 Found 52 hits with Last Name = 'cellai' and Initial = 'l'
Found 52 hits with Last Name = 'cellai' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069245
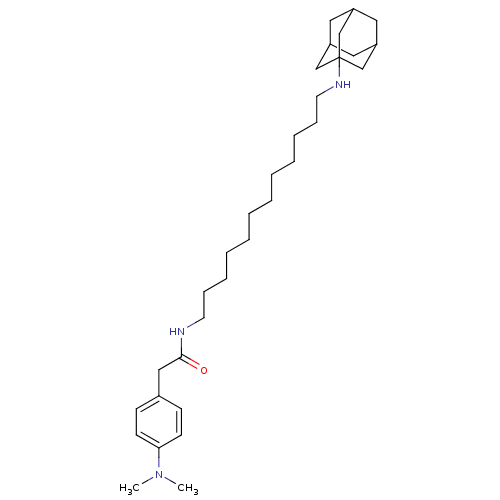
(CHEMBL157336 | N-[12-(Adamantan-1-ylamino)-dodecyl...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:33:24:31:28.27.29,THB:33:28:24.25.32:31,23:24:31:28.27.29,29:28:25:30.32.31,29:30:25:28.33.27| Show InChI InChI=1S/C32H53N3O/c1-35(2)30-15-13-26(14-16-30)22-31(36)33-17-11-9-7-5-3-4-6-8-10-12-18-34-32-23-27-19-28(24-32)21-29(20-27)25-32/h13-16,27-29,34H,3-12,17-25H2,1-2H3,(H,33,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine brain |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069248
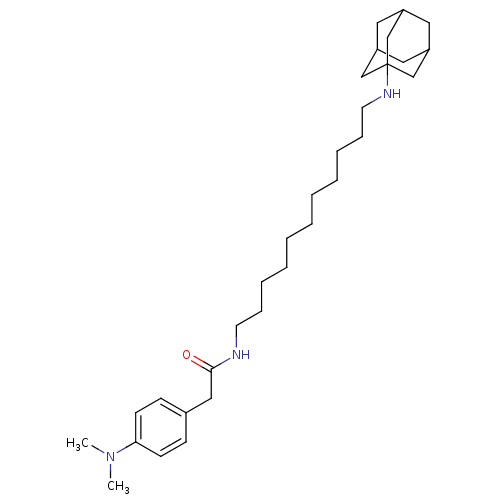
(CHEMBL421974 | N-[11-(Adamantan-1-ylamino)-undecyl...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:32:23:30:27.26.28,THB:32:27:23.24.31:30,22:23:30:27.26.28,28:27:24:29.31.30,28:29:24:27.32.26| Show InChI InChI=1S/C31H51N3O/c1-34(2)29-14-12-25(13-15-29)21-30(35)32-16-10-8-6-4-3-5-7-9-11-17-33-31-22-26-18-27(23-31)20-28(19-26)24-31/h12-15,26-28,33H,3-11,16-24H2,1-2H3,(H,32,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for competitive inhibition constant of acetylcholinesterase (AChE) from Torpedo californica |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069243
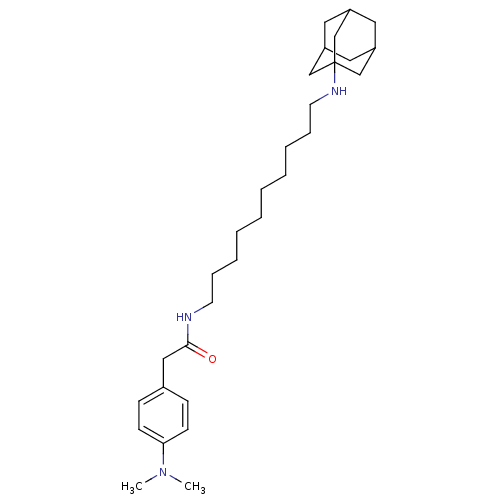
(CHEMBL153934 | N-[10-(Adamantan-1-ylamino)-decyl]-...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:31:22:29:26.25.27,THB:31:26:22.23.30:29,21:22:29:26.25.27,27:26:23:28.30.29,27:28:23:26.31.25| Show InChI InChI=1S/C30H49N3O/c1-33(2)28-13-11-24(12-14-28)20-29(34)31-15-9-7-5-3-4-6-8-10-16-32-30-21-25-17-26(22-30)19-27(18-25)23-30/h11-14,25-27,32H,3-10,15-23H2,1-2H3,(H,31,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for competitive inhibition constant of acetylcholinesterase (AChE) from Torpedo californica |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069247
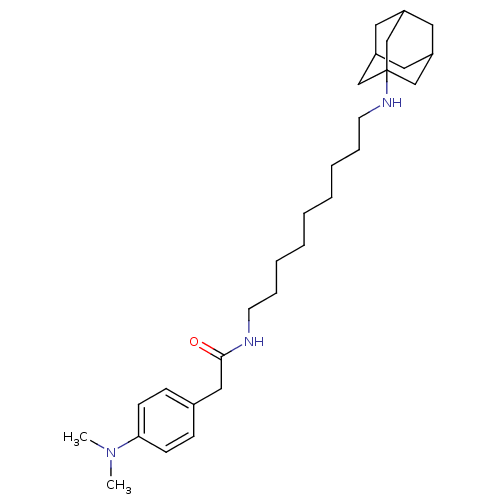
(CHEMBL157429 | N-[9-(Adamantan-1-ylamino)-nonyl]-2...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:30:21:28:25.24.26,THB:30:25:21.22.29:28,20:21:28:25.24.26,26:25:22:27.29.28,26:27:22:25.30.24| Show InChI InChI=1S/C29H47N3O/c1-32(2)27-12-10-23(11-13-27)19-28(33)30-14-8-6-4-3-5-7-9-15-31-29-20-24-16-25(21-29)18-26(17-24)22-29/h10-13,24-26,31H,3-9,14-22H2,1-2H3,(H,30,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for competitive inhibition constant of acetylcholinesterase (AChE) from Torpedo californica |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069244

