 Found 3404 hits with Last Name = 'carruthers' and Initial = 'ni'
Found 3404 hits with Last Name = 'carruthers' and Initial = 'ni' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209809
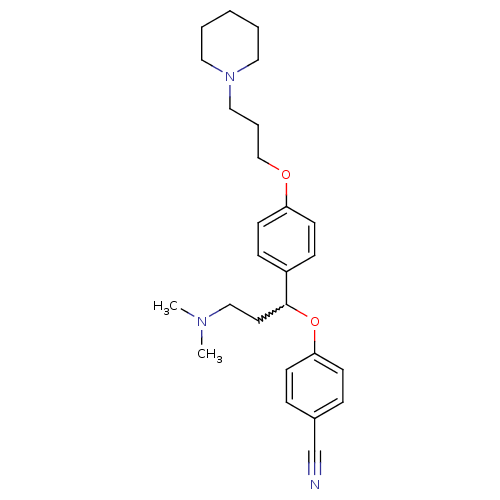
(4-(3-(dimethylamino)-1-(4-(3-(piperidin-1-yl)propo...)Show SMILES CN(C)CCC(Oc1ccc(cc1)C#N)c1ccc(OCCCN2CCCCC2)cc1 |w:5.4| Show InChI InChI=1S/C26H35N3O2/c1-28(2)19-15-26(31-25-11-7-22(21-27)8-12-25)23-9-13-24(14-10-23)30-20-6-18-29-16-4-3-5-17-29/h7-14,26H,3-6,15-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50346209
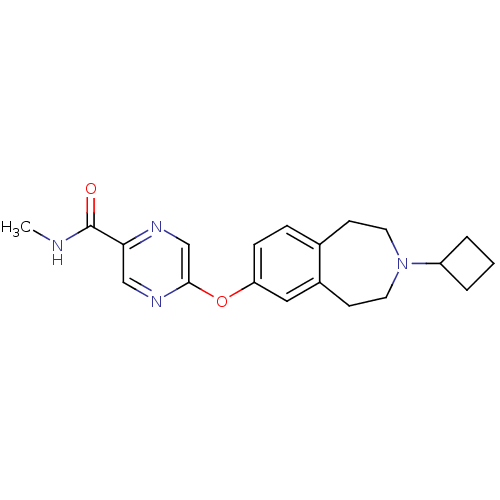
(5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C20H24N4O2/c1-21-20(25)18-12-23-19(13-22-18)26-17-6-5-14-7-9-24(16-3-2-4-16)10-8-15(14)11-17/h5-6,11-13,16H,2-4,7-10H2,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217586
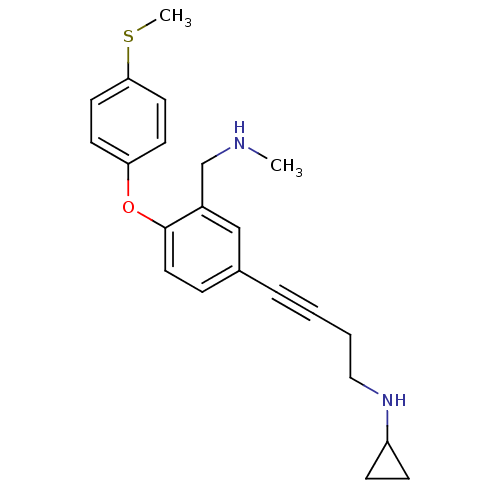
(CHEMBL442080 | N-(4-(3-((methylamino)methyl)-4-(4-...)Show InChI InChI=1S/C22H26N2OS/c1-23-16-18-15-17(5-3-4-14-24-19-7-8-19)6-13-22(18)25-20-9-11-21(26-2)12-10-20/h6,9-13,15,19,23-24H,4,7-8,14,16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50371662
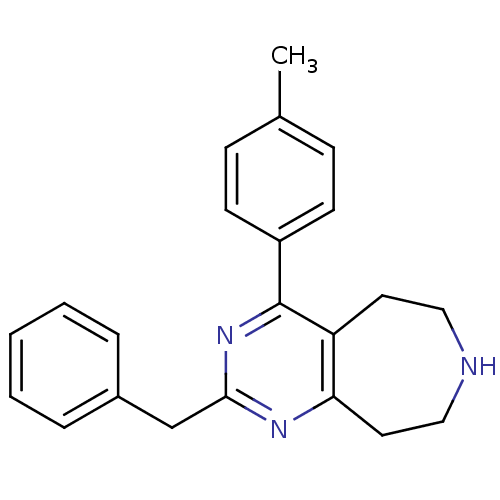
(CHEMBL269974)Show InChI InChI=1S/C22H23N3/c1-16-7-9-18(10-8-16)22-19-11-13-23-14-12-20(19)24-21(25-22)15-17-5-3-2-4-6-17/h2-10,23H,11-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 18: 2103-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.090
BindingDB Entry DOI: 10.7270/Q2571CW5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217575
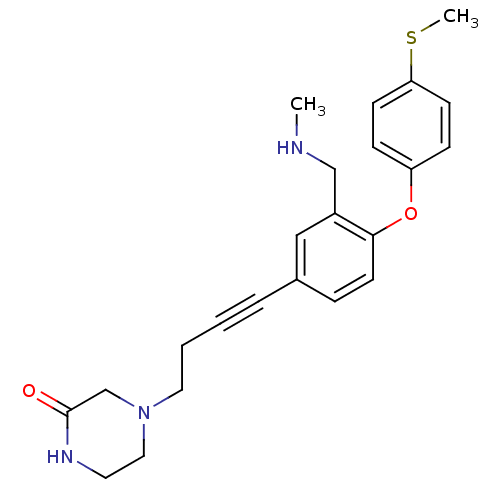
(4-(4-(3-((methylamino)methyl)-4-(4-(methylthio)phe...)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C#CCCN1CCNC(=O)C1 Show InChI InChI=1S/C23H27N3O2S/c1-24-16-19-15-18(5-3-4-13-26-14-12-25-23(27)17-26)6-11-22(19)28-20-7-9-21(29-2)10-8-20/h6-11,15,24H,4,12-14,16-17H2,1-2H3,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217584

((5-(4-(4-isopropylpiperazin-1-yl)butyl)-2-(4-(meth...)Show SMILES CNCc1cc(CCCCN2CCN(CC2)C(C)C)ccc1Oc1ccc(SC)cc1 Show InChI InChI=1S/C26H39N3OS/c1-21(2)29-17-15-28(16-18-29)14-6-5-7-22-8-13-26(23(19-22)20-27-3)30-24-9-11-25(31-4)12-10-24/h8-13,19,21,27H,5-7,14-18,20H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110
