

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli) | BDBM22113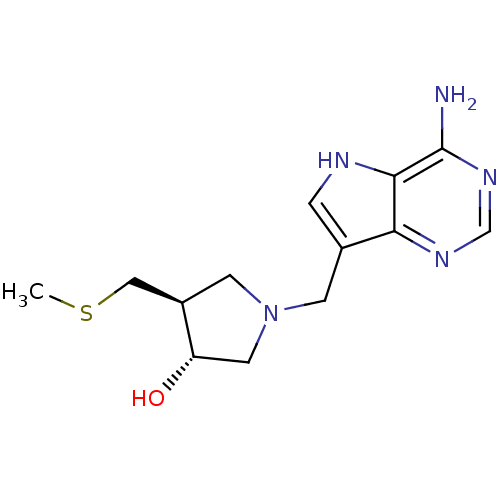 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli MTAN expressed in Escherichia coli BL-21 DE3 using methylthioadenosine as substrate assessed as inhibition... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli) | BDBM22113 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli MTAN expressed in Escherichia coli BL-21 DE3 using methylthioadenosine as substrate by xanthine oxidase co... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50247151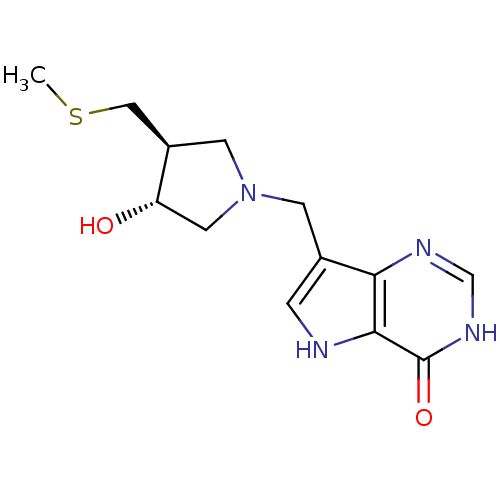 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant human PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-inhibitor complex ... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM22113 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of human MTAP using methylthioadenosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-inhibitor complex... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50247151 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant human PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM22113 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of human MTAP using methylthioadenosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247151 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-in... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli) | BDBM50116357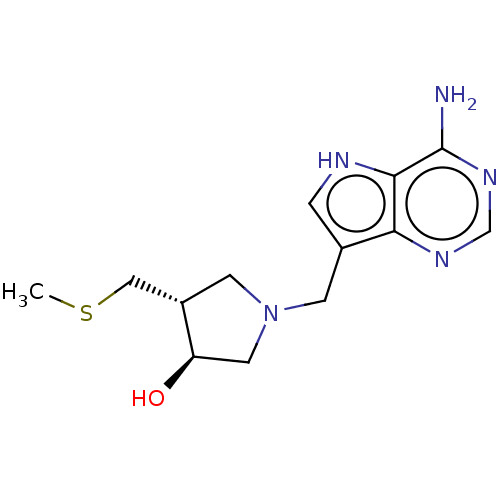 (CHEMBL3604360) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli MTAN expressed in Escherichia coli BL-21 DE3 using methylthioadenosine as substrate by xanthine oxidase co... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247151 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50116358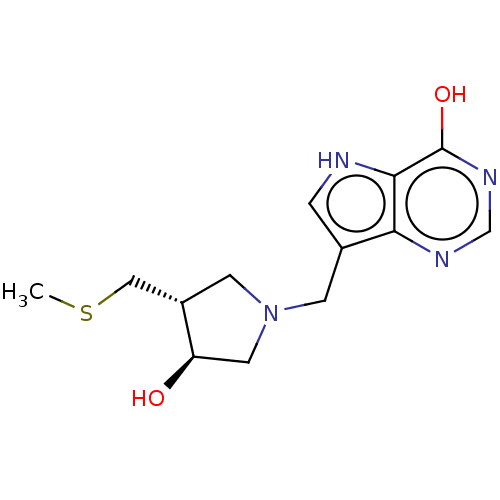 (CHEMBL3604359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant human PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-inhibitor complex ... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50116358 (CHEMBL3604359) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-in... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50116358 (CHEMBL3604359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant human PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23232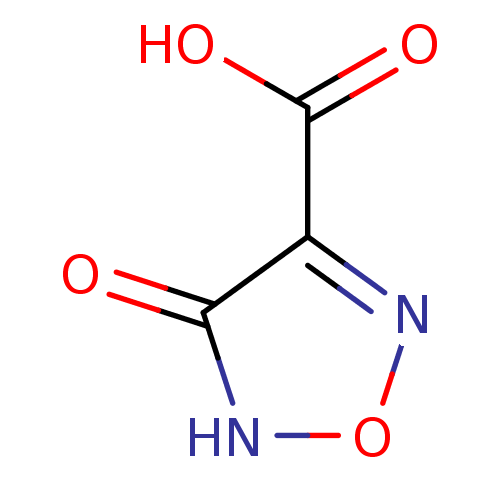 (1,2,5-oxadiazole, OXD1 | 4-hydroxy-1,2,5-oxadiazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 210 | -38.1 | 650 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23251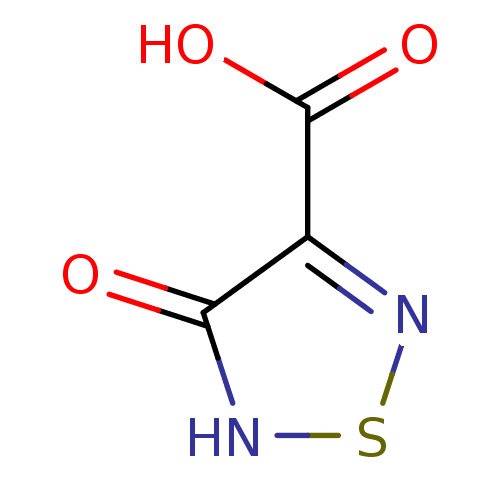 (1,2,5-Thiadiazole, TDA1 | 4-hydroxy-1,2,5-thiadiaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 290 | -37.3 | 140 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50116358 (CHEMBL3604359) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 298 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase (Plasmodium falciparum) | BDBM23242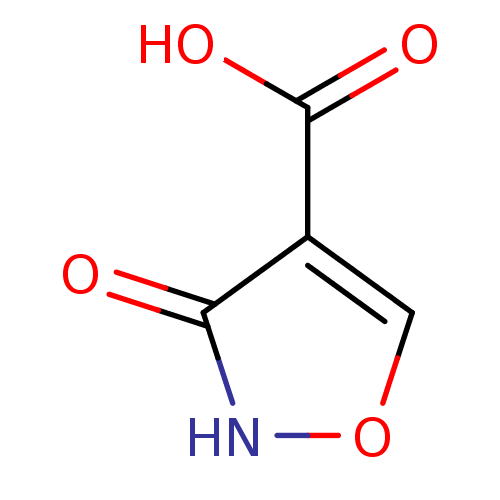 (1,2(1,5)-Isoxazole, IOA1 | 3-hydroxy-1,2-oxazole-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 470 | -36.1 | 1.