(CHEMBL357551 | N-[8-(Adamantan-1-ylamino)-octyl]-2...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:29:20:27:24.23.25,THB:29:24:20.21.28:27,19:20:27:24.23.25,25:24:21:26.28.27,25:26:21:24.29.23| Show InChI InChI=1S/C28H45N3O/c1-31(2)26-11-9-22(10-12-26)18-27(32)29-13-7-5-3-4-6-8-14-30-28-19-23-15-24(20-28)17-25(16-23)21-28/h9-12,23-25,30H,3-8,13-21H2,1-2H3,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for competitive inhibition constant of acetylcholinesterase (AChE) from Torpedo californica |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069246
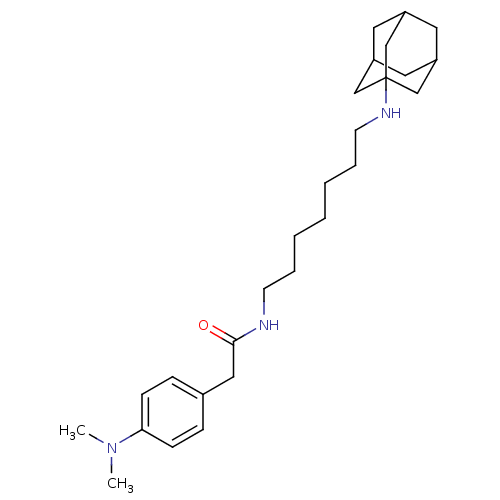
(CHEMBL152361 | N-[7-(Adamantan-1-ylamino)-heptyl]-...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:28:19:26:23.22.24,THB:28:23:19.20.27:26,18:19:26:23.22.24,24:23:20:25.27.26,24:25:20:23.28.22| Show InChI InChI=1S/C27H43N3O/c1-30(2)25-10-8-21(9-11-25)17-26(31)28-12-6-4-3-5-7-13-29-27-18-22-14-23(19-27)16-24(15-22)20-27/h8-11,22-24,29H,3-7,12-20H2,1-2H3,(H,28,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for competitive inhibition constant of acetylcholinesterase (AChE) from Torpedo californica |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine brain |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase in Torpedo californica |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in human erythrocytes |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in Torpedo californica |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in electric eel |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in electric eel |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in Torpedo californica |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in Torpedo californica |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase in Torpedo californica |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine brain |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229834