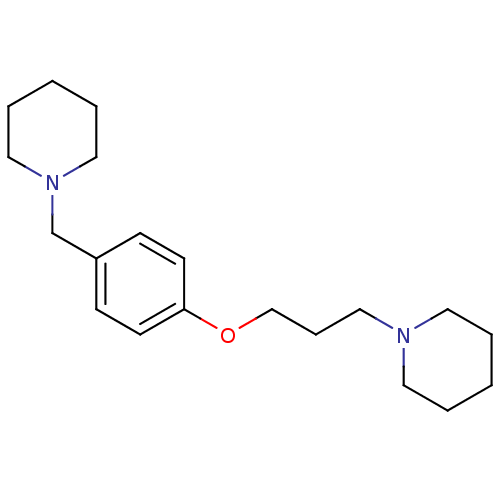
(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Bioorg Med Chem Lett 16: 897-900 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.003
BindingDB Entry DOI: 10.7270/Q2H131M7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50410342
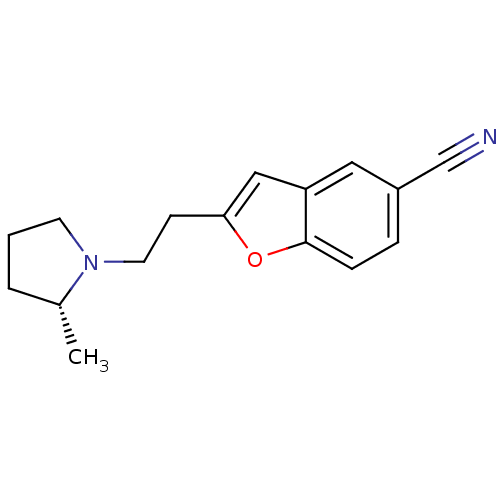
(CHEMBL195408)Show InChI InChI=1S/C16H18N2O/c1-12-3-2-7-18(12)8-6-15-10-14-9-13(11-17)4-5-16(14)19-15/h4-5,9-10,12H,2-3,6-8H2,1H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Mean binding affinity for human H3 receptor |
J Med Chem 48: 2229-38 (2005)
Article DOI: 10.1021/jm049212n
BindingDB Entry DOI: 10.7270/Q2GB258T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50139391
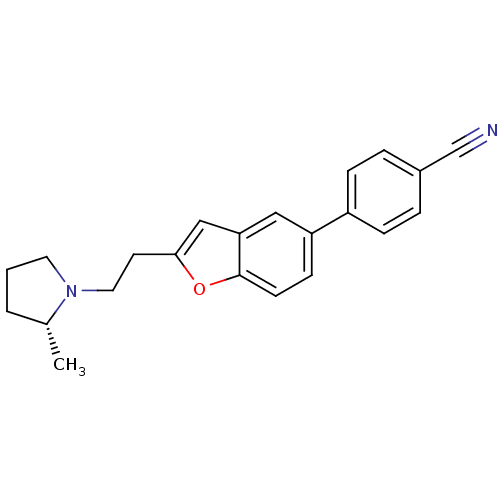
((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H22N2O/c1-16-3-2-11-24(16)12-10-21-14-20-13-19(8-9-22(20)25-21)18-6-4-17(15-23)5-7-18/h4-9,13-14,16H,2-3,10-12H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50028059
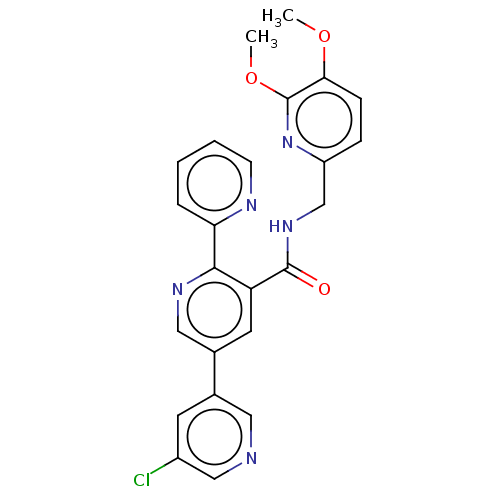
(CHEMBL3338866)Show SMILES COc1ccc(CNC(=O)c2cc(cnc2-c2ccccn2)-c2cncc(Cl)c2)nc1OC Show InChI InChI=1S/C24H20ClN5O3/c1-32-21-7-6-18(30-24(21)33-2)14-29-23(31)19-10-16(15-9-17(25)13-26-11-15)12-28-22(19)20-5-3-4-8-27-20/h3-13H,14H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-((3H)-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2 rec... |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50177731
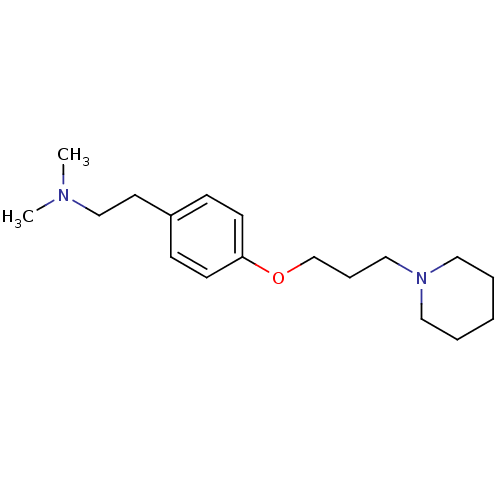
(CHEMBL204872 | dimethyl-{2-[4-(3-piperidin-1-yl-pr...)Show InChI InChI=1S/C18H30N2O/c1-19(2)15-11-17-7-9-18(10-8-17)21-16-6-14-20-12-4-3-5-13-20/h7-10H,3-6,11-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Bioorg Med Chem Lett 16: 897-900 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.003
BindingDB Entry DOI: 10.7270/Q2H131M7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217593
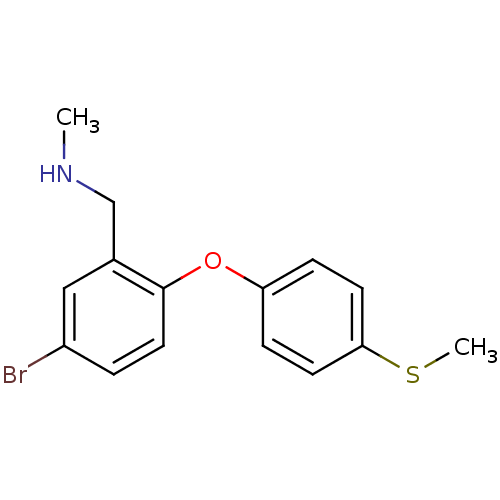
((5-bromo-2-(4-(methylthio)phenoxy)phenyl)-N-methyl...)Show InChI InChI=1S/C15H16BrNOS/c1-17-10-11-9-12(16)3-8-15(11)18-13-4-6-14(19-2)7-5-13/h3-9,17H,10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371305
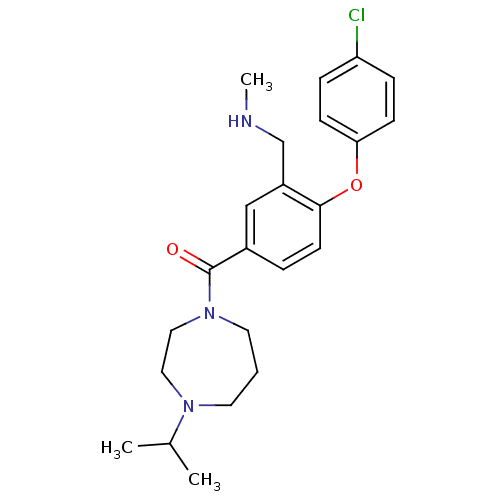