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Bristol | Assay Description An LDH enzymatic assay developed for high throughput format was used. The dehydrogenase reaction was run in the lactate to pyruvate direction and cou... | J Biol Chem 279: 31429-39 (2004) Article DOI: 10.1074/jbc.M402433200 BindingDB Entry DOI: 10.7270/Q2CR5RN4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50116357 (CHEMBL3604360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of human MTAP using methylthioadenosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7167 (3-(4-sulfamoylphenyl)-3,4,10,11-tetraazatricyclo[7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | Bioorg Med Chem Lett 15: 1315-9 (2005) Article DOI: 10.1016/j.bmcl.2005.01.023 BindingDB Entry DOI: 10.7270/Q2N58JKH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM31541 (pyrazolo[4,3-h]quinazoline-3-carboxamide, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... | J Med Chem 52: 5152-63 (2009) Article DOI: 10.1021/jm9006559 BindingDB Entry DOI: 10.7270/Q2JH3JJP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7177 (3-(4-methanesulfonylphenyl)-3,4,10,11-tetraazatric...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | Bioorg Med Chem Lett 15: 1315-9 (2005) Article DOI: 10.1016/j.bmcl.2005.01.023 BindingDB Entry DOI: 10.7270/Q2N58JKH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM31539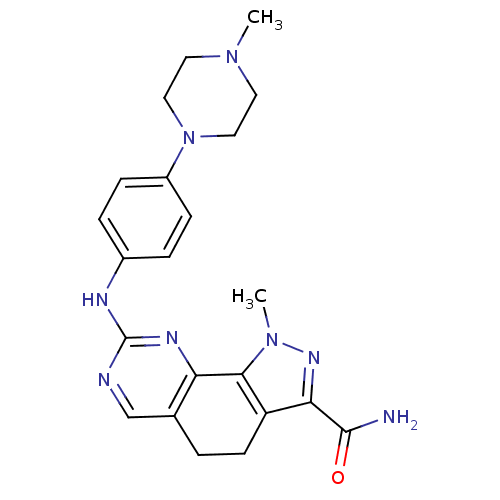 (pyrazolo[4,3-h]quinazoline-3-carboxamide, 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... | J Med Chem 52: 5152-63 (2009) Article DOI: 10.1021/jm9006559 BindingDB Entry DOI: 10.7270/Q2JH3JJP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM31532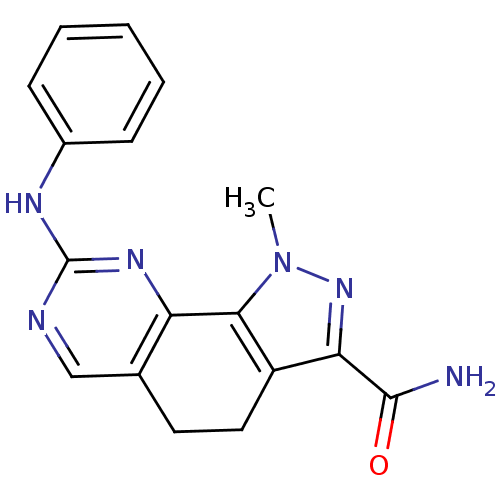 (pyrazolo[4,3-h]quinazoline-3-carboxamide, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Nerviano Medical Sciences Srl | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat... | J