

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019010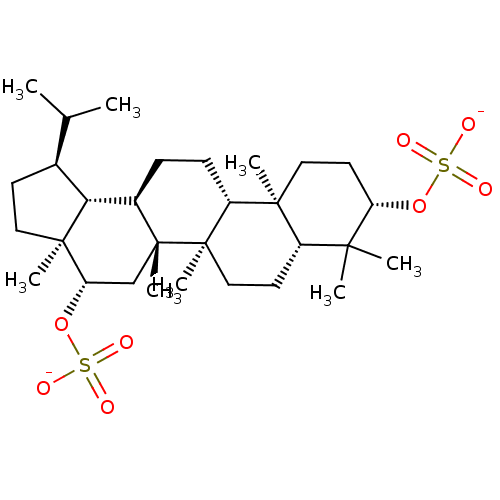 (CHEMBL3288082) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Uncompetitive inhibition of Pacific electric ray AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019013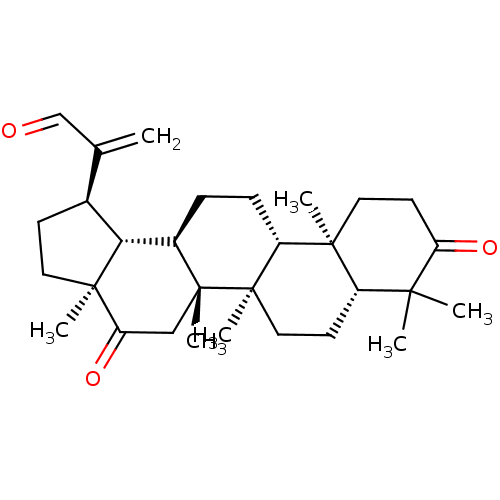 (CHEMBL3288094) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019010 (CHEMBL3288082) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019003 (CHEMBL3288074) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019012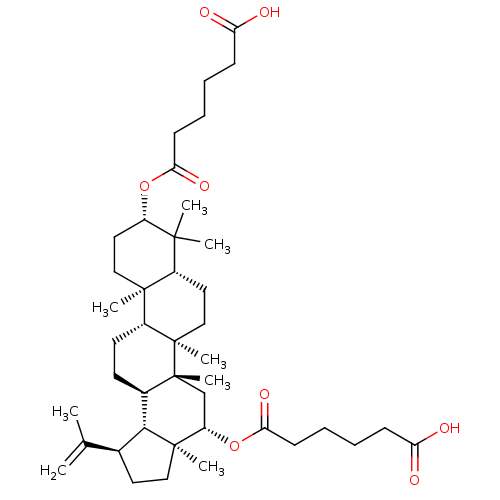 (CHEMBL3288092) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019010 (CHEMBL3288082) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019009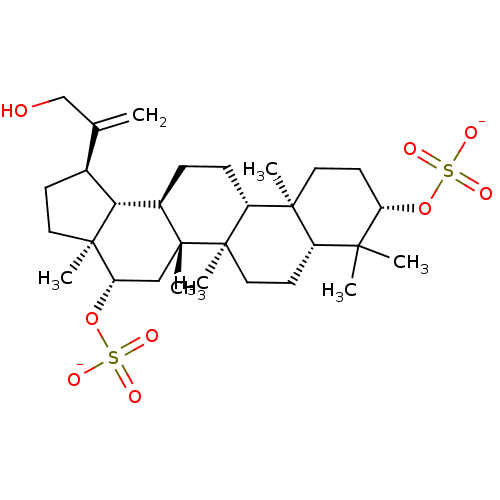 (CHEMBL3288081) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019003 (CHEMBL3288074) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019004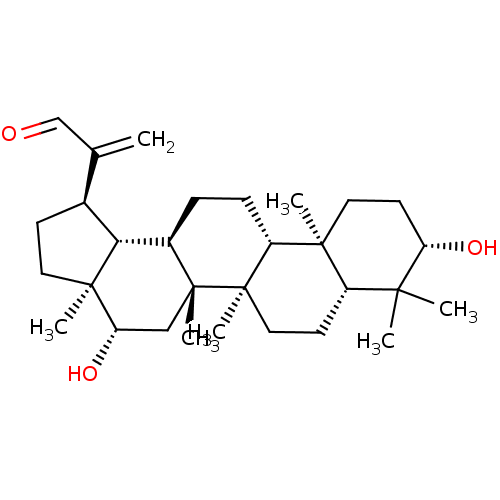 (CHEMBL3288075) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019002 (Calenduladiol) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019008 (CHEMBL3288080) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019011 (CHEMBL3288083) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019014 (CHEMBL3288085) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019015 (CHEMBL3288086) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50019016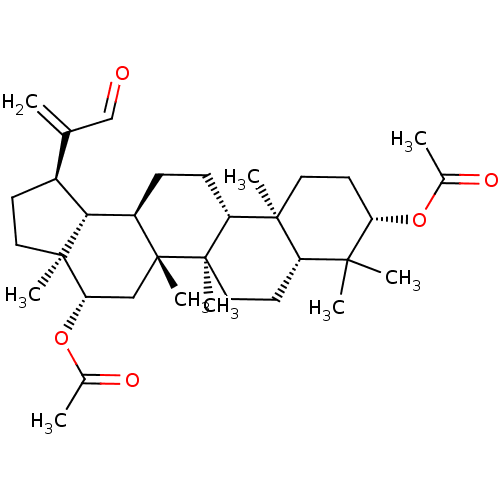 (CHEMBL3288087) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of BChE in horse serum using butyrylthiocholine iodide as substrate after 120 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019002 (Calenduladiol) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019005 (CHEMBL3288076) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019013 (CHEMBL3288094) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019012 (CHEMBL3288092) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019011 (CHEMBL3288083) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019009 (CHEMBL3288081) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019006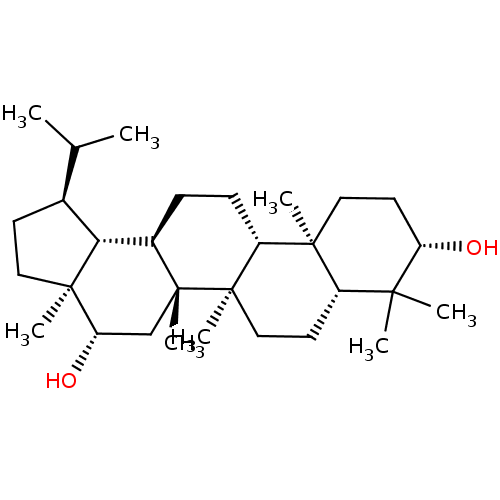 (CHEMBL3288077) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019007 (CHEMBL3288078) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50019008 (CHEMBL3288080) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional del Sur Curated by ChEMBL | Assay Description Inhibition of Pacific electric ray AChE using acetylthiocholine iodide as substrate after 60 mins by Ellman's method | Bioorg Med Chem 22: 3341-50 (2014) Article DOI: 10.1016/j.bmc.2014.04.050 BindingDB Entry DOI: 10.7270/Q28P6220 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||