

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against specific binding of [125I]-MIP-1 alpha to human CCR5 receptor | Bioorg Med Chem Lett 11: 265-70 (2001) BindingDB Entry DOI: 10.7270/Q2668CFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141931 ((R)-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141911 ((R)-2-[(2S,3S)-3-{4-[5-(4-Cyano-benzyl)-2-ethyl-2H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141908 ((R)-2-[(2S,3S)-3-{4-[5-(4-tert-Butyl-benzyl)-2-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141905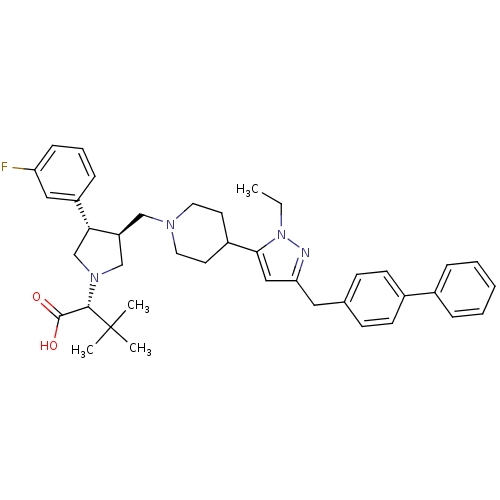 ((R)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141978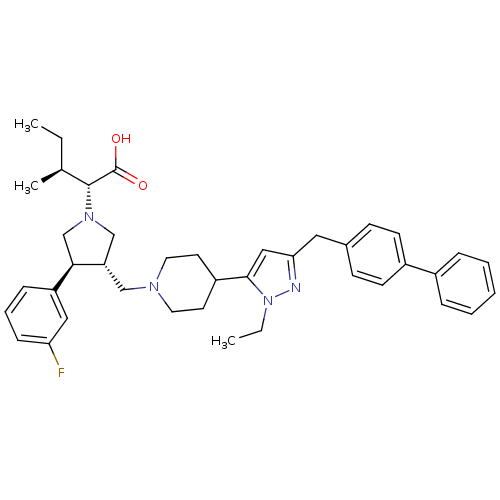 ((2R,4S)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141935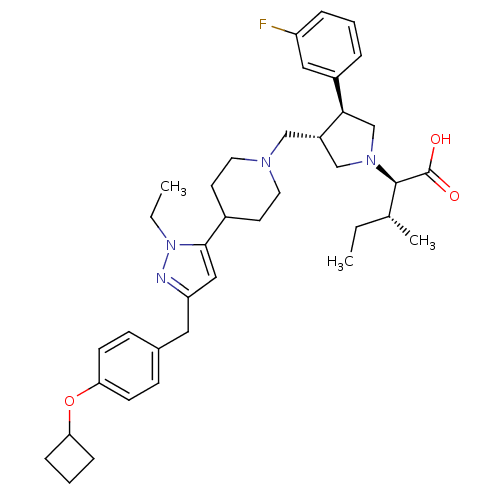 ((2R,4R)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141951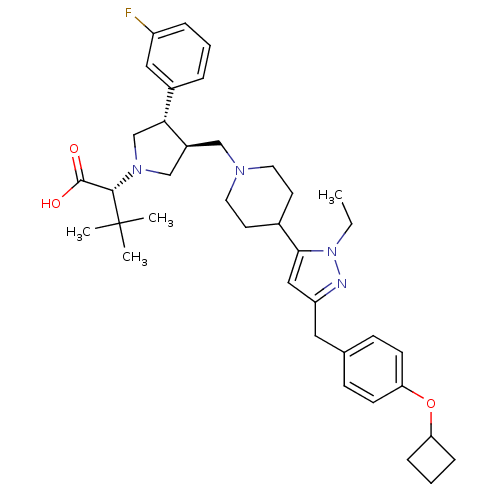 ((R)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-2-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141913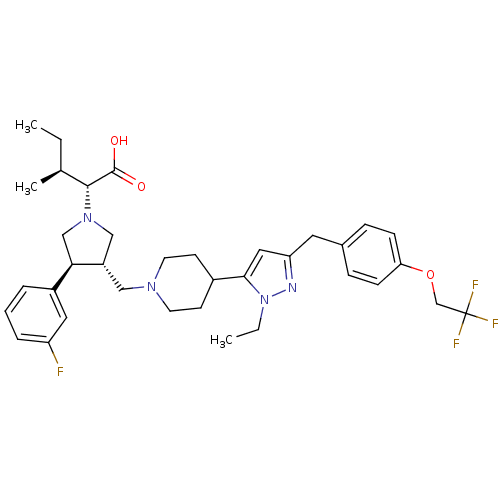 ((2R,4S)-2-[(2S,3S)-3-(4-{2-Ethyl-5-[4-(2,2,2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141941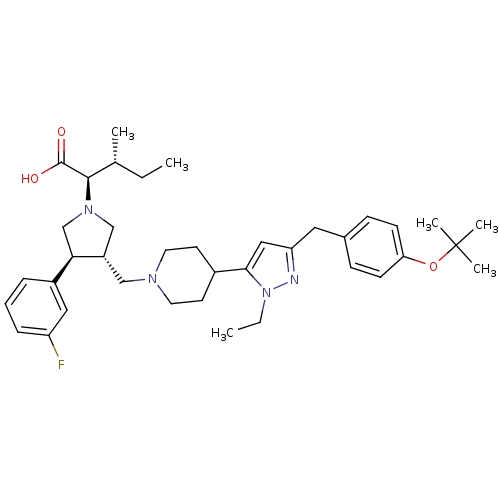 ((2R,4R)-2-[(2S,3S)-3-{4-[5-(4-tert-Butoxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141906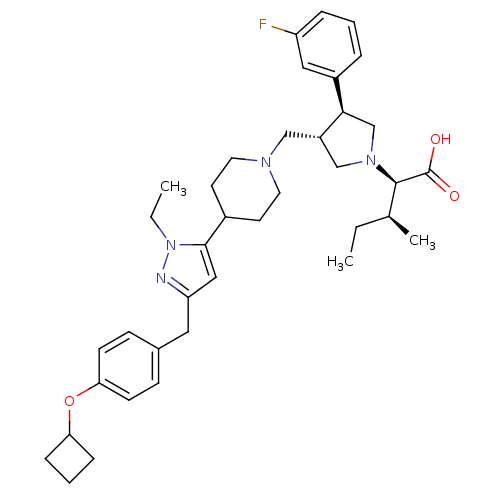 ((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141973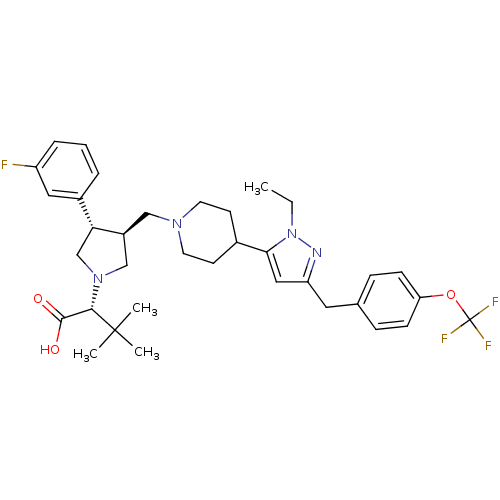 (2-[3-{4-[2-Ethyl-5-(4-trifluoromethoxy-benzyl)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141910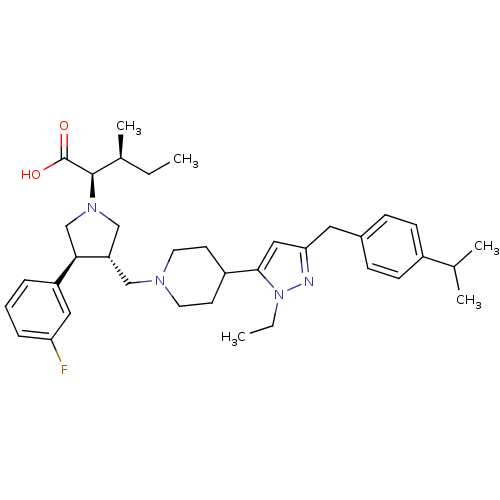 ((2R,4S)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-isopropyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141979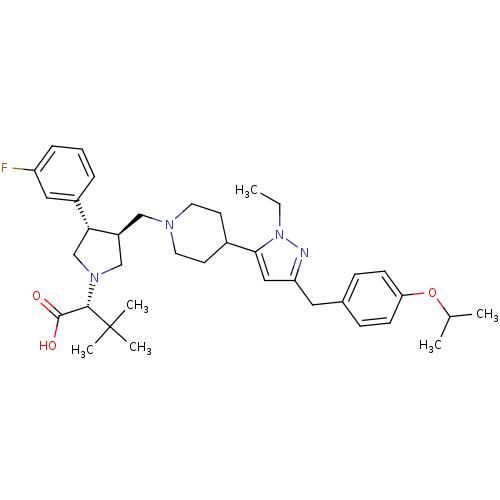 ((R)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-isopropoxy-benzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141984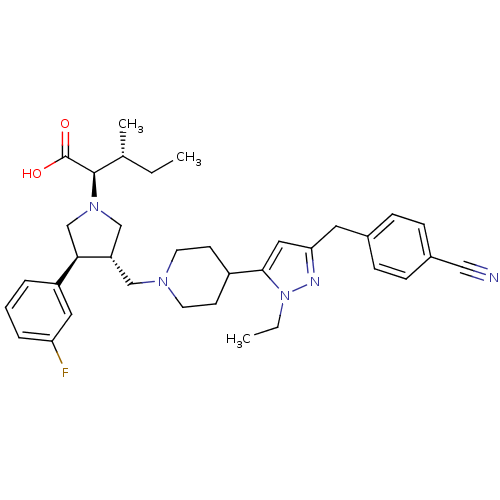 ((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Cyano-benzyl)-2-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141883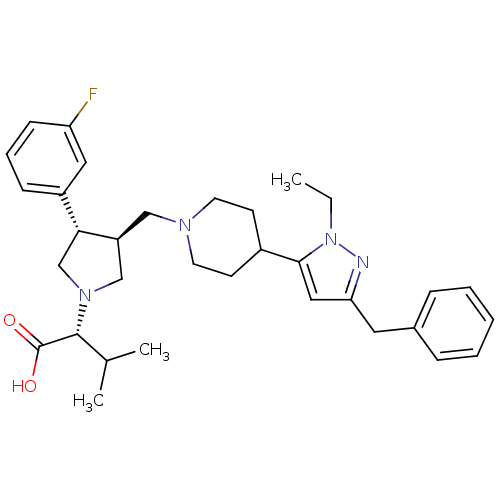 ((R)-2-((3S,4S)-3-((4-(3-benzyl-1-ethyl-1H-pyrazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141972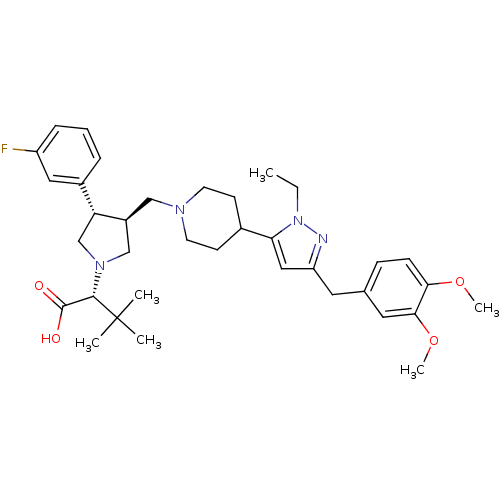 ((R)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-2-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141874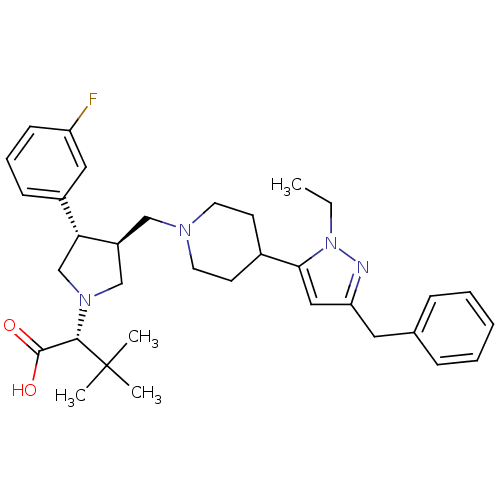 ((R)-2-((3S,4S)-3-((4-(3-benzyl-1-ethyl-1H-pyrazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141945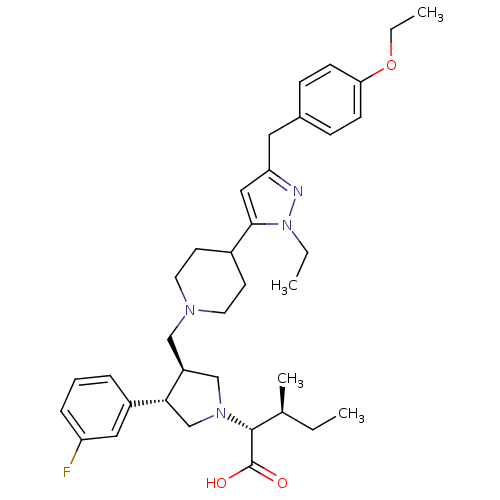 ((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Ethoxy-benzyl)-2-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141914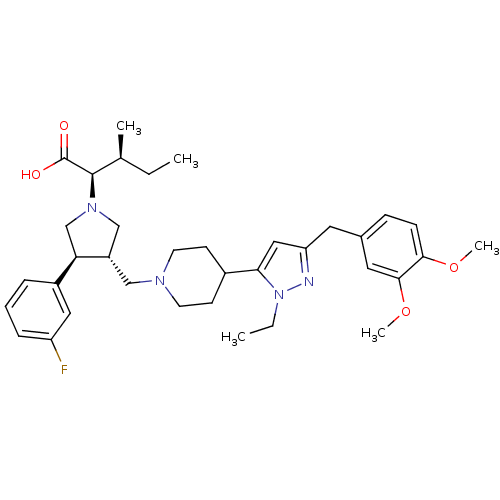 ((2R,4S)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141875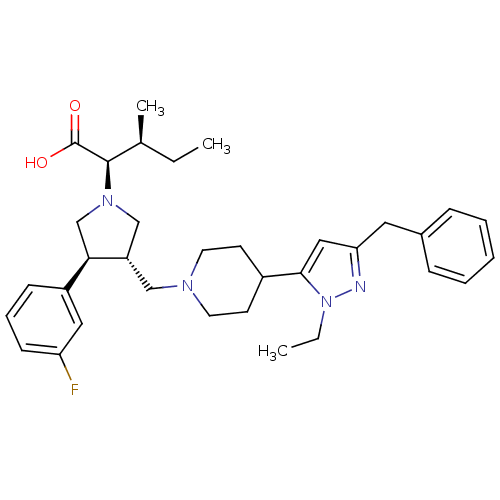 ((2R,3S)-2-[(2S,3S)-3-[4-(5-Benzyl-2-ethyl-2H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50141887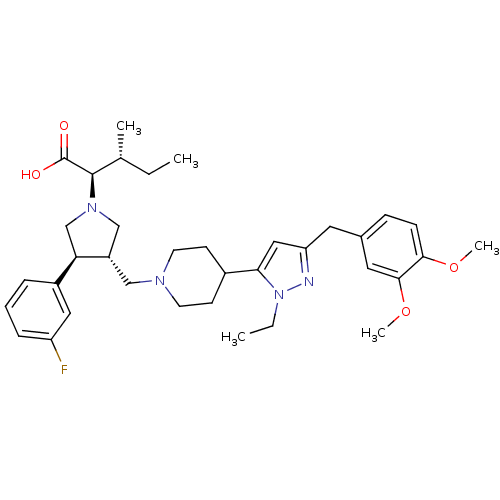 ((2R,4R)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 | Bioorg Med Chem Lett 14: 947-52 (2004) Article DOI: 10.1016/j.bmcl.2003.12.006 BindingDB Entry DOI: 10.7270/Q2F18Z5V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9091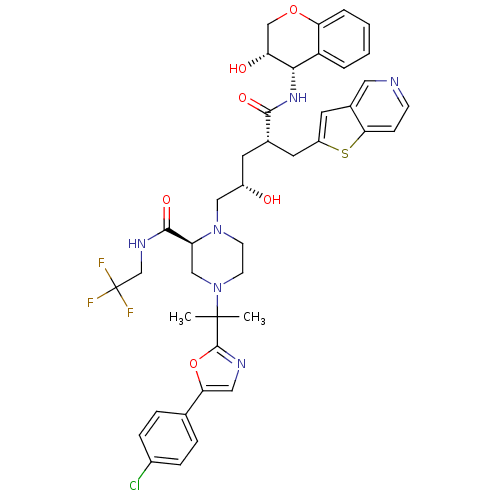 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9097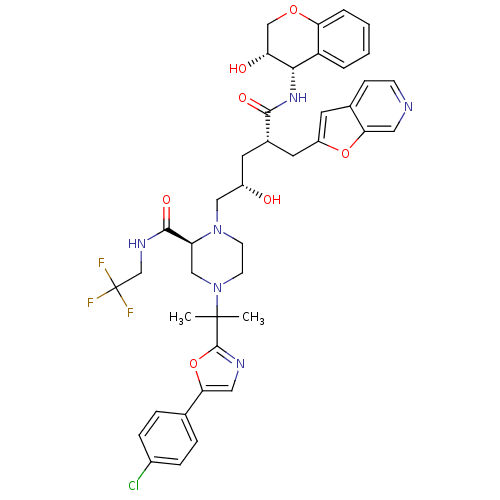 