Med Chem 52: 5152-63 (2009) Article DOI: 10.1021/jm9006559 BindingDB Entry DOI: 10.7270/Q2JH3JJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7107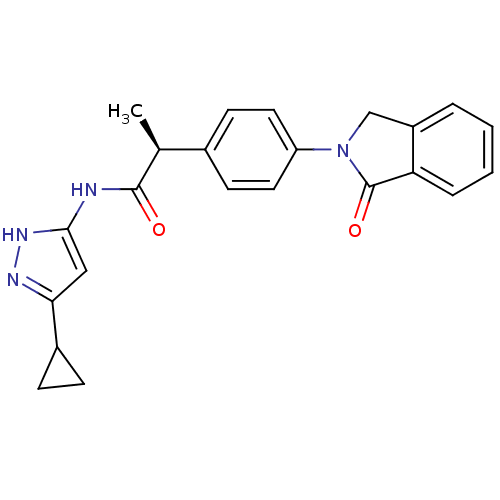 ((2S)-N-(5-Cyclopropyl-1H-pyrazol-3-yl)-2-[4-(1-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 48: 2944-56 (2005) Article DOI: 10.1021/jm0408870 BindingDB Entry DOI: 10.7270/Q2WQ020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7173 (3-[4-(butylsulfamoyl)phenyl]-3,4,10,11-tetraazatri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | Bioorg Med Chem Lett 15: 1315-9 (2005) Article DOI: 10.1016/j.bmcl.2005.01.023 BindingDB Entry DOI: 10.7270/Q2N58JKH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7169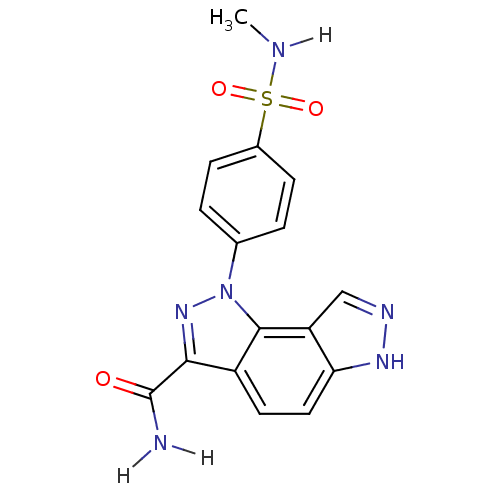 (3-[4-(methylsulfamoyl)phenyl]-3,4,10,11-tetraazatr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | Bioorg Med Chem Lett 15: 1315-9 (2005) Article DOI: 10.1016/j.bmcl.2005.01.023 BindingDB Entry DOI: 10.7270/Q2N58JKH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7111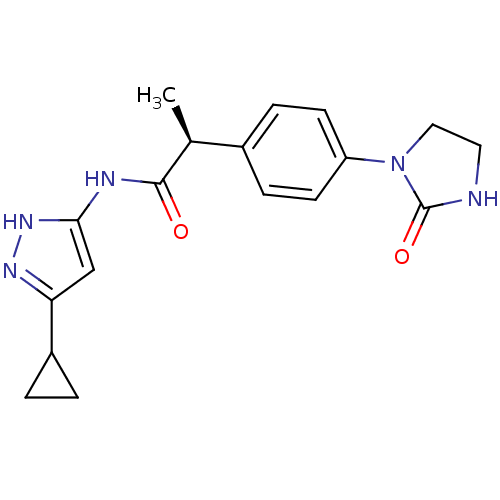 ((2S)-N-(5-Cyclopropyl-1H-pyrazol-3-yl)-2-[4-(2-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 48: 2944-56 (2005) Article DOI: 10.1021/jm0408870 BindingDB Entry DOI: 10.7270/Q2WQ020R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7214 (3-(2,2,2-trifluoroethyl)-3,4,10,11-tetraazatricycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | Bioorg Med Chem Lett 15: 1315-9 (2005) Article DOI: 10.1016/j.bmcl.2005.01.023 BindingDB Entry DOI: 10.7270/Q2N58JKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7163 (3-Phenylacetamidoaminopyrazole deriv. 