(CHEMBL272077)Show SMILES CNCc1cc(ccc1Oc1ccc(Cl)cc1)C(=O)N1CCCN(CC1)C(C)C Show InChI InChI=1S/C23H30ClN3O2/c1-17(2)26-11-4-12-27(14-13-26)23(28)18-5-10-22(19(15-18)16-25-3)29-21-8-6-20(24)7-9-21/h5-10,15,17,25H,4,11-14,16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217592
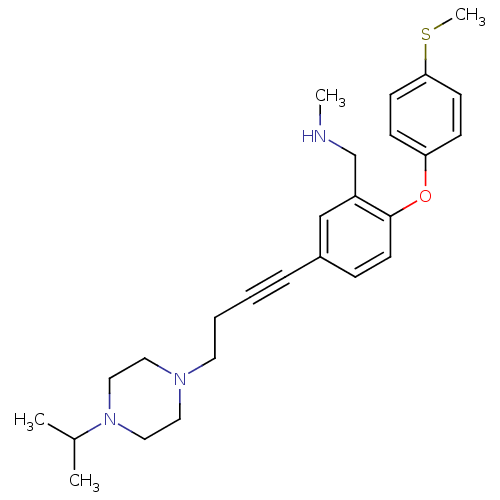
((5-(4-(4-isopropylpiperazin-1-yl)but-1-ynyl)-2-(4-...)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C#CCCN1CCN(CC1)C(C)C Show InChI InChI=1S/C26H35N3OS/c1-21(2)29-17-15-28(16-18-29)14-6-5-7-22-8-13-26(23(19-22)20-27-3)30-24-9-11-25(31-4)12-10-24/h8-13,19,21,27H,6,14-18,20H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50200636
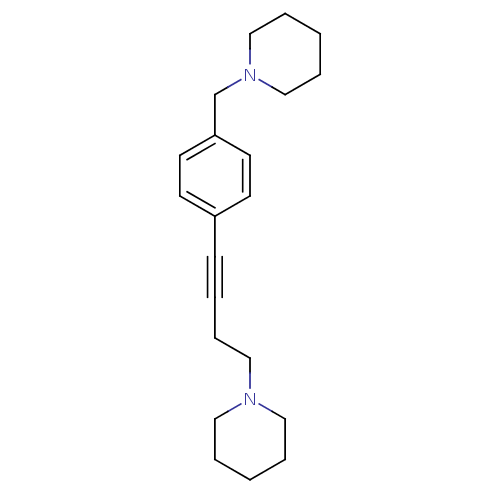
(1-(4-(4-(piperidin-1-ylmethyl)phenyl)but-3-ynyl)pi...)Show InChI InChI=1S/C21H30N2/c1-4-14-22(15-5-1)16-8-3-9-20-10-12-21(13-11-20)19-23-17-6-2-7-18-23/h10-13H,1-2,4-8,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414797
(CHEMBL582977)Show InChI InChI=1S/C22H31N3O/c23-22(26)21-10-15-25(16-11-21)18-20-9-6-8-19(17-20)7-2-5-14-24-12-3-1-4-13-24/h6,8-9,17,21H,1,3-5,10-16,18H2,(H2,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development, LLC
Curated by PDSP Ki Database
| |
Br J Pharmacol 143: 649-61 (2004)
Article DOI: 10.1038/sj.bjp.0705964
BindingDB Entry DOI: 10.7270/Q2319TF2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217590
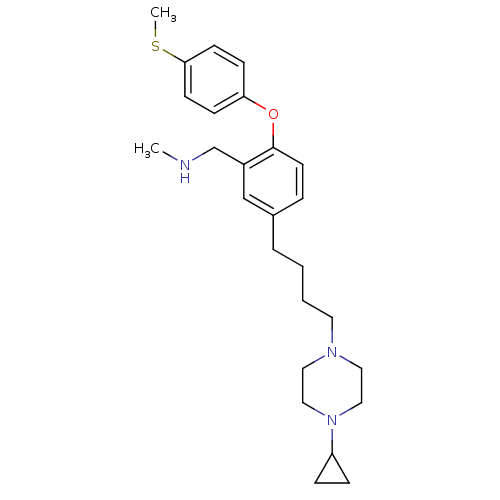
((5-(4-(4-cyclopropylpiperazin-1-yl)butyl)-2-(4-(me...)Show SMILES CNCc1cc(CCCCN2CCN(CC2)C2CC2)ccc1Oc1ccc(SC)cc1 Show InChI InChI=1S/C26H37N3OS/c1-27-20-22-19-21(6-13-26(22)30-24-9-11-25(31-2)12-10-24)5-3-4-14-28-15-17-29(18-16-28)23-7-8-23/h6,9-13,19,23,27H,3-5,7-8,14-18,20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209802
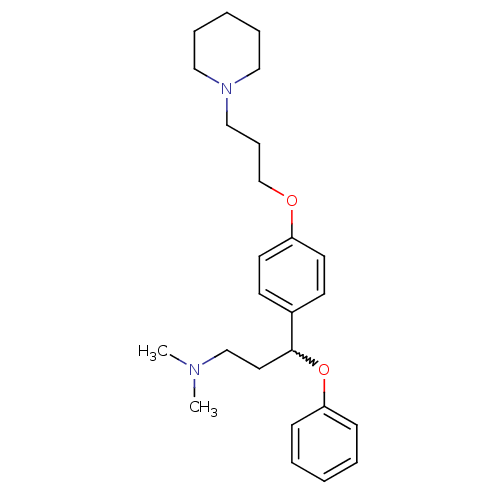
(CHEMBL438490 | N,N-dimethyl-3-phenoxy-3-(4-(3-(pip...)Show SMILES CN(C)CCC(Oc1ccccc1)c1ccc(OCCCN2CCCCC2)cc1 |w:5.5| Show InChI InChI=1S/C25H36N2O2/c1-26(2)20-16-25(29-24-10-5-3-6-11-24)22-12-14-23(15-13-22)28-21-9-19-27-17-7-4-8-18-27/h3,5-6,10-15,25H,4,7-9,16-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217579

((5-(4-(4-fluoropiperidin-1-yl)but-1-ynyl)-2-(4-(me...)Show InChI InChI=1S/C24H29FN2OS/c1-26-18-20-17-19(5-3-4-14-27-15-12-21(25)13-16-27)6-11-24(20)28-22-7-9-23(29-2)10-8-22/h6-11,17,21,26H,4,12-16,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodoproxyfan from human recombinant histamine H3 receptor expressed in human SK-N-MC cells after 1 hr by fluid scintillation co... |