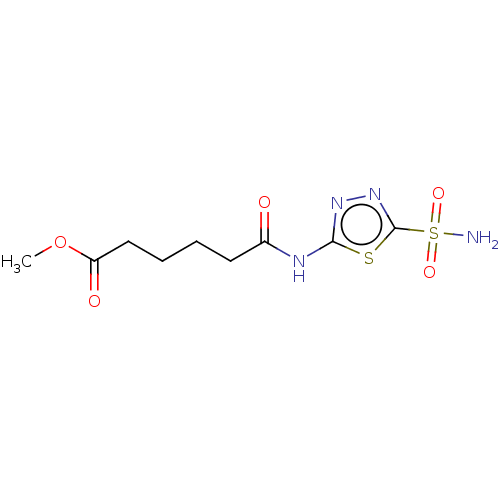
(CHEMBL328270)Show InChI InChI=1S/C9H14N4O5S2/c1-18-7(15)5-3-2-4-6(14)11-8-12-13-9(19-8)20(10,16)17/h2-5H2,1H3,(H2,10,16,17)(H,11,12,14) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229838
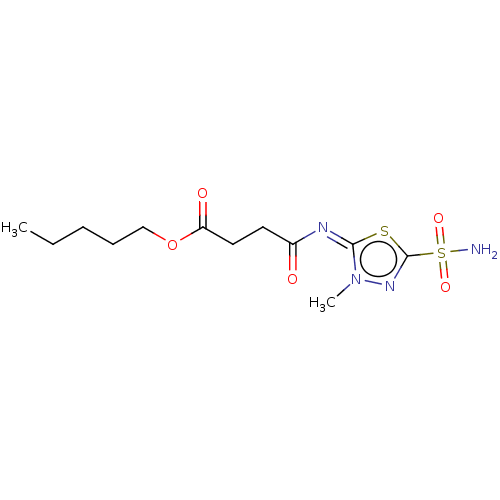
(CHEMBL88183)Show InChI InChI=1S/C12H20N4O5S2/c1-3-4-5-8-21-10(18)7-6-9(17)14-11-16(2)15-12(22-11)23(13,19)20/h3-8H2,1-2H3,(H2,13,19,20)/b14-11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229841
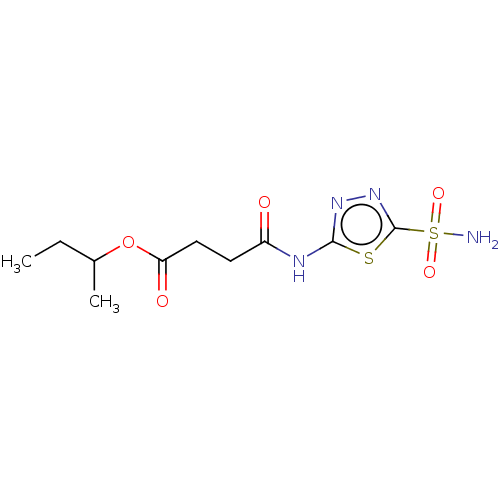
(CHEMBL420799)Show InChI InChI=1S/C10H16N4O5S2/c1-3-6(2)19-8(16)5-4-7(15)12-9-13-14-10(20-9)21(11,17)18/h6H,3-5H2,1-2H3,(H2,11,17,18)(H,12,13,15) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229842
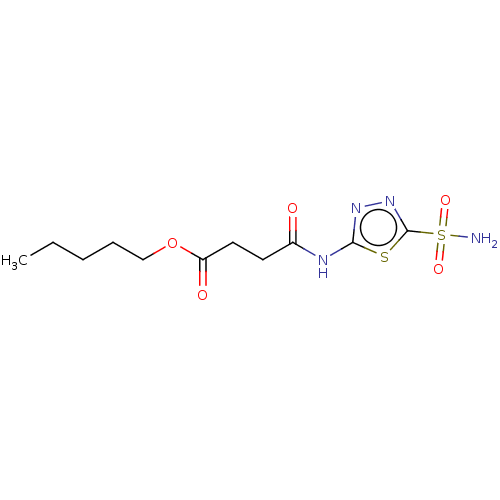
(CHEMBL90119)Show InChI InChI=1S/C11H18N4O5S2/c1-2-3-4-7-20-9(17)6-5-8(16)13-10-14-15-11(21-10)22(12,18)19/h2-7H2,1H3,(H2,12,18,19)(H,13,14,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50212309
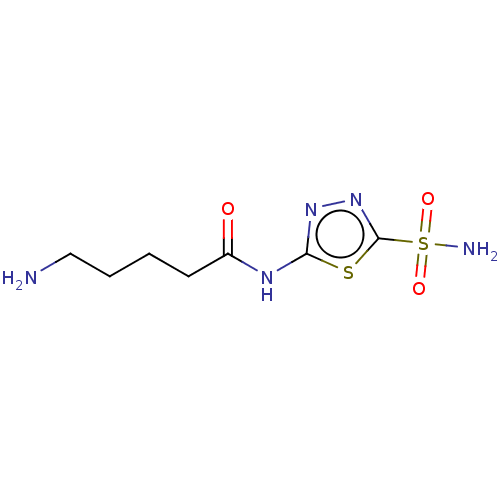
(CHEMBL91302)Show InChI InChI=1S/C7H13N5O3S2/c8-4-2-1-3-5(13)10-6-11-12-7(16-6)17(9,14)15/h1-4,8H2,(H2,9,14,15)(H,10,11,13) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229847
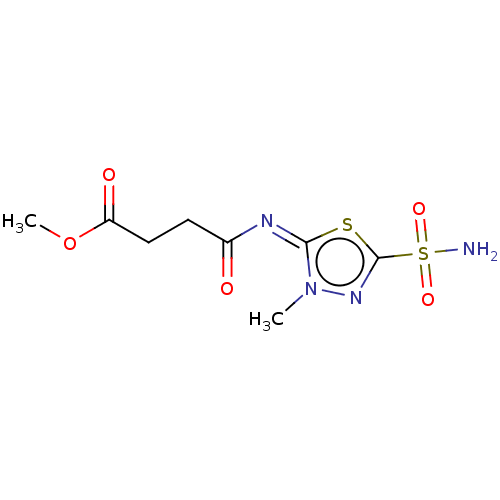
(CHEMBL91512)Show InChI InChI=1S/C8H12N4O5S2/c1-12-7(18-8(11-12)19(9,15)16)10-5(13)3-4-6(14)17-2/h3-4H2,1-2H3,(H2,9,15,16)/b10-7+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229846
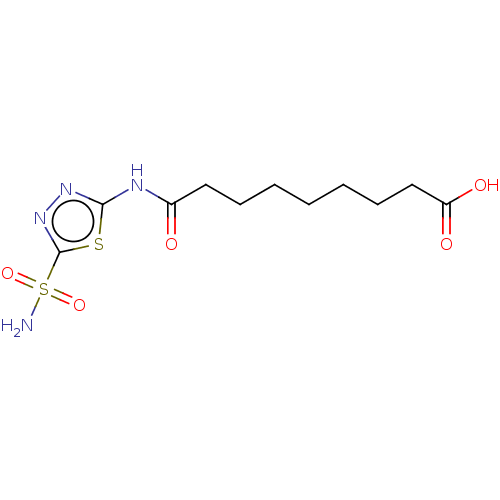
(CHEMBL264307)Show InChI InChI=1S/C11H18N4O5S2/c12-22(19,20)11-15-14-10(21-11)13-8(16)6-4-2-1-3-5-7-9(17)18/h1-7H2,(H,17,18)(H2,12,19,20)(H,13,14,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229845