40 | CS10 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 47: 3367-80 (2004) Article DOI: 10.1021/jm031145u BindingDB Entry DOI: 10.7270/Q2RX998G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7166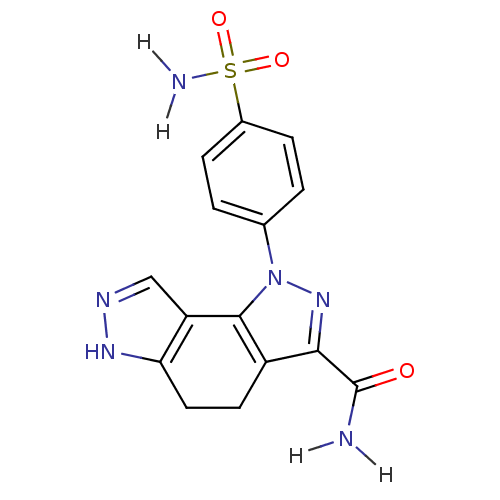 (3-(4-sulfamoylphenyl)-3,4,10,11-tetraazatricyclo[7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | Bioorg Med Chem Lett 15: 1315-9 (2005) Article DOI: 10.1016/j.bmcl.2005.01.023 BindingDB Entry DOI: 10.7270/Q2N58JKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7159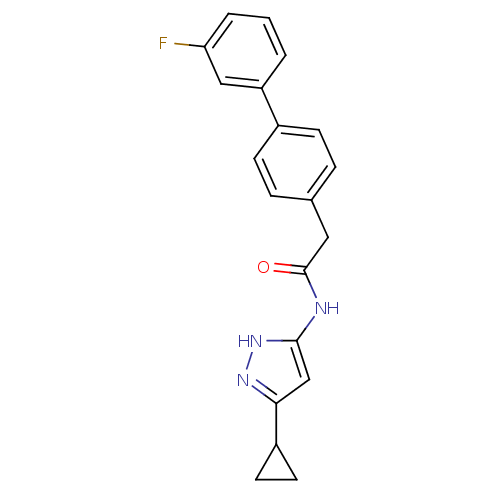 (3-Phenylacetamidoaminopyrazole deriv. 36 | N-(5-Cy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 47: 3367-80 (2004) Article DOI: 10.1021/jm031145u BindingDB Entry DOI: 10.7270/Q2RX998G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7162 (3-Phenylacetamidoaminopyrazole deriv. 39 | 4 -{2-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 47: 3367-80 (2004) Article DOI: 10.1021/jm031145u BindingDB Entry DOI: 10.7270/Q2RX998G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7195 (3-(4-cyanophenyl)-3,4,10,11-tetraazatricyclo[7.3.0...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | Bioorg Med Chem Lett 15: 1315-9 (2005) Article DOI: 10.1016/j.bmcl.2005.01.023 BindingDB Entry DOI: 10.7270/Q2N58JKH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50327930 (3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology Curated by ChEMBL | Assay Description Inhibition of AurA | Bioorg Med Chem 18: 7113-20 (2010) Article DOI: 10.1016/j.bmc.2010.07.048 BindingDB Entry DOI: 10.7270/Q2Q240GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12103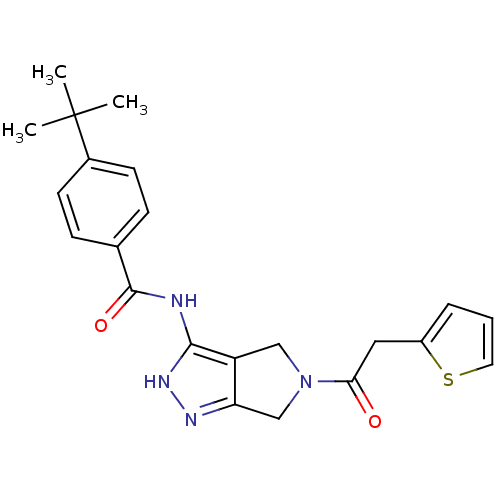 (1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 11 | 4-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 48: 3080-4 (2005) Article DOI: 10.1021/jm049076m BindingDB Entry DOI: 10.7270/Q2FF3QKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50327929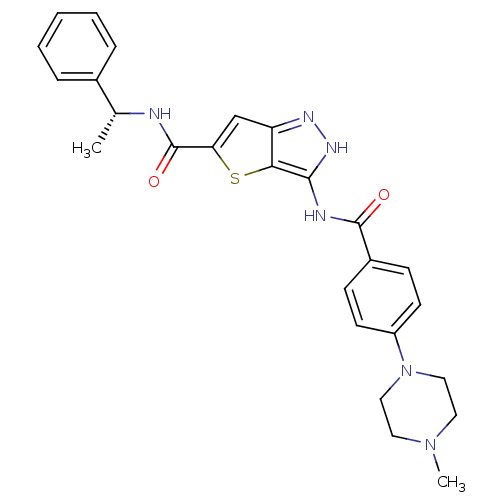 (3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology Curated by ChEMBL | Assay Description Inhibition of AurA | Bioorg Med Chem 18: 7113-20 (2010) Article DOI: 10.1016/j.bmc.2010.07.048 BindingDB Entry DOI: 10.7270/Q2Q240GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12983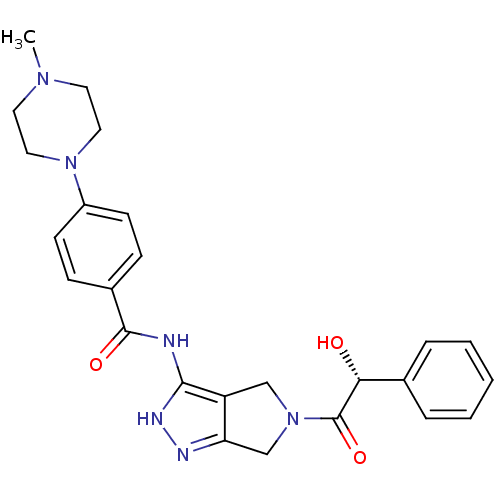 (5-Amido-pyrrolopyrazole 9b | CHEMBL385872 | N-{5-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50281297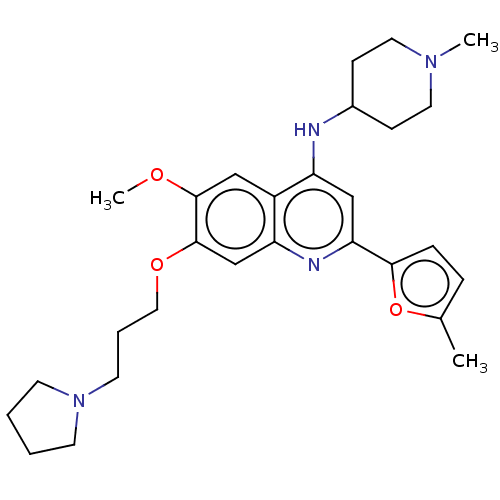 (CHEMBL4170114) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of G9a (unknown origin) using biotinylated histone monomethyl-H3K9 peptide by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01609 BindingDB Entry DOI: 10.7270/Q2GH9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7206 (3-(pyridin-2-yl)-3,4,10,11-tetraazatricyclo[7.3.0....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | Bioorg Med Chem Lett 15: 1315-9 (2005) Article DOI: 10.1016/j.bmcl.2005.01.023 BindingDB Entry DOI: 10.7270/Q2N58JKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7187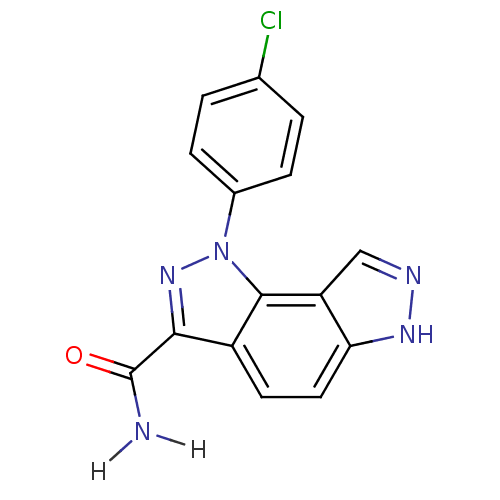 (3-(4-chlorophenyl)-3,4,10,11-tetraazatricyclo[7.3....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | Bioorg Med Chem Lett 15: 1315-9 (2005) Article DOI: 10.1016/j.bmcl.2005.01.023 BindingDB Entry DOI: 10.7270/Q2N58JKH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7160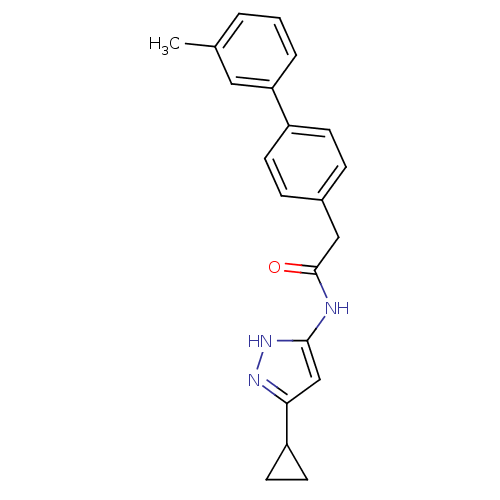 (3-Phenylacetamidoaminopyrazole deriv. 