Bioorg Med Chem Lett 20: 6226-30 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.103
BindingDB Entry DOI: 10.7270/Q2CF9QB3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50371682
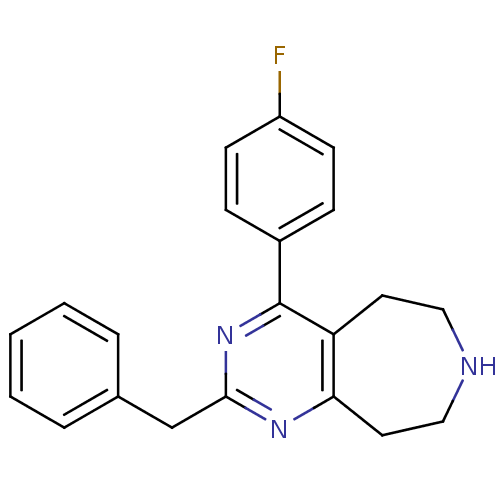
(CHEMBL270188)Show InChI InChI=1S/C21H20FN3/c22-17-8-6-16(7-9-17)21-18-10-12-23-13-11-19(18)24-20(25-21)14-15-4-2-1-3-5-15/h1-9,23H,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 18: 2103-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.090
BindingDB Entry DOI: 10.7270/Q2571CW5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414804
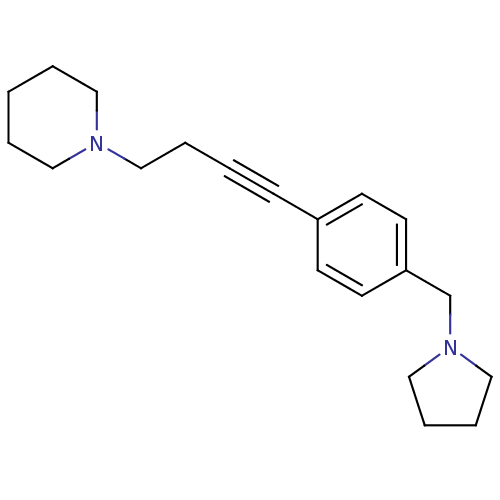
(CHEMBL574712)Show InChI InChI=1S/C20H28N2/c1-3-13-21(14-4-1)15-5-2-8-19-9-11-20(12-10-19)18-22-16-6-7-17-22/h9-12H,1,3-7,13-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50410347
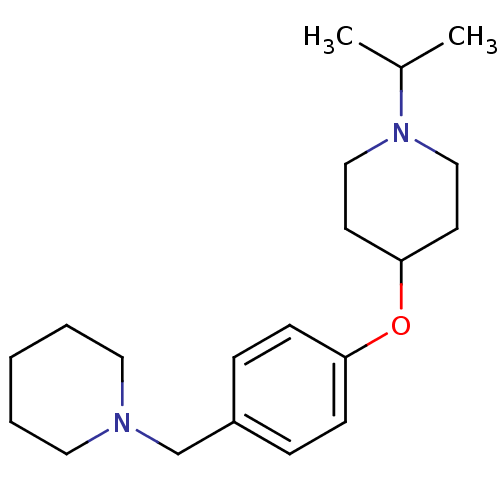
(CHEMBL194441)Show InChI InChI=1S/C20H32N2O/c1-17(2)22-14-10-20(11-15-22)23-19-8-6-18(7-9-19)16-21-12-4-3-5-13-21/h6-9,17,20H,3-5,10-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Mean binding affinity for human H3 receptor |
J Med Chem 48: 2229-38 (2005)
Article DOI: 10.1021/jm049212n
BindingDB Entry DOI: 10.7270/Q2GB258T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414811
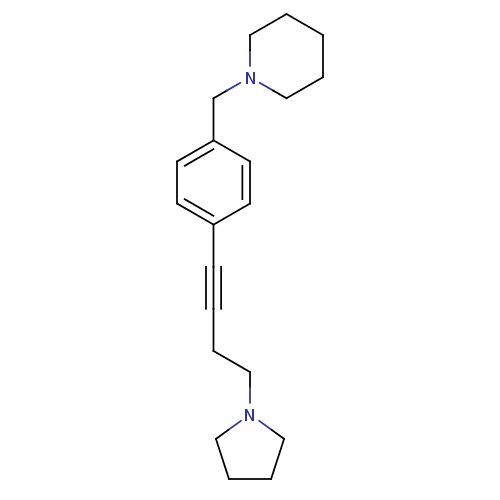
(CHEMBL574721)Show InChI InChI=1S/C20H28N2/c1-3-16-22(17-4-1)18-20-11-9-19(10-12-20)8-2-5-13-21-14-6-7-15-21/h9-12H,1,3-7,13-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50089369
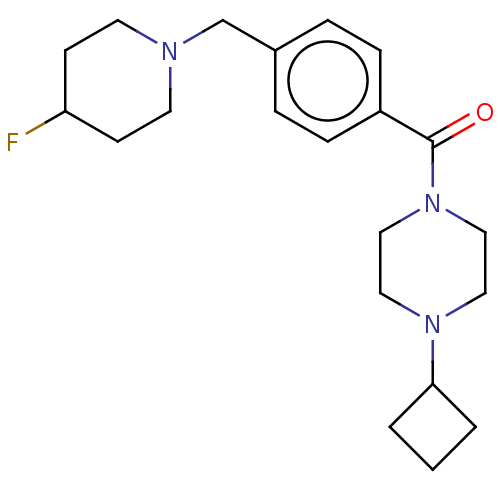
(CHEMBL3577959)Show SMILES FC1CCN(Cc2ccc(cc2)C(=O)N2CCN(CC2)C2CCC2)CC1 Show InChI InChI=1S/C21H30FN3O/c22-19-8-10-23(11-9-19)16-17-4-6-18(7-5-17)21(26)25-14-12-24(13-15-25)20-2-1-3-20/h4-7,19-20H,1-3,8-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from human histamine H3 receptor expressed in human SK-N-MC cells after 45 mins |
ACS Med Chem Lett 6: 450-4 (2015)
Article DOI: 10.1021/ml5005156
BindingDB Entry DOI: 10.7270/Q22F7Q59 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50206229