(CHEMBL91517)Show InChI InChI=1S/C7H10N4O5S2/c8-18(15,16)7-11-10-6(17-7)9-4(12)2-1-3-5(13)14/h1-3H2,(H,13,14)(H2,8,15,16)(H,9,10,12) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229839
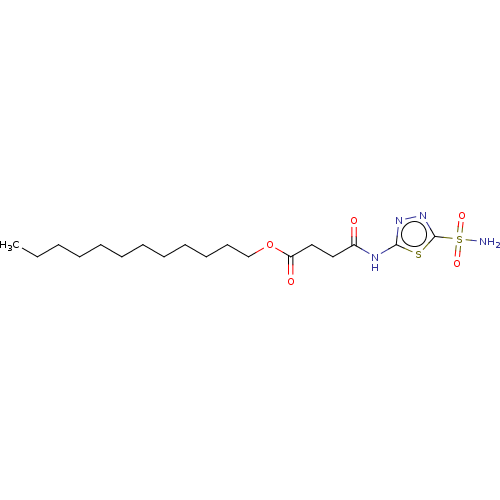
(CHEMBL91622)Show SMILES CCCCCCCCCCCCOC(=O)CCC(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C18H32N4O5S2/c1-2-3-4-5-6-7-8-9-10-11-14-27-16(24)13-12-15(23)20-17-21-22-18(28-17)29(19,25)26/h2-14H2,1H3,(H2,19,25,26)(H,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229835
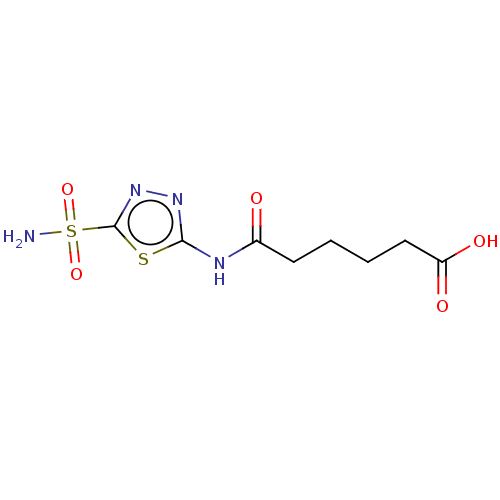
(CHEMBL90857)Show InChI InChI=1S/C8H12N4O5S2/c9-19(16,17)8-12-11-7(18-8)10-5(13)3-1-2-4-6(14)15/h1-4H2,(H,14,15)(H2,9,16,17)(H,10,11,13) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50185303
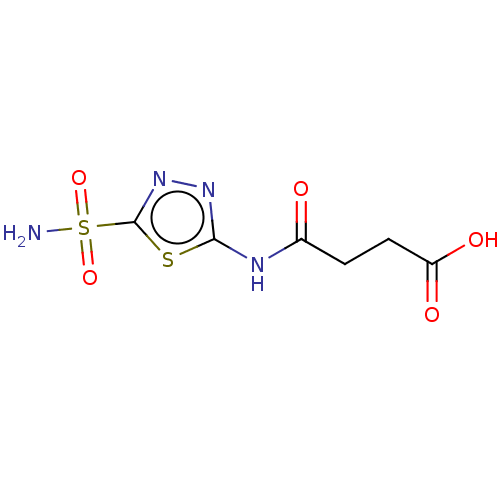
(CHEMBL88115)Show InChI InChI=1S/C6H8N4O5S2/c7-17(14,15)6-10-9-5(16-6)8-3(11)1-2-4(12)13/h1-2H2,(H,12,13)(H2,7,14,15)(H,8,9,11) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229836
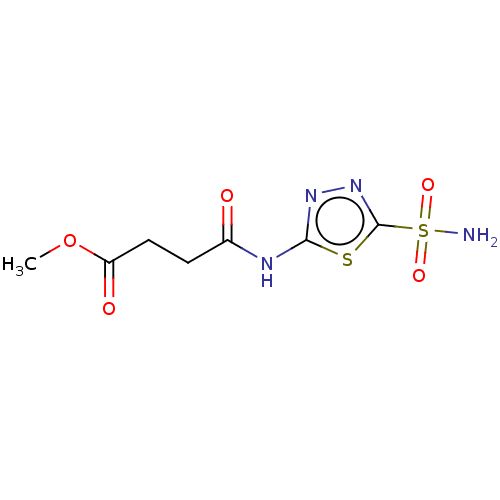
(CHEMBL92500)Show InChI InChI=1S/C7H10N4O5S2/c1-16-5(13)3-2-4(12)9-6-10-11-7(17-6)18(8,14)15/h2-3H2,1H3,(H2,8,14,15)(H,9,10,12) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229843
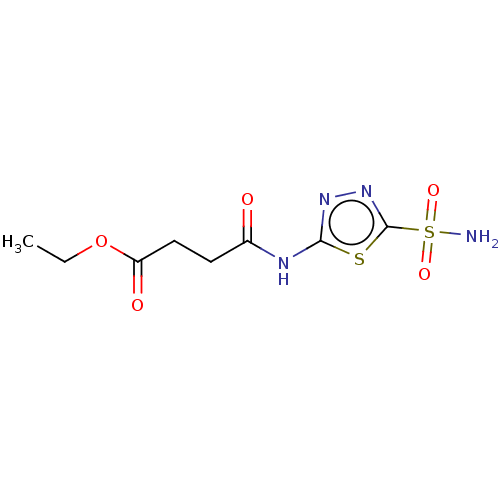
(CHEMBL91131)Show InChI InChI=1S/C8H12N4O5S2/c1-2-17-6(14)4-3-5(13)10-7-11-12-8(18-7)19(9,15)16/h2-4H2,1H3,(H2,9,15,16)(H,10,11,13) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM50333128