37 | N-(5-Cy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 47: 3367-80 (2004) Article DOI: 10.1021/jm031145u BindingDB Entry DOI: 10.7270/Q2RX998G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7200 (3-(3-methylphenyl)-3,4,10,11-tetraazatricyclo[7.3....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | Bioorg Med Chem Lett 15: 1315-9 (2005) Article DOI: 10.1016/j.bmcl.2005.01.023 BindingDB Entry DOI: 10.7270/Q2N58JKH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7185 (3-(4-methoxyphenyl)-3,4,10,11-tetraazatricyclo[7.3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | Bioorg Med Chem Lett 15: 1315-9 (2005) Article DOI: 10.1016/j.bmcl.2005.01.023 BindingDB Entry DOI: 10.7270/Q2N58JKH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12982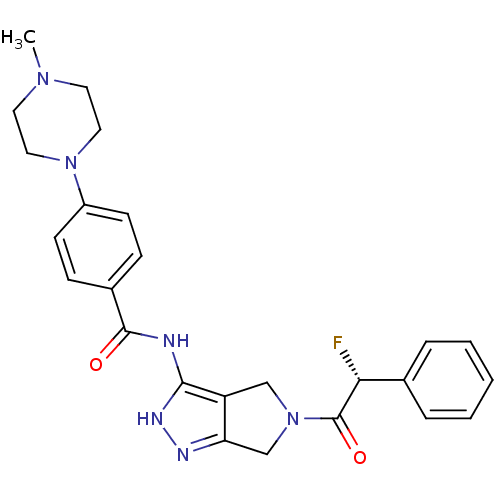 (5-Amido-pyrrolopyrazole 9a | CHEMBL385266 | N-{5-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50327928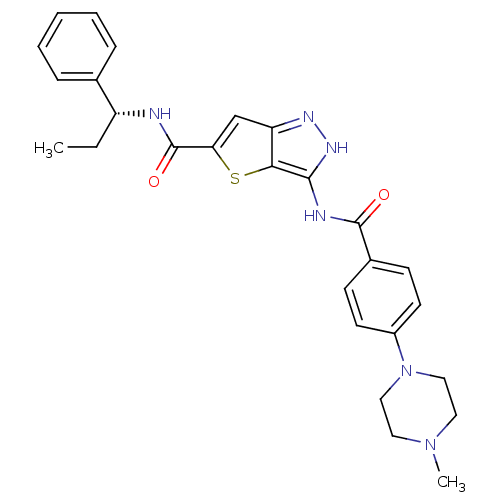 (3-({[4-(4-METHYLPIPERAZIN-1-YL)PHENYL]CARBONYL}AMI...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology Curated by ChEMBL | Assay Description Inhibition of AurA | Bioorg Med Chem 18: 7113-20 (2010) Article DOI: 10.1016/j.bmc.2010.07.048 BindingDB Entry DOI: 10.7270/Q2Q240GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50327923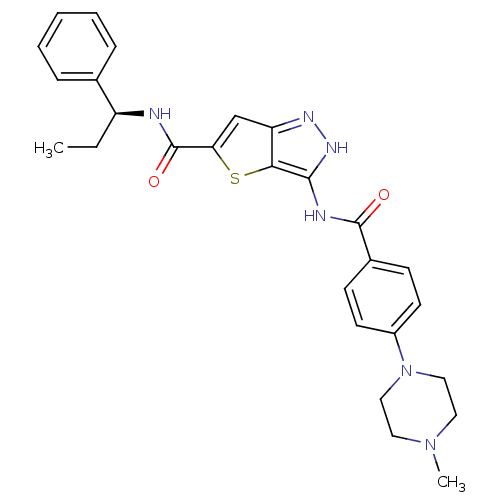 (3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology Curated by ChEMBL | Assay Description Inhibition of AurA | Bioorg Med Chem 18: 7113-20 (2010) Article DOI: 10.1016/j.bmc.2010.07.048 BindingDB Entry DOI: 10.7270/Q2Q240GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7171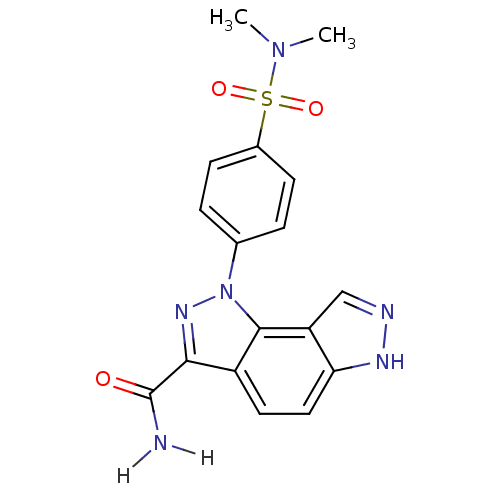 (3-[4-(dimethylsulfamoyl)phenyl]-3,4,10,11-tetraaza...