(3-(9-(3-(piperidin-1-yl)propoxy)-1,2,3,5,6,10b-hex...)Show SMILES Oc1cccc(c1)C1CN2CCCC2c2cc(OCCCN3CCCCC3)ccc12 |w:13.13,7.7| Show InChI InChI=1S/C26H34N2O2/c29-21-8-4-7-20(17-21)25-19-28-15-5-9-26(28)24-18-22(10-11-23(24)25)30-16-6-14-27-12-2-1-3-13-27/h4,7-8,10-11,17-18,25-26,29H,1-3,5-6,9,12-16,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in SK-N-MC cells assessed as effect on cAMP accumulation |
Bioorg Med Chem Lett 17: 2603-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.106
BindingDB Entry DOI: 10.7270/Q2HH6JR6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50216250
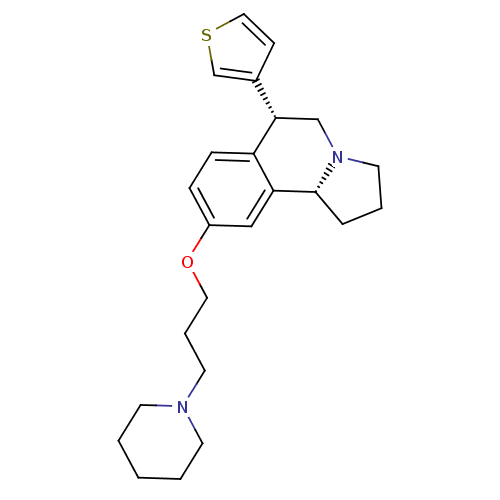
((6R,10bR)-9-(3-(piperidin-1-yl)propoxy)-6-(thiophe...)Show SMILES C(COc1ccc2[C@@H](CN3CCC[C@@H]3c2c1)c1ccsc1)CN1CCCCC1 Show InChI InChI=1S/C24H32N2OS/c1-2-10-25(11-3-1)12-5-14-27-20-7-8-21-22(16-20)24-6-4-13-26(24)17-23(21)19-9-15-28-18-19/h7-9,15-16,18,23-24H,1-6,10-14,17H2/t23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 17: 4374-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.043
BindingDB Entry DOI: 10.7270/Q22Z157G |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50216250

((6R,10bR)-9-(3-(piperidin-1-yl)propoxy)-6-(thiophe...)Show SMILES C(COc1ccc2[C@@H](CN3CCC[C@@H]3c2c1)c1ccsc1)CN1CCCCC1 Show InChI InChI=1S/C24H32N2OS/c1-2-10-25(11-3-1)12-5-14-27-20-7-8-21-22(16-20)24-6-4-13-26(24)17-23(21)19-9-15-28-18-19/h7-9,15-16,18,23-24H,1-6,10-14,17H2/t23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4374-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.043
BindingDB Entry DOI: 10.7270/Q22Z157G |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50217572
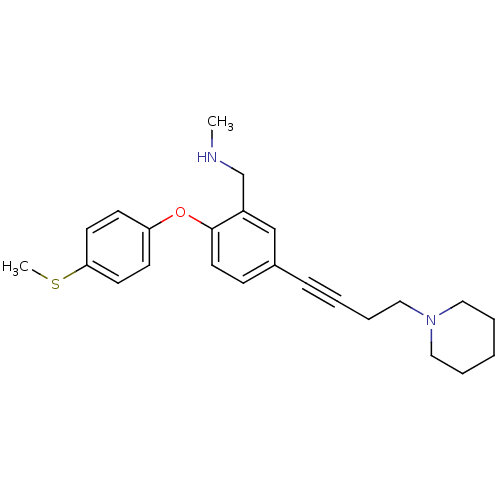
(CHEMBL393036 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C24H30N2OS/c1-25-19-21-18-20(8-4-7-17-26-15-5-3-6-16-26)9-14-24(21)27-22-10-12-23(28-2)13-11-22/h9-14,18,25H,3,5-7,15-17,19H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217565
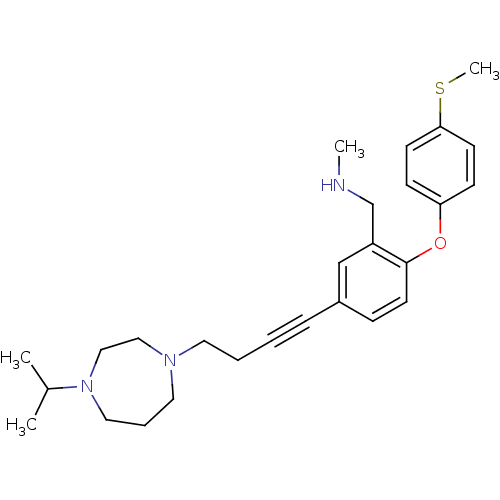
((5-(4-(4-isopropyl-1,4-diazepan-1-yl)but-1-ynyl)-2...)Show SMILES CNCc1cc(ccc1Oc1ccc(SC)cc1)C#CCCN1CCCN(CC1)C(C)C Show InChI InChI=1S/C27H37N3OS/c1-22(2)30-17-7-16-29(18-19-30)15-6-5-8-23-9-14-27(24(20-23)21-28-3)31-25-10-12-26(32-4)13-11-25/h9-14,20,22,28H,6-7,15-19,21H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50217572