(((2S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-4-...)Show SMILES Nc1ccn([C@@H]2O[C@H](CO[P@@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)C[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C9H16N3O13P3/c10-7-1-2-12(9(14)11-7)8-6(13)3-5(23-8)4-22-27(18,19)25-28(20,21)24-26(15,16)17/h1-2,5-6,8,13H,3-4H2,(H,18,19)(H,20,21)(H2,10,11,14)(H2,15,16,17)/t5-,6+,8+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS5B polymerase S282T mutant assessed as blocking of full length RNA product formation by single-nucleotide incorporation assay |
Antimicrob Agents Chemother 51: 2920-8 (2007)
Article DOI: 10.1128/aac.00186-07
BindingDB Entry DOI: 10.7270/Q2HH6NVS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
(Homo sapiens (Human)) | BDBM50229844
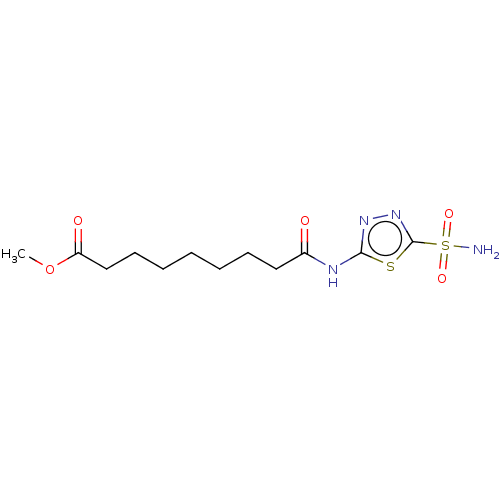
(CHEMBL328566)Show InChI InChI=1S/C12H20N4O5S2/c1-21-10(18)8-6-4-2-3-5-7-9(17)14-11-15-16-12(22-11)23(13,19)20/h2-8H2,1H3,(H2,13,19,20)(H,14,15,17) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
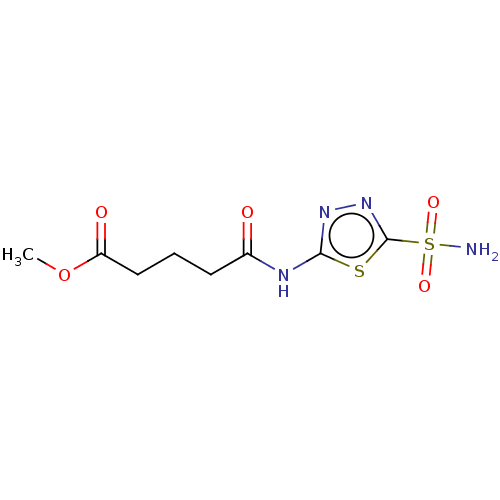
(Homo sapiens (Human)) | BDBM50229837
(CHEMBL89489)Show InChI InChI=1S/C8H12N4O5S2/c1-17-6(14)4-2-3-5(13)10-7-11-12-8(18-7)19(9,15)16/h2-4H2,1H3,(H2,9,15,16)(H,10,11,13) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1/12/13/14/2/3/4/5A, mitochondrial/5B, mitochondrial/6/7/9
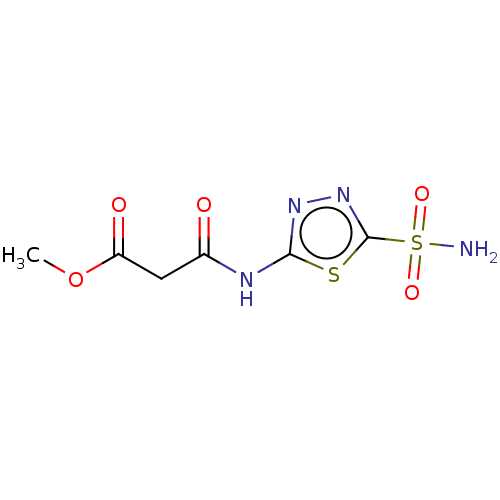
(Homo sapiens (Human)) | BDBM50229840
(CHEMBL329625)Show InChI InChI=1S/C6H8N4O5S2/c1-15-4(12)2-3(11)8-5-9-10-6(16-5)17(7,13)14/h2H2,1H3,(H2,7,13,14)(H,8,9,11) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Chimico Internazionale Dr. G. Rende
Curated by ChEMBL
| Assay Description
Inhibition of Carbonic anhydrase enzyme in rabbits |
J Med Chem 35: 2697-703 (1992)
BindingDB Entry DOI: 10.7270/Q2K076G9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069245