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9098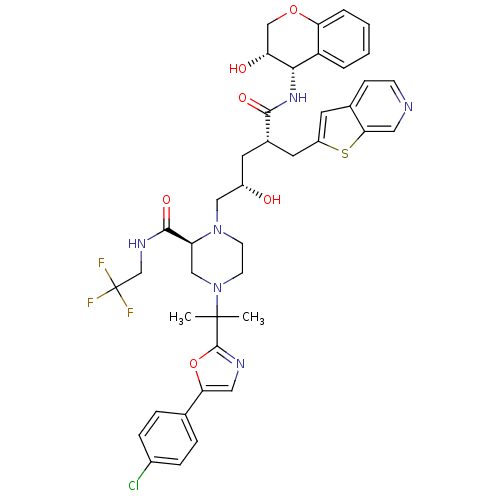 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9099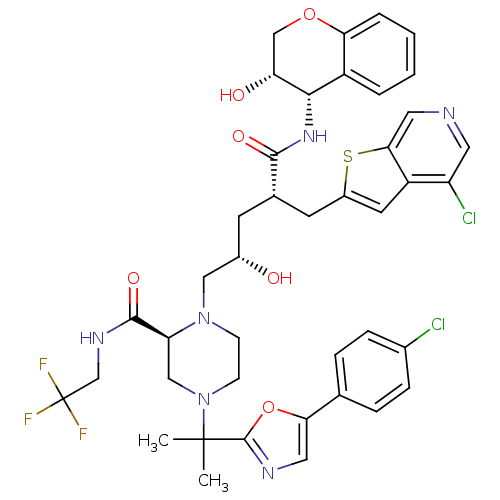 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127969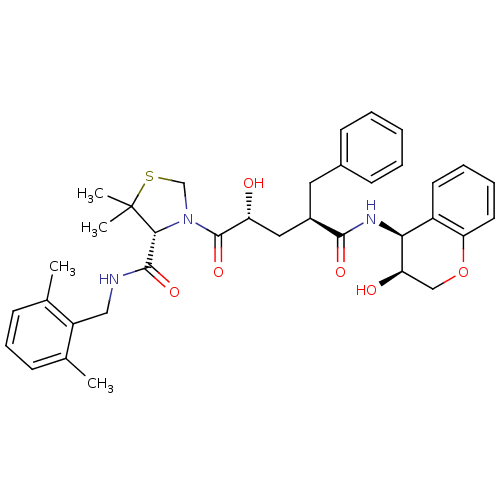 ((R)-3-[(2R,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9093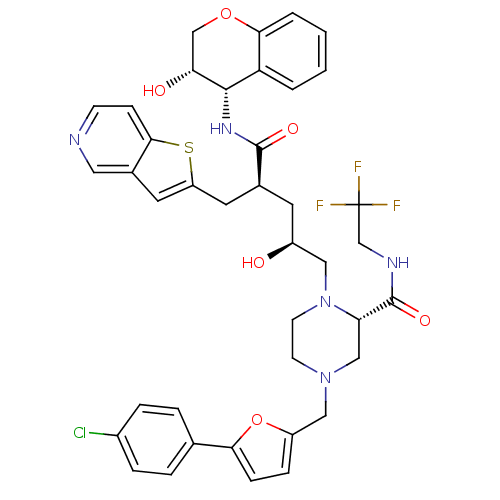 ((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9102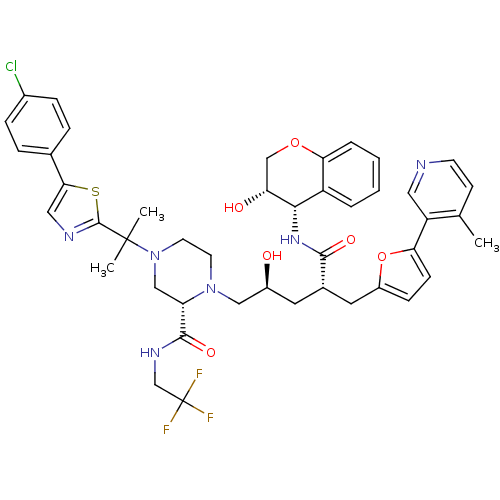 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-thiazol-2-yl]pro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9104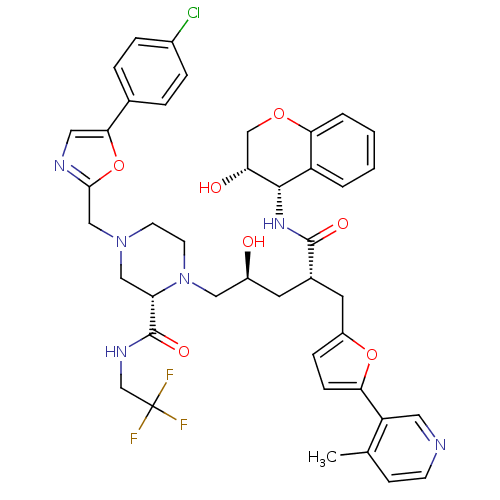 ((2S)-4-{[5-(4-chlorophenyl)-1,3-oxazol-2-yl]methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127974 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9092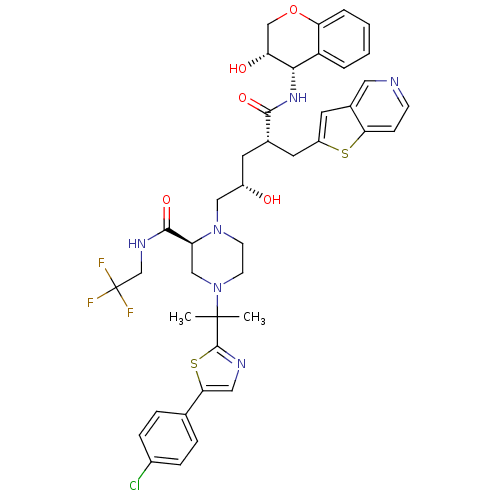 