(CHEMBL393036 | N-methyl(2-(4-(methylthio)phenoxy)-...)Show InChI InChI=1S/C24H30N2OS/c1-25-19-21-18-20(8-4-7-17-26-15-5-3-6-16-26)9-14-24(21)27-22-10-12-23(28-2)13-11-22/h9-14,18,25H,3,5-7,15-17,19H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50217589
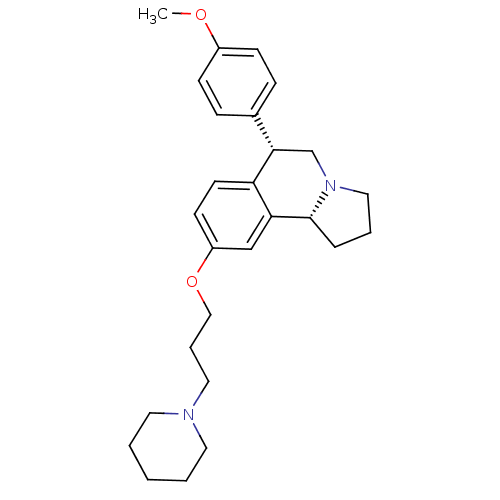
((6S,10bR)-6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl...)Show SMILES COc1ccc(cc1)[C@@H]1CN2CCC[C@@H]2c2cc(OCCCN3CCCCC3)ccc12 Show InChI InChI=1S/C27H36N2O2/c1-30-22-10-8-21(9-11-22)26-20-29-17-5-7-27(29)25-19-23(12-13-24(25)26)31-18-6-16-28-14-3-2-4-15-28/h8-13,19,26-27H,2-7,14-18,20H2,1H3/t26-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 17: 4799-803 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.061
BindingDB Entry DOI: 10.7270/Q2F18ZFF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209810
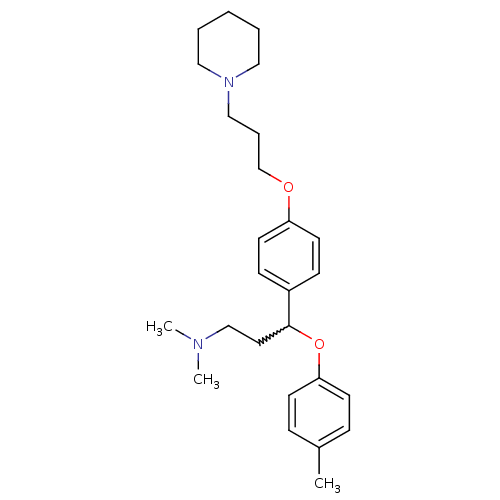
(CHEMBL245516 | N,N-dimethyl-3-(4-(3-(piperidin-1-y...)Show SMILES CN(C)CCC(Oc1ccc(C)cc1)c1ccc(OCCCN2CCCCC2)cc1 |w:5.4| Show InChI InChI=1S/C26H38N2O2/c1-22-8-12-25(13-9-22)30-26(16-20-27(2)3)23-10-14-24(15-11-23)29-21-7-19-28-17-5-4-6-18-28/h8-15,26H,4-7,16-21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209806

(CHEMBL245307 | N,N-dimethyl-3-phenyl-3-(3-(3-(pipe...)Show SMILES CN(C)CCC(Oc1cccc(OCCCN2CCCCC2)c1)c1ccccc1 |w:5.4| Show InChI InChI=1S/C25H36N2O2/c1-26(2)19-15-25(22-11-5-3-6-12-22)29-24-14-9-13-23(21-24)28-20-10-18-27-16-7-4-8-17-27/h3,5-6,9,11-14,21,25H,4,7-8,10,15-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50209815
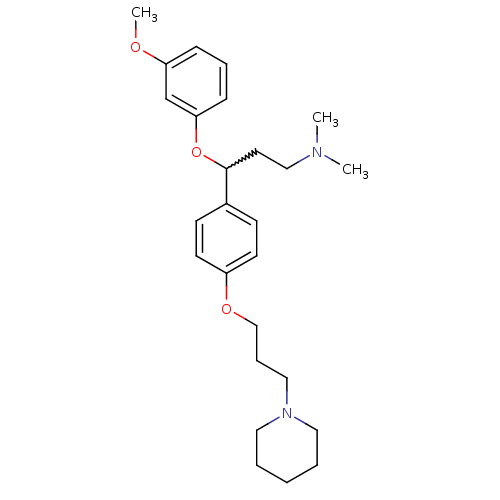
(3-(3-methoxyphenoxy)-N,N-dimethyl-3-(4-(3-(piperid...)Show SMILES COc1cccc(OC(CCN(C)C)c2ccc(OCCCN3CCCCC3)cc2)c1 |w:8.8| Show InChI InChI=1S/C26H38N2O3/c1-27(2)19-15-26(31-25-10-7-9-24(21-25)29-3)22-11-13-23(14-12-22)30-20-8-18-28-16-5-4-6-17-28/h7,9-14,21,26H,4-6,8,15-20H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 receptor |
Bioorg Med Chem Lett 17: 3130-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.034
BindingDB Entry DOI: 10.7270/Q2H131QK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50346205
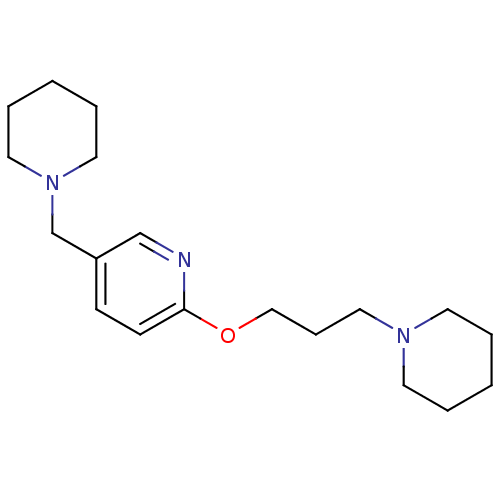
(5-Piperidin-1-ylmethyl-2-(3-piperidin-1-yl-propoxy...)Show InChI InChI=1S/C19H31N3O/c1-3-10-21(11-4-1)14-7-15-23-19-9-8-18(16-20-19)17-22-12-5-2-6-13-22/h8-9,16H,1-7,10-15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4413-25 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.007