(CHEMBL157336 | N-[12-(Adamantan-1-ylamino)-dodecyl...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:33:24:31:28.27.29,THB:33:28:24.25.32:31,23:24:31:28.27.29,29:28:25:30.32.31,29:30:25:28.33.27| Show InChI InChI=1S/C32H53N3O/c1-35(2)30-15-13-26(14-16-30)22-31(36)33-17-11-9-7-5-3-4-6-8-10-12-18-34-32-23-27-19-28(24-32)21-29(20-27)25-32/h13-16,27-29,34H,3-12,17-25H2,1-2H3,(H,33,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in Torpedo californica |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069243

(CHEMBL153934 | N-[10-(Adamantan-1-ylamino)-decyl]-...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:31:22:29:26.25.27,THB:31:26:22.23.30:29,21:22:29:26.25.27,27:26:23:28.30.29,27:28:23:26.31.25| Show InChI InChI=1S/C30H49N3O/c1-33(2)28-13-11-24(12-14-28)20-29(34)31-15-9-7-5-3-4-6-8-10-16-32-30-21-25-17-26(22-30)19-27(18-25)23-30/h11-14,25-27,32H,3-10,15-23H2,1-2H3,(H,31,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine erythrocytes |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM86487
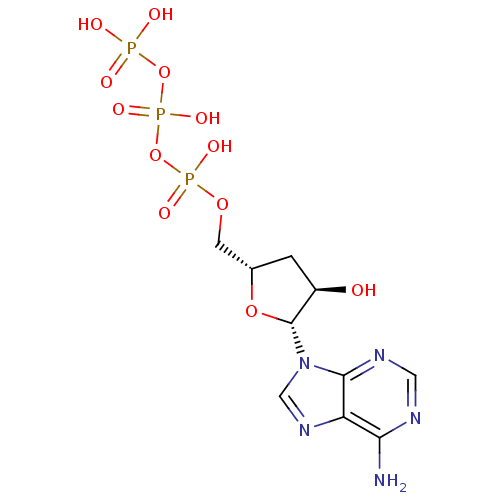
(ATP,3'-deoxy | CAS_0 | NSC_0)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C[C@H]1O |r| Show InChI InChI=1S/C10H16N5O12P3/c11-8-7-9(13-3-12-8)15(4-14-7)10-6(16)1-5(25-10)2-24-29(20,21)27-30(22,23)26-28(17,18)19/h3-6,10,16H,1-2H2,(H,20,21)(H,22,23)(H2,11,12,13)(H2,17,18,19)/t5-,6+,10+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS5B polymerase S282T mutant assessed as blocking of full length RNA product formation by single-nucleotide incorporation assay |
Antimicrob Agents Chemother 51: 2920-8 (2007)
Article DOI: 10.1128/aac.00186-07
BindingDB Entry DOI: 10.7270/Q2HH6NVS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50069245