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-thiazol-2-yl]pro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9103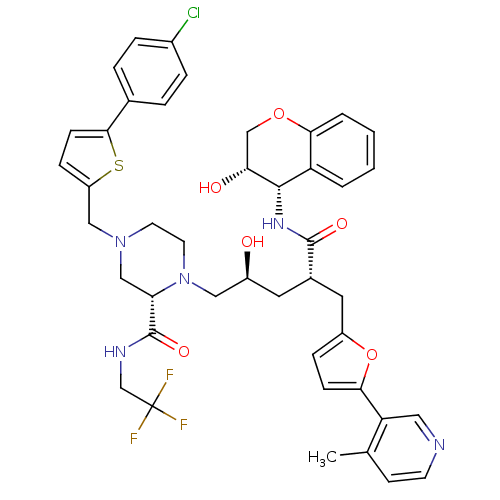 ((2S)-4-{[5-(4-chlorophenyl)thiophen-2-yl]methyl}-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127970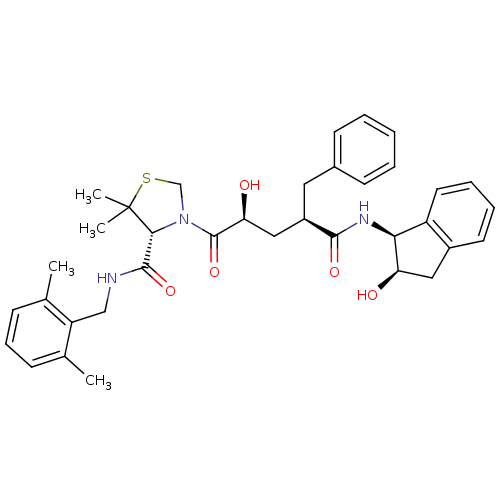 (3-[(1R,4S)-2-Hydroxy-4-((1S,2R)-2-hydroxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127976 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9094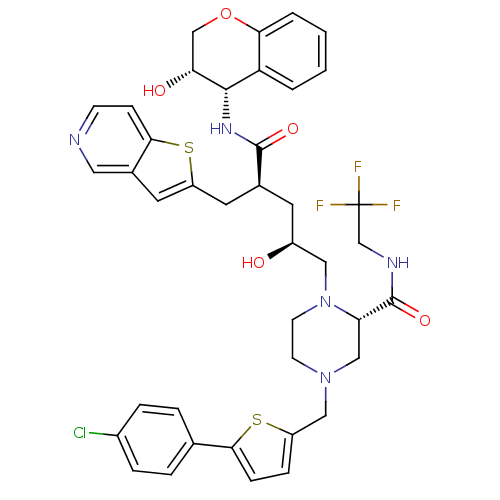 ((2S)-4-{[5-(4-chlorophenyl)thiophen-2-yl]methyl}-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9096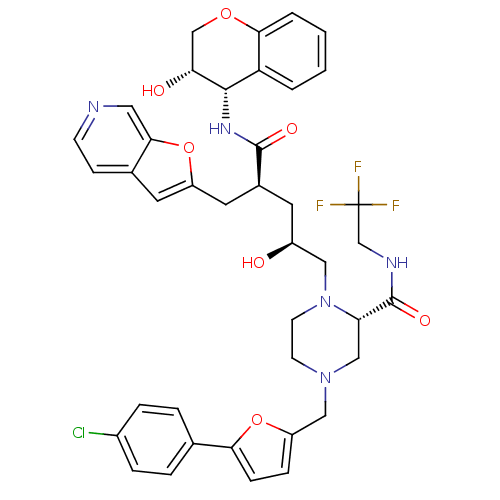 ((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127977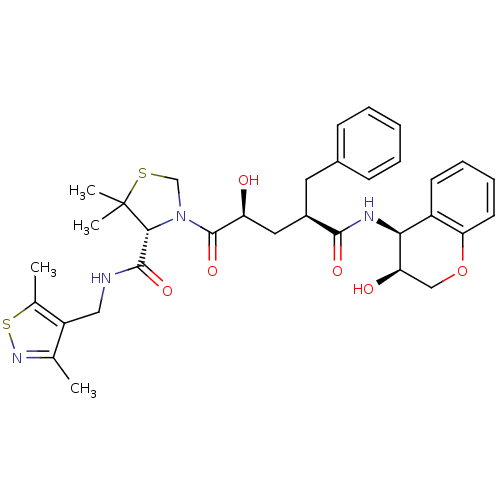 