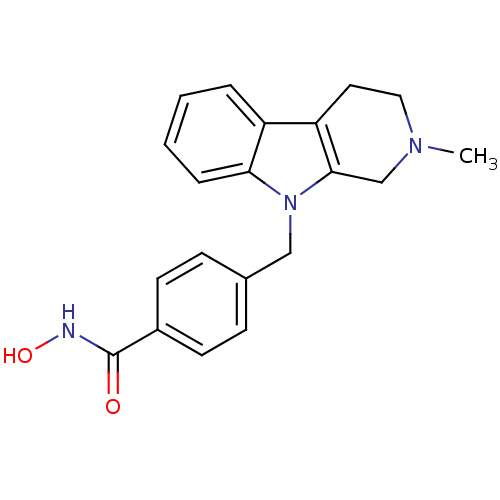 (CHEMBL2018451) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM124188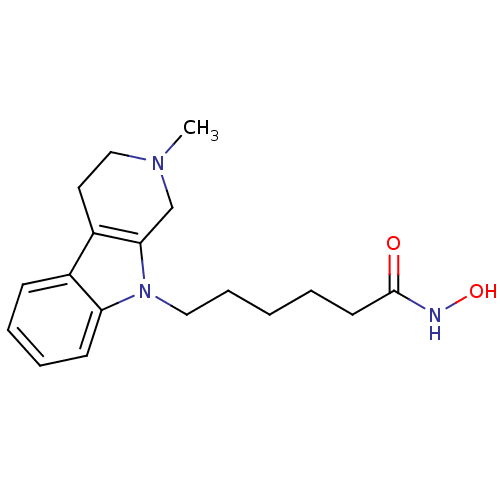 (US8748451, 3 | US8748451, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
The Board of Trustees of The University of Illinois US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US8748451 (2014) BindingDB Entry DOI: 10.7270/Q26D5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380394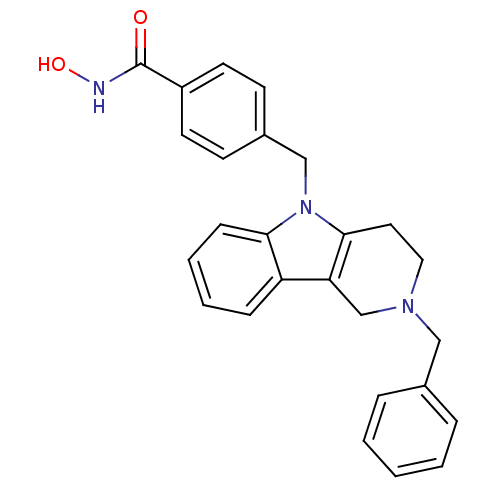 (CHEMBL2018443) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM218178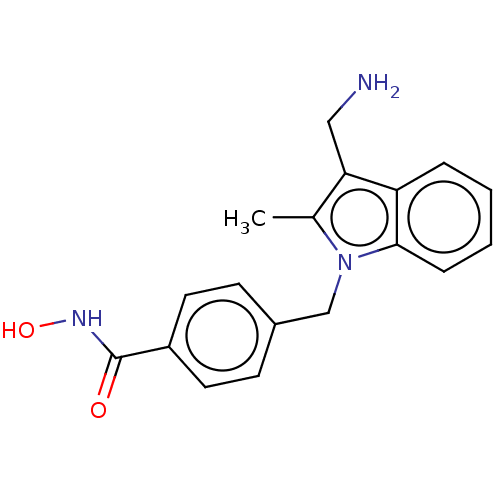 (US9249087, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.46 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM218227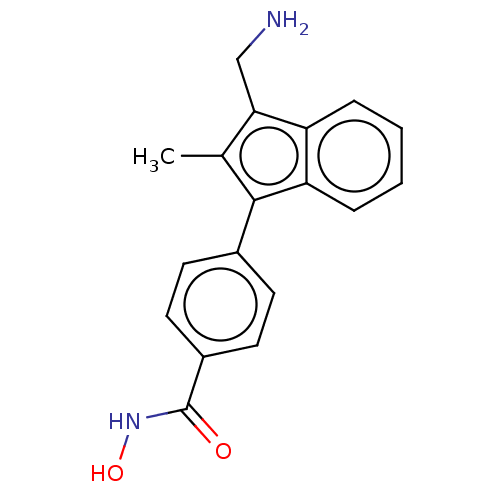 (US9249087, Table 1 , Compound 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.46 