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | Bioorg Med Chem Lett 15: 1315-9 (2005) Article DOI: 10.1016/j.bmcl.2005.01.023 BindingDB Entry DOI: 10.7270/Q2N58JKH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50327927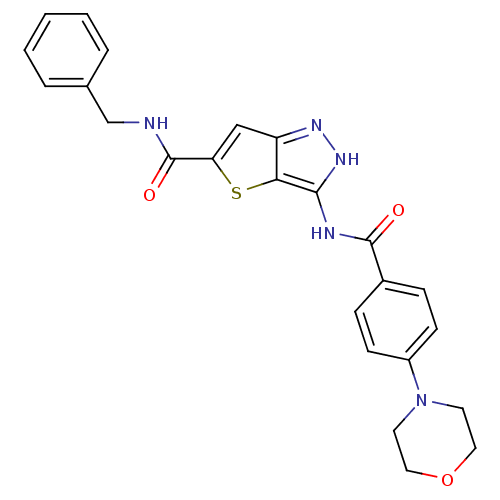 (3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology Curated by ChEMBL | Assay Description Inhibition of AurA | Bioorg Med Chem 18: 7113-20 (2010) Article DOI: 10.1016/j.bmc.2010.07.048 BindingDB Entry DOI: 10.7270/Q2Q240GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7161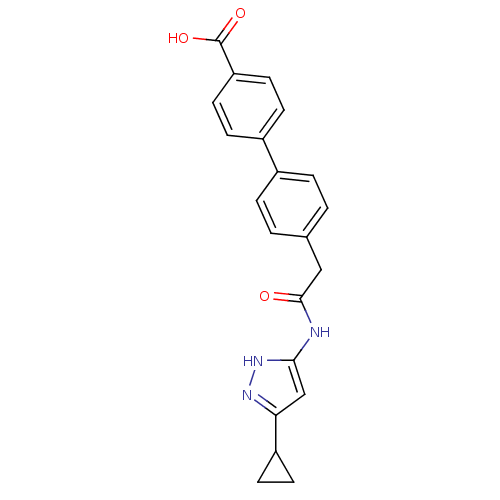 (3-Phenylacetamidoaminopyrazole deriv. 38 | 4 -{2-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 47: 3367-80 (2004) Article DOI: 10.1021/jm031145u BindingDB Entry DOI: 10.7270/Q2RX998G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM7114 (3-Aminopyrazole deriv. 27 | N-(5-Cyclopropyl-1H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 48: 2944-56 (2005) Article DOI: 10.1021/jm0408870 BindingDB Entry DOI: 10.7270/Q2WQ020R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM12985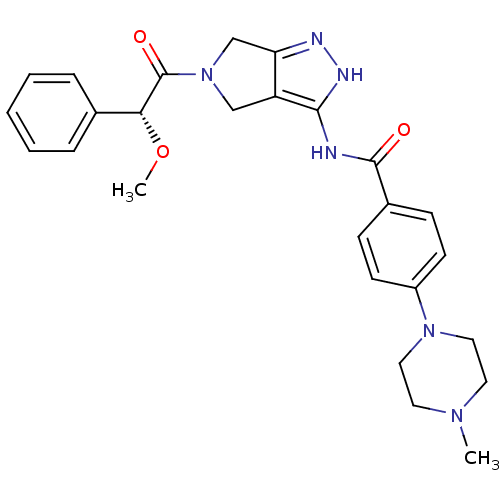 (5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences | Assay Description The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... | J Med Chem 49: 7247-51 (2006) Article DOI: 10.1021/jm060897w BindingDB Entry DOI: 10.7270/Q2NS0S4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 555 total ) | Next | Last >> |