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50121838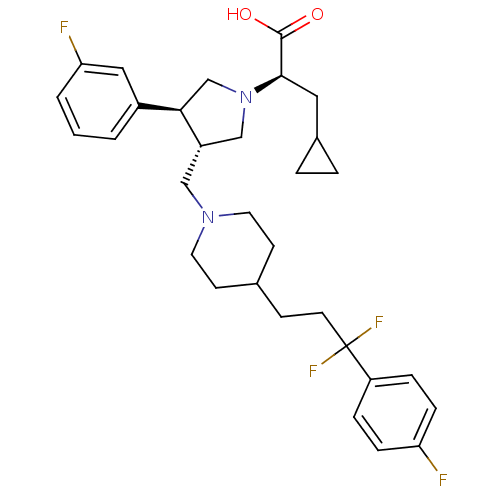 ((R)-3-cyclopropyl-2-((3S,4S)-3-((4-(3,3-difluoro-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127970 (3-[(1R,4S)-2-Hydroxy-4-((1S,2R)-2-hydroxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127970 (3-[(1R,4S)-2-Hydroxy-4-((1S,2R)-2-hydroxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9095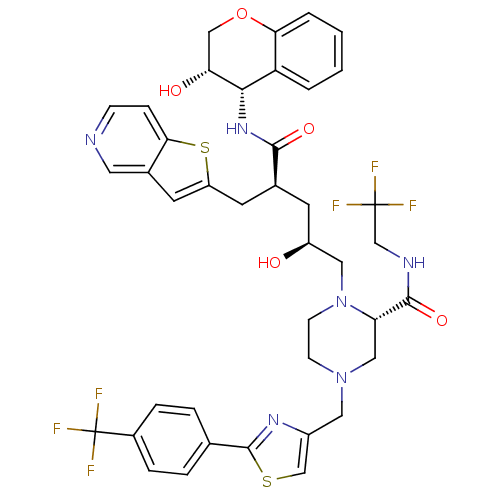 ((2S)-1-[(2S,4S)-2-hydroxy-4-{[(3S,4S)-3-hydroxy-3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127972 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127968 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9101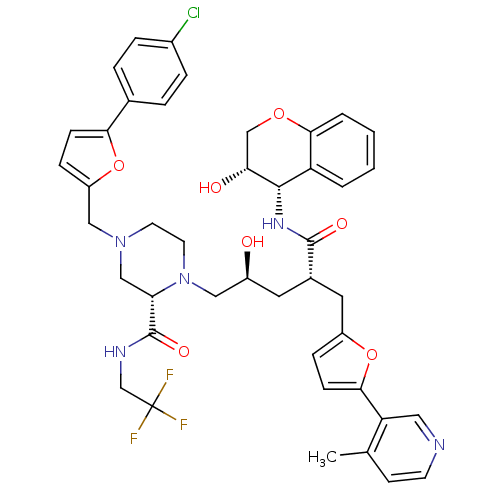 ((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127969 ((R)-3-[(2R,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127974 