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380398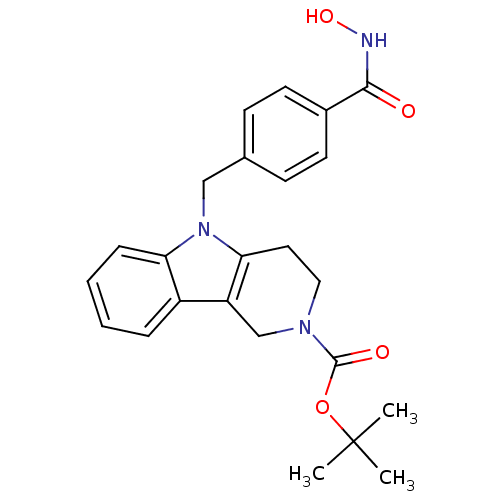 (CHEMBL2018303) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380388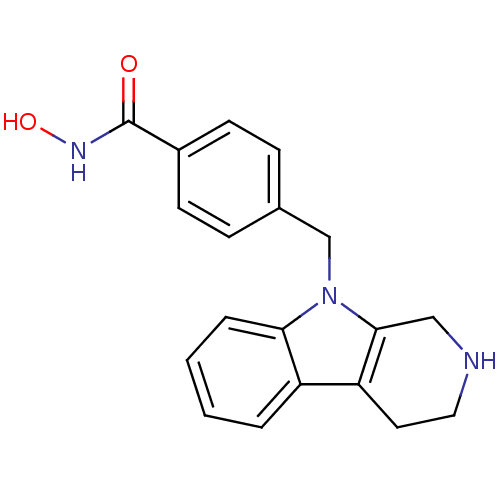 (CHEMBL2018450) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM218225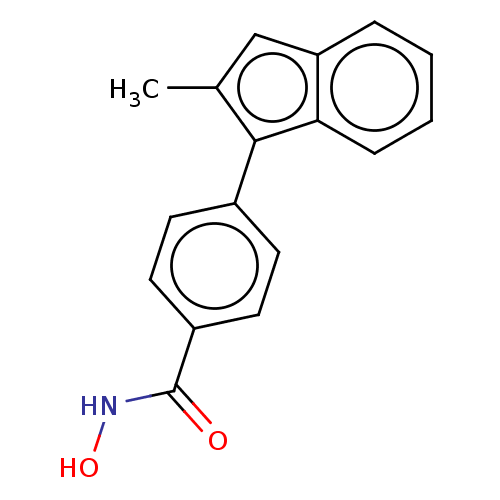 (US9249087, Table 1 , Compound 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM218161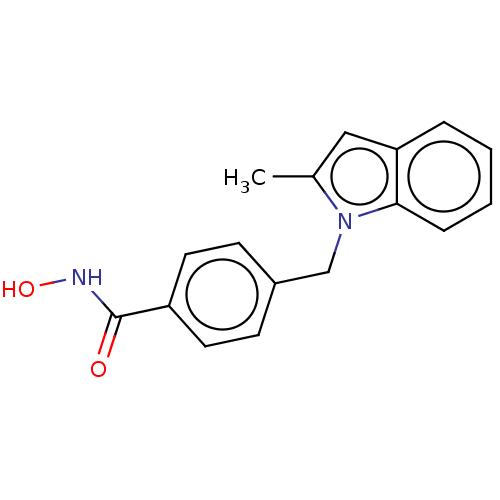 (US9249087, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM218174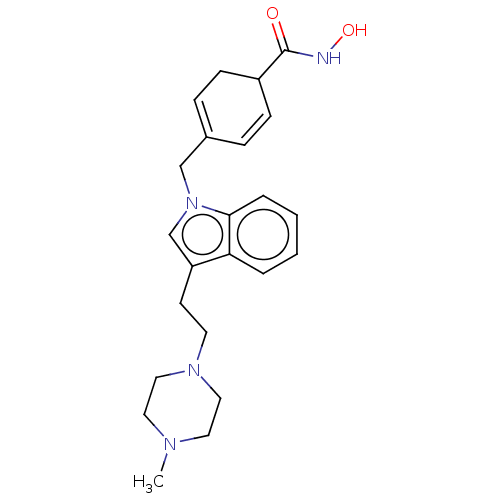 (US9249087, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.94 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM218230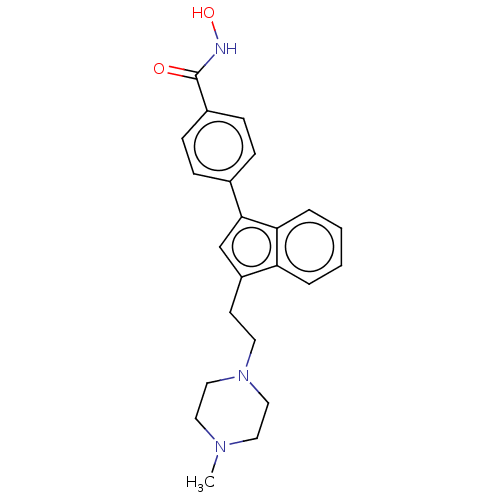 (US9249087, Table 1 , Compound 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.94 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380393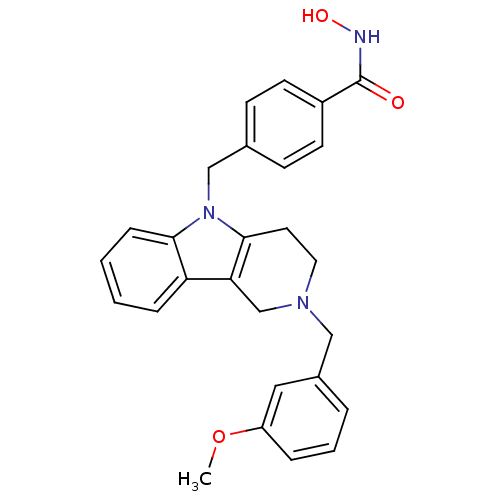 (CHEMBL2018444) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.96 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380396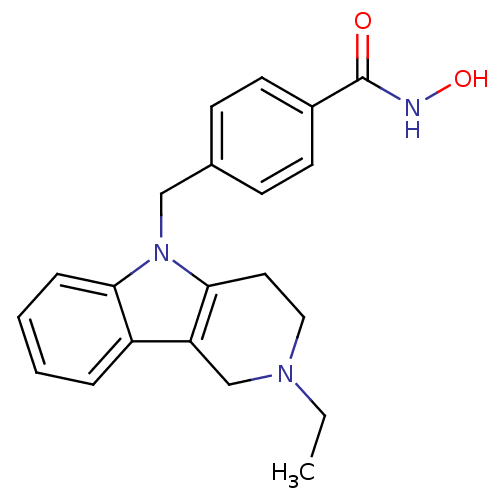 (CHEMBL2018305) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.99 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380392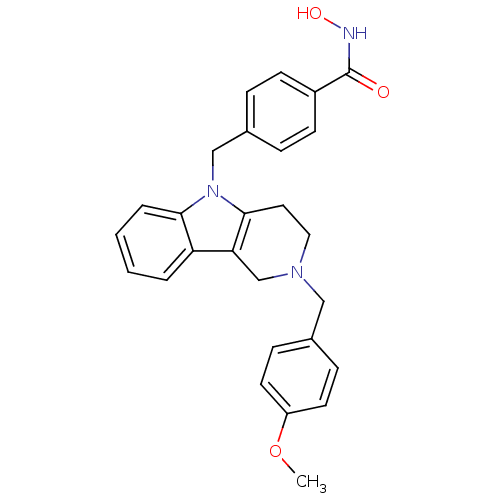 (CHEMBL2018445) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.05 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380402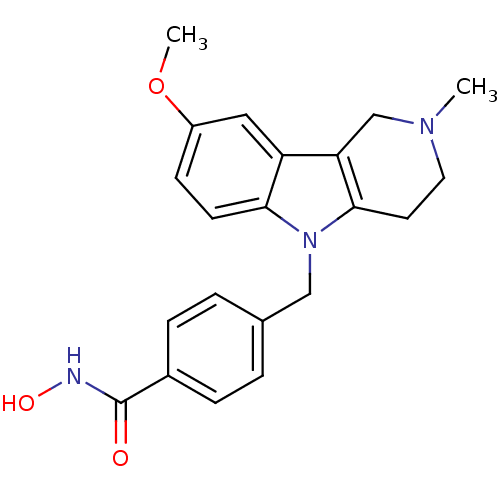 (CHEMBL2018299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM218231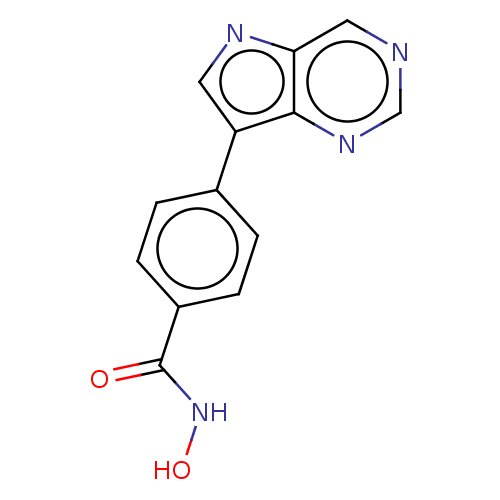 (US9249087, Table 1 , Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.48 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM218169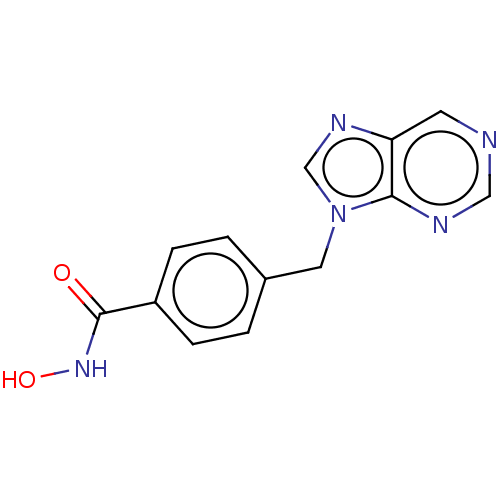 (US9249087, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.48 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380399 (CHEMBL2018302 | Tubastatin A | US10227295, Compoun...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380389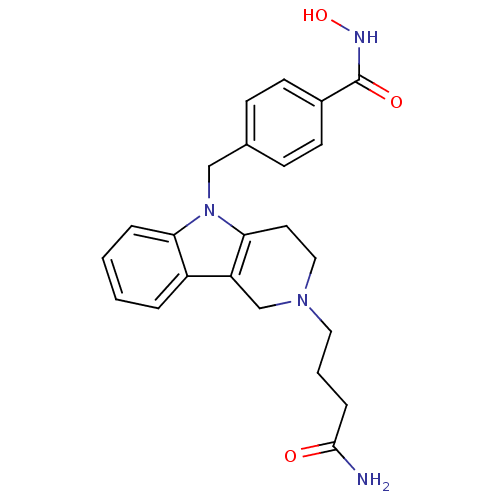 (CHEMBL2018449) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.06 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM218165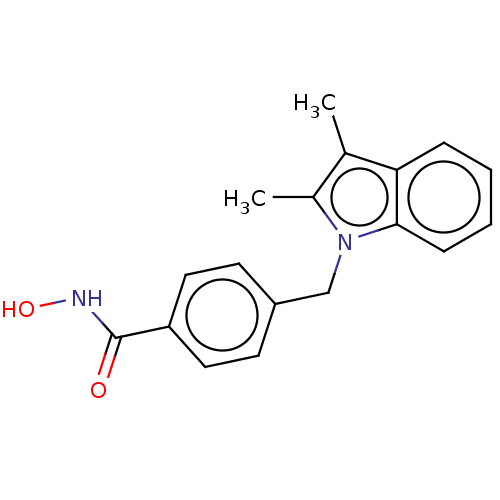 (US9249087, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.08 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380405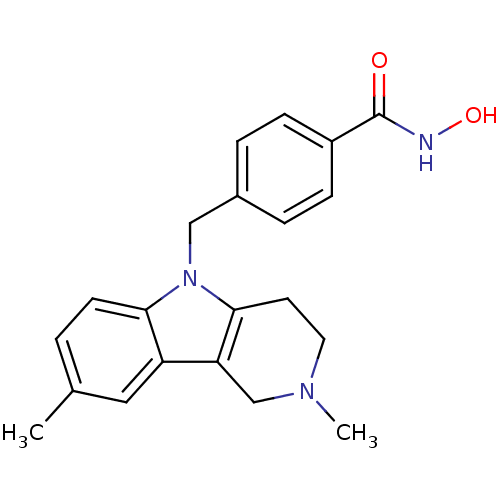 (CHEMBL2018296) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM218163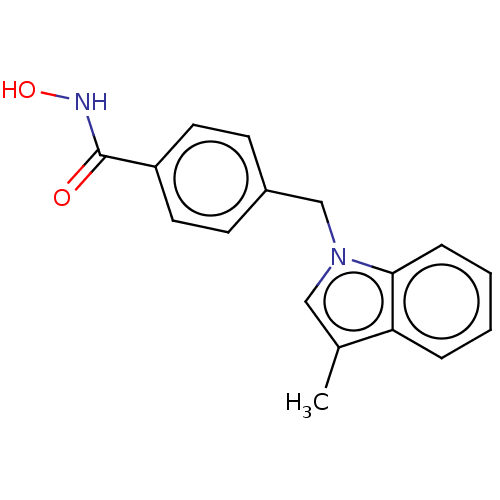 (US9249087, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.11 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM218226 (US9249087, Table 1 , Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.11 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US9249087 (2016) BindingDB Entry DOI: 10.7270/Q2Q23Z3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM124194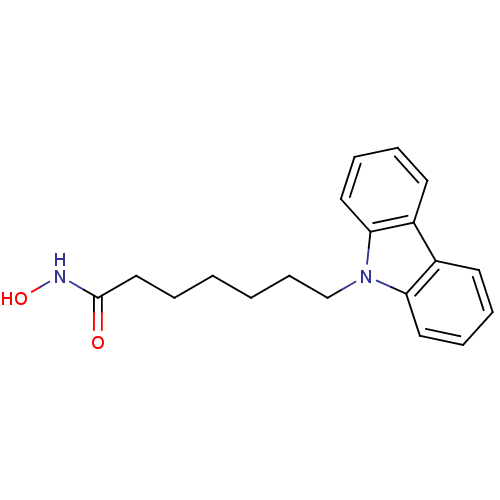 (US8748451, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 8.0 | 25 |
The Board of Trustees of The University of Illinois US Patent | Assay Description HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... | US Patent US8748451 (2014) BindingDB Entry DOI: 10.7270/Q26D5RPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50380397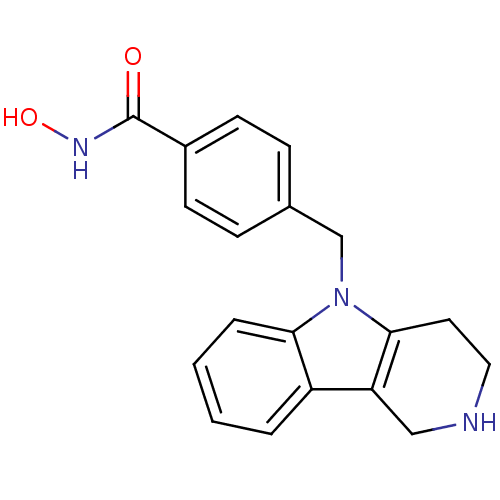 (CHEMBL2018304) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs | J Med Chem 55: 639-51 (2012) Article DOI: 10.1021/jm200773h BindingDB Entry DOI: 10.7270/Q2CJ8FG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 361 total ) | Next | Last >> |