(CHEMBL157336 | N-[12-(Adamantan-1-ylamino)-dodecyl...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:33:24:31:28.27.29,THB:33:28:24.25.32:31,23:24:31:28.27.29,29:28:25:30.32.31,29:30:25:28.33.27| Show InChI InChI=1S/C32H53N3O/c1-35(2)30-15-13-26(14-16-30)22-31(36)33-17-11-9-7-5-3-4-6-8-10-12-18-34-32-23-27-19-28(24-32)21-29(20-27)25-32/h13-16,27-29,34H,3-12,17-25H2,1-2H3,(H,33,36) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in electric eel |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50069244

(CHEMBL357551 | N-[8-(Adamantan-1-ylamino)-octyl]-2...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:29:20:27:24.23.25,THB:29:24:20.21.28:27,19:20:27:24.23.25,25:24:21:26.28.27,25:26:21:24.29.23| Show InChI InChI=1S/C28H45N3O/c1-31(2)26-11-9-22(10-12-26)18-27(32)29-13-7-5-3-4-6-8-14-30-28-19-23-15-24(20-28)17-25(16-23)21-28/h9-12,23-25,30H,3-8,13-21H2,1-2H3,(H,29,32) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for competitive inhibition constant of acetylcholinesterase (AChE) from Torpedo californica |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50069243

(CHEMBL153934 | N-[10-(Adamantan-1-ylamino)-decyl]-...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:31:22:29:26.25.27,THB:31:26:22.23.30:29,21:22:29:26.25.27,27:26:23:28.30.29,27:28:23:26.31.25| Show InChI InChI=1S/C30H49N3O/c1-33(2)28-13-11-24(12-14-28)20-29(34)31-15-9-7-5-3-4-6-8-10-16-32-30-21-25-17-26(22-30)19-27(18-25)23-30/h11-14,25-27,32H,3-10,15-23H2,1-2H3,(H,31,34) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase in electric eel |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50069245

(CHEMBL157336 | N-[12-(Adamantan-1-ylamino)-dodecyl...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:33:24:31:28.27.29,THB:33:28:24.25.32:31,23:24:31:28.27.29,29:28:25:30.32.31,29:30:25:28.33.27| Show InChI InChI=1S/C32H53N3O/c1-35(2)30-15-13-26(14-16-30)22-31(36)33-17-11-9-7-5-3-4-6-8-10-12-18-34-32-23-27-19-28(24-32)21-29(20-27)25-32/h13-16,27-29,34H,3-12,17-25H2,1-2H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine erythrocytes |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50069245

(CHEMBL157336 | N-[12-(Adamantan-1-ylamino)-dodecyl...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:33:24:31:28.27.29,THB:33:28:24.25.32:31,23:24:31:28.27.29,29:28:25:30.32.31,29:30:25:28.33.27| Show InChI InChI=1S/C32H53N3O/c1-35(2)30-15-13-26(14-16-30)22-31(36)33-17-11-9-7-5-3-4-6-8-10-12-18-34-32-23-27-19-28(24-32)21-29(20-27)25-32/h13-16,27-29,34H,3-12,17-25H2,1-2H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine brain |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50069244

(CHEMBL357551 | N-[8-(Adamantan-1-ylamino)-octyl]-2...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:29:20:27:24.23.25,THB:29:24:20.21.28:27,19:20:27:24.23.25,25:24:21:26.28.27,25:26:21:24.29.23| Show InChI InChI=1S/C28H45N3O/c1-31(2)26-11-9-22(10-12-26)18-27(32)29-13-7-5-3-4-6-8-14-30-28-19-23-15-24(20-28)17-25(16-23)21-28/h9-12,23-25,30H,3-8,13-21H2,1-2H3,(H,29,32) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in electric eel |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM50478972