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119338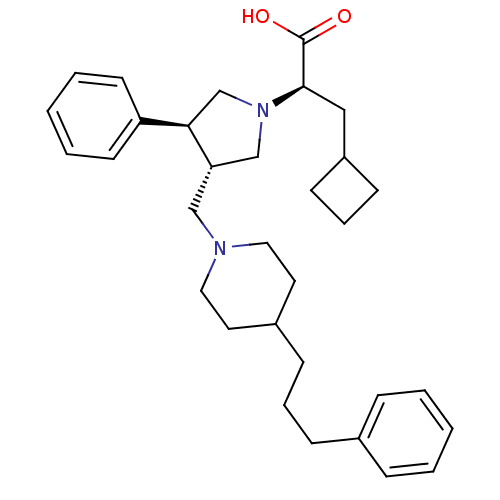 ((R)-3-Cyclobutyl-2-{(3S,4S)-3-phenyl-4-[4-(3-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1 alpha from human C-C chemokine receptor type 5 expressed on CHO cell membranes | Bioorg Med Chem Lett 15: 2129-34 (2005) Article DOI: 10.1016/j.bmcl.2005.02.030 BindingDB Entry DOI: 10.7270/Q21C1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127969 ((R)-3-[(2R,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50119338 ((R)-3-Cyclobutyl-2-{(3S,4S)-3-phenyl-4-[4-(3-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to displace [125I]- labeled MIP-1alpha from CCR5 receptor expressed on CHO cell membrane | Bioorg Med Chem Lett 13: 119-23 (2002) BindingDB Entry DOI: 10.7270/Q2CN7382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 835 total ) | Next | Last >> |