

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384793 (CHEMBL2037384) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50541071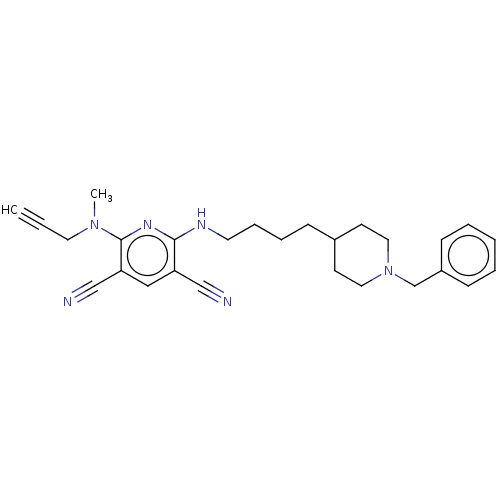 (CHEMBL4528766) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50541069 (CHEMBL4635389) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of rat MAO B using kynuramine as substrate preincubated for 30 mins followed by substrate addition | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50359391 (CHEMBL1929421) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of human MAO A | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50541052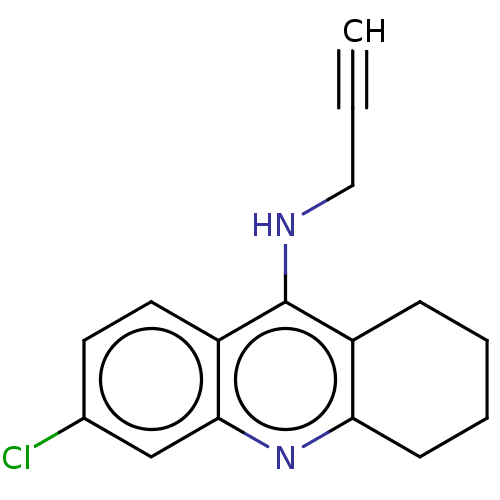 (CHEMBL4632951) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 min inter... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50541052 (CHEMBL4632951) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 mi... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 mi... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50541051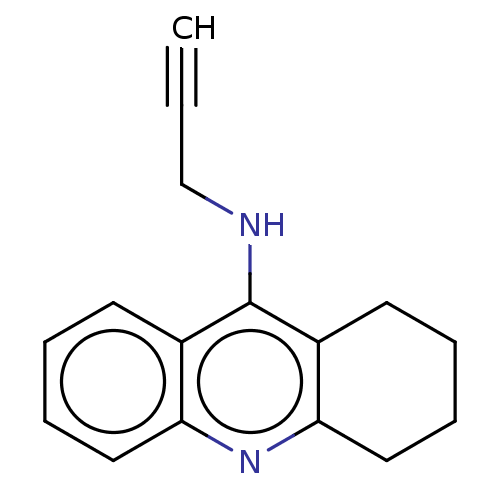 (CHEMBL4633540) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 min inter... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 mi... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50541051 (CHEMBL4633540) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 mi... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384793 (CHEMBL2037384) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50541051 (CHEMBL4633540) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 mi... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50541052 (CHEMBL4632951) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 mi... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50359391 (CHEMBL1929421) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of human MAO B | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50541068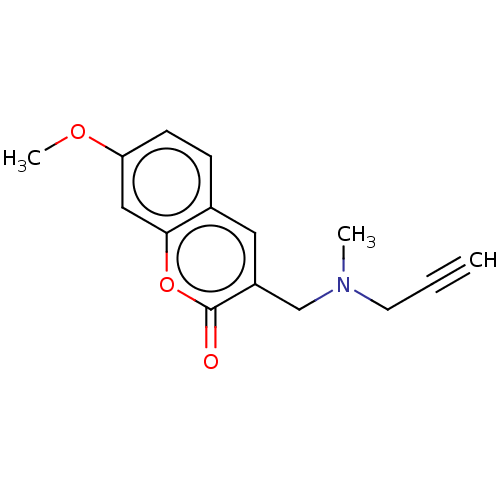 (CHEMBL4648667) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of rat MAO B using kynuramine as substrate preincubated for 30 mins followed by substrate addition | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50541054 (CHEMBL4637721) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 mi... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384792 (CHEMBL2037385) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50541064 (CHEMBL4635698) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50541065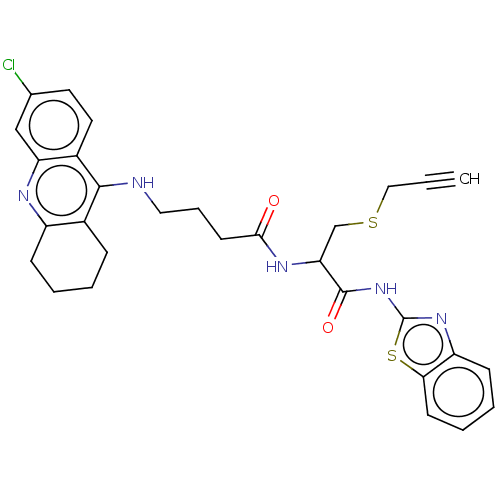 (CHEMBL4633417) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50541061 (CHEMBL4635342) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50541063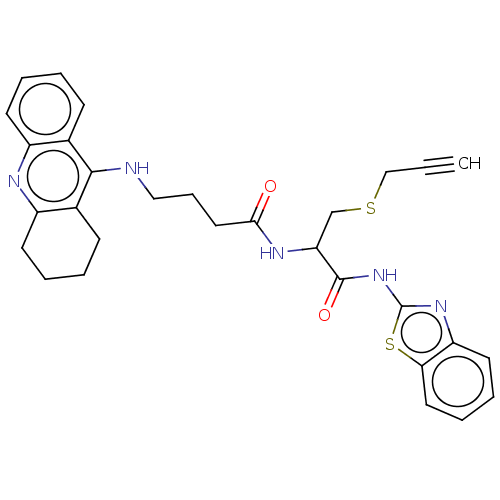 (CHEMBL4637185) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50541066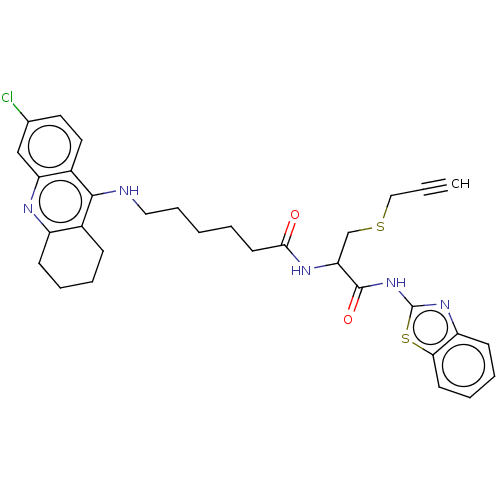 (CHEMBL4641182) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50541059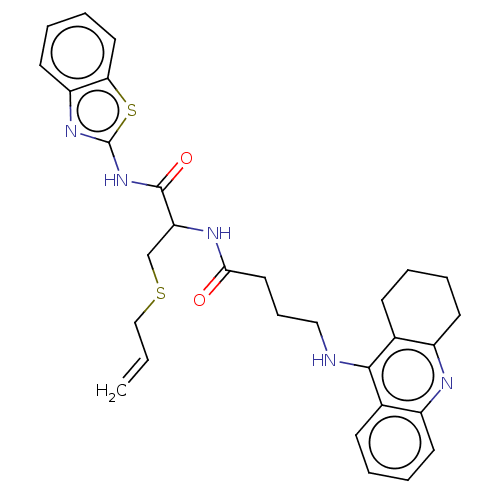 (CHEMBL4648566) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50541060 (CHEMBL4642573) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50541062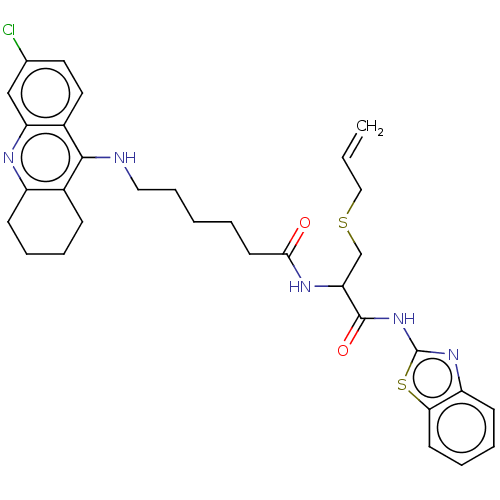 (CHEMBL4640059) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50541057 (CHEMBL4639993) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50541055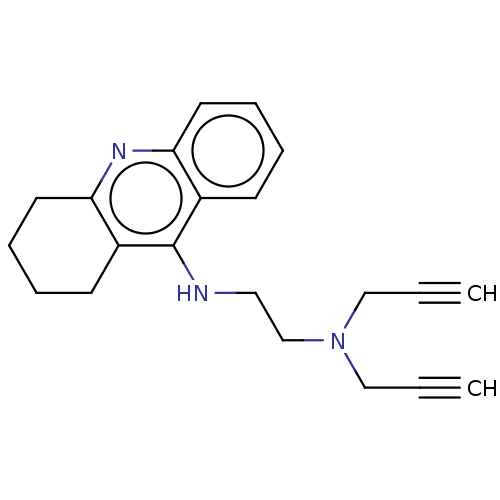 (CHEMBL4647462) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 339 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 mi... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50541055 (CHEMBL4647462) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 353 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 mi... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50541067 (CHEMBL4638612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 377 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of rat MAO B using kynuramine as substrate preincubated for 30 mins followed by substrate addition | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50541058 (CHEMBL4639503) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384794 (CHEMBL2037380) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50541071 (CHEMBL4528766) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384796 (CHEMBL2037382) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50541068 (CHEMBL4648667) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 669 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of rat MAO A using kynuramine as substrate preincubated for 30 mins followed by substrate addition | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50359391 (CHEMBL1929421) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of human AChE | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50541056 (CHEMBL4633036) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50541053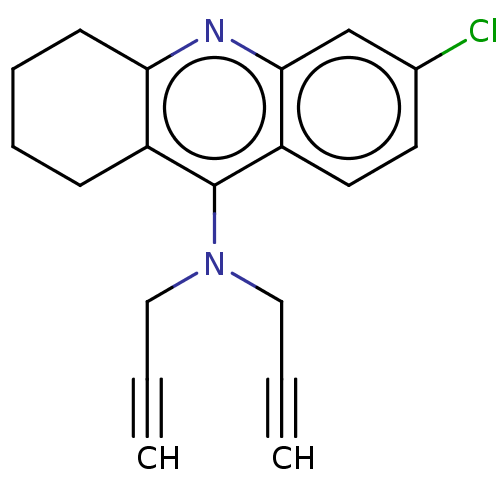 (CHEMBL4642125) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 884 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 mi... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50541050 (CHEMBL4647757) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured ... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384795 (CHEMBL2037381) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50359391 (CHEMBL1929421) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of human BuChE | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50541070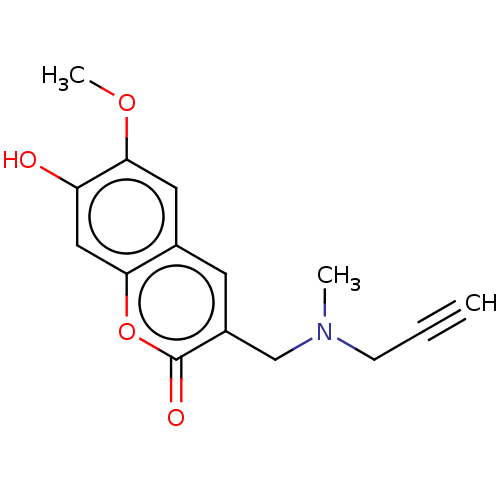 (CHEMBL4647954) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50541068 (CHEMBL4648667) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50541067 (CHEMBL4638612) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50541071 (CHEMBL4528766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of human MAO B | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50541053 (CHEMBL4642125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride as substrate peincubated for 15 mins followed by substrate addition measured at 1 mi... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126880 BindingDB Entry DOI: 10.7270/Q2639T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384796 (CHEMBL2037382) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50384793 (CHEMBL2037384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 57 total ) | Next | Last >> |