(2''C-Me-CTP | CHEMBL223585)Show SMILES C[C@@]1(O)[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]1n1ccc(N)nc1=O |r| Show InChI InChI=1S/C10H18N3O14P3/c1-10(16)7(14)5(25-8(10)13-3-2-6(11)12-9(13)15)4-24-29(20,21)27-30(22,23)26-28(17,18)19/h2-3,5,7-8,14,16H,4H2,1H3,(H,20,21)(H,22,23)(H2,11,12,15)(H2,17,18,19)/t5-,7-,8-,10-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS5B polymerase S282T mutant assessed as blocking of full length RNA product formation by single-nucleotide incorporation assay |
Antimicrob Agents Chemother 51: 2920-8 (2007)
Article DOI: 10.1128/aac.00186-07
BindingDB Entry DOI: 10.7270/Q2HH6NVS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50069243

(CHEMBL153934 | N-[10-(Adamantan-1-ylamino)-decyl]-...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:31:22:29:26.25.27,THB:31:26:22.23.30:29,21:22:29:26.25.27,27:26:23:28.30.29,27:28:23:26.31.25| Show InChI InChI=1S/C30H49N3O/c1-33(2)28-13-11-24(12-14-28)20-29(34)31-15-9-7-5-3-4-6-8-10-16-32-30-21-25-17-26(22-30)19-27(18-25)23-30/h11-14,25-27,32H,3-10,15-23H2,1-2H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine erythrocytes |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50069243

(CHEMBL153934 | N-[10-(Adamantan-1-ylamino)-decyl]-...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:31:22:29:26.25.27,THB:31:26:22.23.30:29,21:22:29:26.25.27,27:26:23:28.30.29,27:28:23:26.31.25| Show InChI InChI=1S/C30H49N3O/c1-33(2)28-13-11-24(12-14-28)20-29(34)31-15-9-7-5-3-4-6-8-10-16-32-30-21-25-17-26(22-30)19-27(18-25)23-30/h11-14,25-27,32H,3-10,15-23H2,1-2H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine erythrocytes |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50069243

(CHEMBL153934 | N-[10-(Adamantan-1-ylamino)-decyl]-...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:31:22:29:26.25.27,THB:31:26:22.23.30:29,21:22:29:26.25.27,27:26:23:28.30.29,27:28:23:26.31.25| Show InChI InChI=1S/C30H49N3O/c1-33(2)28-13-11-24(12-14-28)20-29(34)31-15-9-7-5-3-4-6-8-10-16-32-30-21-25-17-26(22-30)19-27(18-25)23-30/h11-14,25-27,32H,3-10,15-23H2,1-2H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in human erythrocytes |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50069245

(CHEMBL157336 | N-[12-(Adamantan-1-ylamino)-dodecyl...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:33:24:31:28.27.29,THB:33:28:24.25.32:31,23:24:31:28.27.29,29:28:25:30.32.31,29:30:25:28.33.27| Show InChI InChI=1S/C32H53N3O/c1-35(2)30-15-13-26(14-16-30)22-31(36)33-17-11-9-7-5-3-4-6-8-10-12-18-34-32-23-27-19-28(24-32)21-29(20-27)25-32/h13-16,27-29,34H,3-12,17-25H2,1-2H3,(H,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in electric eel |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50069244

(CHEMBL357551 | N-[8-(Adamantan-1-ylamino)-octyl]-2...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:29:20:27:24.23.25,THB:29:24:20.21.28:27,19:20:27:24.23.25,25:24:21:26.28.27,25:26:21:24.29.23| Show InChI InChI=1S/C28H45N3O/c1-31(2)26-11-9-22(10-12-26)18-27(32)29-13-7-5-3-4-6-8-14-30-28-19-23-15-24(20-28)17-25(16-23)21-28/h9-12,23-25,30H,3-8,13-21H2,1-2H3,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in human erythrocytes |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50069244

(CHEMBL357551 | N-[8-(Adamantan-1-ylamino)-octyl]-2...)Show SMILES CN(C)c1ccc(CC(=O)NCCCCCCCCNC23CC4CC(CC(C4)C2)C3)cc1 |TLB:29:20:27:24.23.25,THB:29:24:20.21.28:27,19:20:27:24.23.25,25:24:21:26.28.27,25:26:21:24.29.23| Show InChI InChI=1S/C28H45N3O/c1-31(2)26-11-9-22(10-12-26)18-27(32)29-13-7-5-3-4-6-8-14-30-28-19-23-15-24(20-28)17-25(16-23)21-28/h9-12,23-25,30H,3-8,13-21H2,1-2H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Strutturistica Chimica G. Giacomello
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of acetylcholinesterase (AChE) in bovine brain |
Bioorg Med Chem Lett 8: 575-80 (1999)
BindingDB Entry DOI: 10.7270/Q2S181N2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data