BindingDB Entry DOI: 10.7270/Q28S4Q7C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to the human histamine H3 receptor |
J Med Chem 46: 3957-60 (2003)
Article DOI: 10.1021/jm0341047
BindingDB Entry DOI: 10.7270/Q2QJ7J1C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50371669
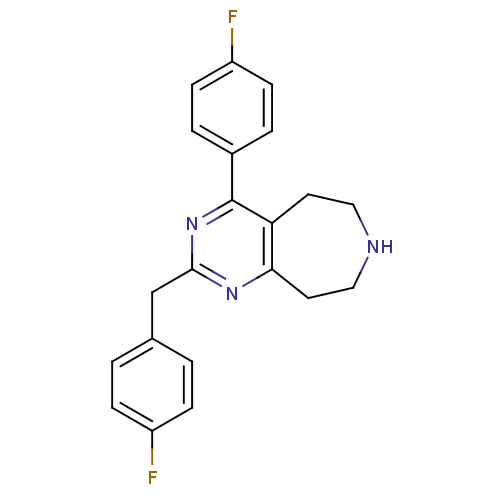
(CHEMBL402164)Show InChI InChI=1S/C21H19F2N3/c22-16-5-1-14(2-6-16)13-20-25-19-10-12-24-11-9-18(19)21(26-20)15-3-7-17(23)8-4-15/h1-8,24H,9-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5HT2A receptor expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 18: 2103-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.090
BindingDB Entry DOI: 10.7270/Q2571CW5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371294
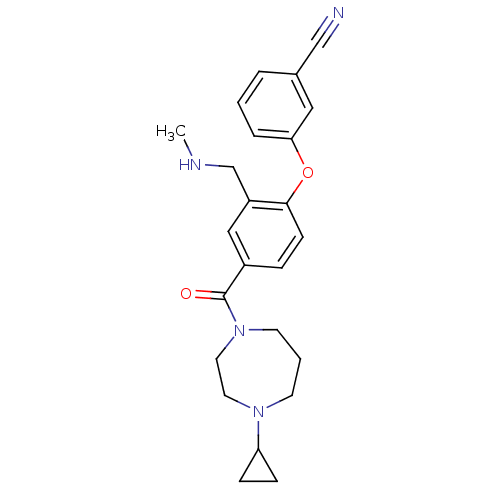
(CHEMBL257208)Show SMILES CNCc1cc(ccc1Oc1cccc(c1)C#N)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C24H28N4O2/c1-26-17-20-15-19(6-9-23(20)30-22-5-2-4-18(14-22)16-25)24(29)28-11-3-10-27(12-13-28)21-7-8-21/h2,4-6,9,14-15,21,26H,3,7-8,10-13,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50217589

((6S,10bR)-6-(4-methoxyphenyl)-9-(3-(piperidin-1-yl...)Show SMILES COc1ccc(cc1)[C@@H]1CN2CCC[C@@H]2c2cc(OCCCN3CCCCC3)ccc12 Show InChI InChI=1S/C27H36N2O2/c1-30-22-10-8-21(9-11-22)26-20-29-17-5-7-27(29)25-19-23(12-13-24(25)26)31-18-6-16-28-14-3-2-4-15-28/h8-13,19,26-27H,2-7,14-18,20H2,1H3/t26-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50209802

(CHEMBL438490 | N,N-dimethyl-3-phenoxy-3-(4-(3-(pip...)Show SMILES CN(C)CCC(Oc1ccccc1)c1ccc(OCCCN2CCCCC2)cc1 |w:5.5| Show InChI InChI=1S/C25H36N2O2/c1-26(2)20-16-25(29-24-10-5-3-6-11-24)22-12-14-23(15-13-22)28-21-9-19-27-17-7-4-8-18-27/h3,5-6,10-15,25H,4,7-9,16-21H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity at human histamine H3 |
Bioorg Med Chem Lett 17: 5325-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.017
BindingDB Entry DOI: 10.7270/Q24749K4 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414798
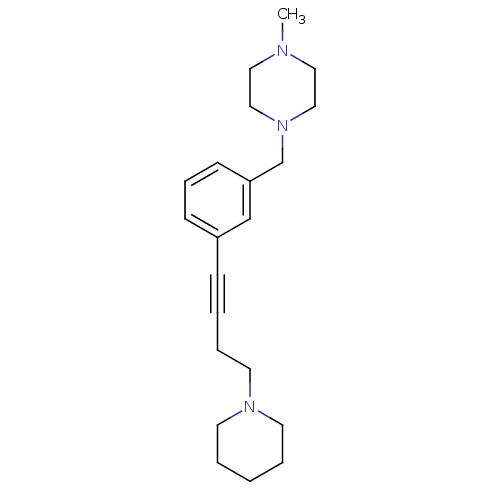
(CHEMBL575172)Show InChI InChI=1S/C21H31N3/c1-22-14-16-24(17-15-22)19-21-10-7-9-20(18-21)8-3-6-13-23-11-4-2-5-12-23/h7,9-10,18H,2,4-6,11-17,19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50198598
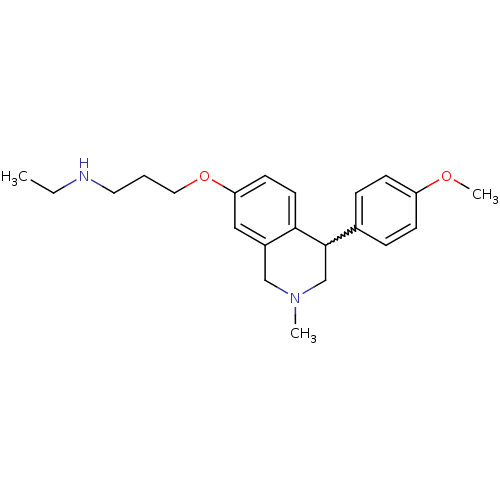
(CHEMBL396945 | N-ethyl-3-(4-(4-methoxyphenyl)-2-me...)Show SMILES CCNCCCOc1ccc2C(CN(C)Cc2c1)c1ccc(OC)cc1 |w:11.19| Show InChI InChI=1S/C22H30N2O2/c1-4-23-12-5-13-26-20-10-11-21-18(14-20)15-24(2)16-22(21)17-6-8-19(25-3)9-7-17/h6-11,14,22-23H,4-5,12-13,15-16H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Binding affinity to rat SERT |
Bioorg Med Chem Lett 17: 702-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.089
BindingDB Entry DOI: 10.7270/Q23B5ZSS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50410346
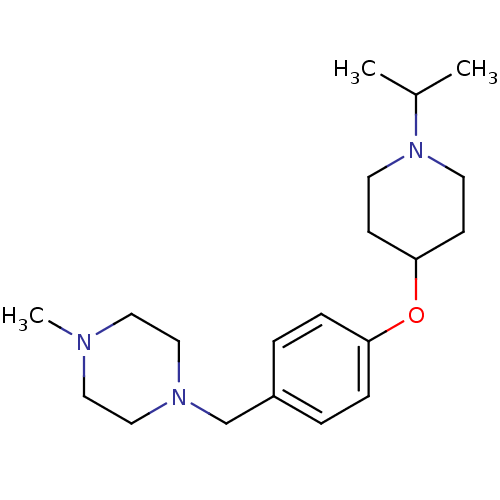
(CHEMBL372471)Show InChI InChI=1S/C20H33N3O/c1-17(2)23-10-8-20(9-11-23)24-19-6-4-18(5-7-19)16-22-14-12-21(3)13-15-22/h4-7,17,20H,8-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Mean binding affinity for human H3 receptor |
J Med Chem 48: 2229-38 (2005)
Article DOI: 10.1021/jm049212n
BindingDB Entry DOI: 10.7270/Q2GB258T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414800
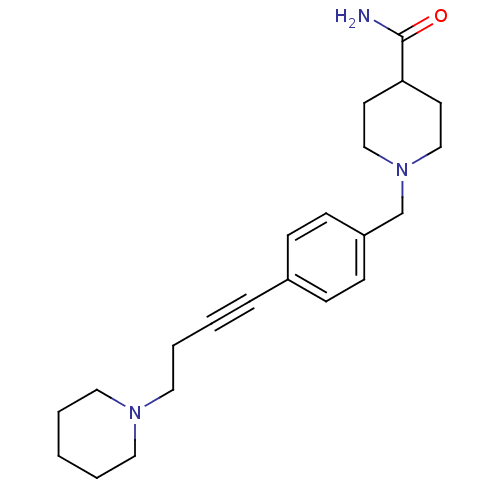
(CHEMBL583182)Show InChI InChI=1S/C22H31N3O/c23-22(26)21-11-16-25(17-12-21)18-20-9-7-19(8-10-20)6-2-5-15-24-13-3-1-4-14-24/h7-10,21H,1,3-5,11-18H2,(H2,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50414792
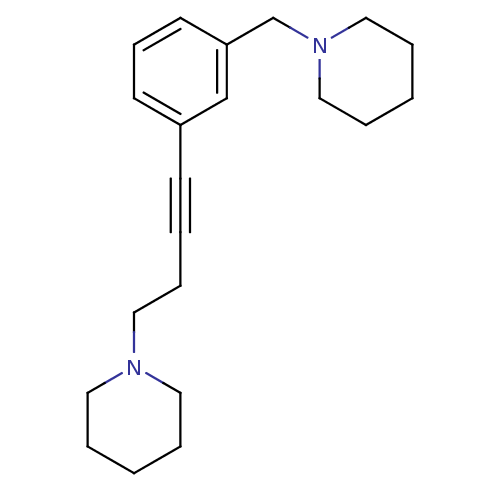
(CHEMBL573817)Show InChI InChI=1S/C21H30N2/c1-4-13-22(14-5-1)15-8-3-10-20-11-9-12-21(18-20)19-23-16-6-2-7-17-23/h9,11-12,18H,1-2,4-8,13-17,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in SK-N-MC cells |
Eur J Med Chem 44: 4098-106 (2009)
Article DOI: 10.1016/j.ejmech.2009.04.049
BindingDB Entry DOI: 10.7270/Q2VH5Q3G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50371290
(CHEMBL401683)Show SMILES CNCc1cc(ccc1Oc1ccccc1)C(=O)N1CCCN(CC1)C1CC1 Show InChI InChI=1S/C23H29N3O2/c1-24-17-19-16-18(8-11-22(19)28-21-6-3-2-4-7-21)23(27)26-13-5-12-25(14-15-26)20-9-10-20/h2-4,6-8,11,16,20,24H,5,9-10,12-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H3 receptor |
Bioorg Med Chem Lett 18: 39-43 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.016
BindingDB Entry DOI: 10.7270/Q2R49RMD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data