

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 reverse transcriptase | Eur J Med Chem 44: 2190-201 (2009) Article DOI: 10.1016/j.ejmech.2008.10.032 BindingDB Entry DOI: 10.7270/Q2X069TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant wild type reverse transcriptase | Bioorg Med Chem 16: 4173-85 (2008) Article DOI: 10.1016/j.bmc.2007.12.046 BindingDB Entry DOI: 10.7270/Q2WW7MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of HIV1 virion-associated reverse transcriptase | Bioorg Med Chem 16: 6353-63 (2008) Article DOI: 10.1016/j.bmc.2008.05.010 BindingDB Entry DOI: 10.7270/Q2B56NJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168052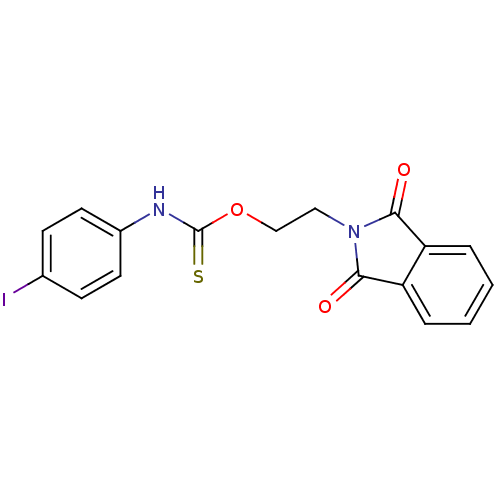 ((4-Iodo-phenyl)-thiocarbamic acid 2-(1,3-dioxo-1,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50480227 (CHEMBL520032) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 reverse transcriptase | Eur J Med Chem 44: 2190-201 (2009) Article DOI: 10.1016/j.ejmech.2008.10.032 BindingDB Entry DOI: 10.7270/Q2X069TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103N+Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168128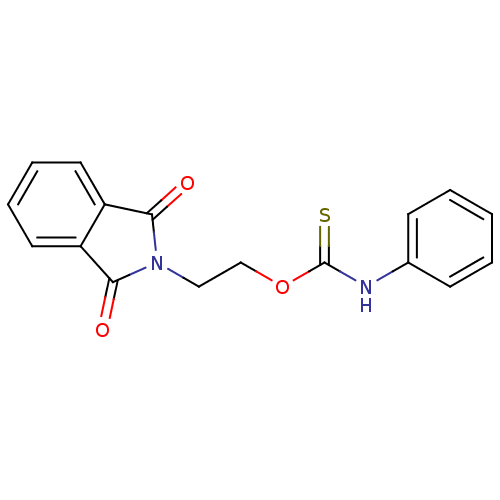 (CHEMBL191655 | O-2-(1,3-dioxoisoindolin-2-yl)ethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 virion reverse transcriptase | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50480228 (CHEMBL484982) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 reverse transcriptase | Eur J Med Chem 44: 2190-201 (2009) Article DOI: 10.1016/j.ejmech.2008.10.032 BindingDB Entry DOI: 10.7270/Q2X069TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478732 (CHEMBL443403) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of HIV1 virion-associated reverse transcriptase | Bioorg Med Chem 16: 6353-63 (2008) Article DOI: 10.1016/j.bmc.2008.05.010 BindingDB Entry DOI: 10.7270/Q2B56NJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478749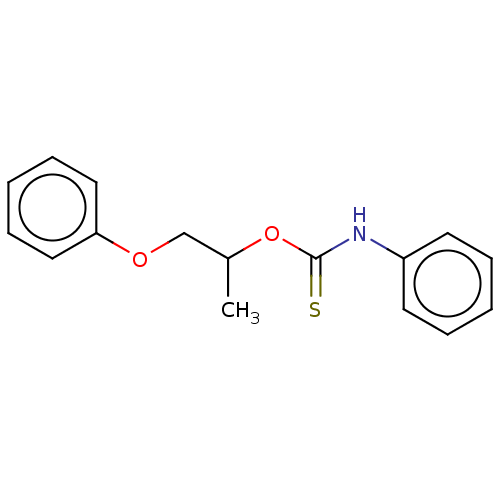 (CHEMBL471541) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant wild type reverse transcriptase | Bioorg Med Chem 16: 4173-85 (2008) Article DOI: 10.1016/j.bmc.2007.12.046 BindingDB Entry DOI: 10.7270/Q2WW7MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant wild type reverse transcriptase | Bioorg Med Chem 16: 4173-85 (2008) Article DOI: 10.1016/j.bmc.2007.12.046 BindingDB Entry DOI: 10.7270/Q2WW7MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of HIV1 virion-associated reverse transcriptase | Bioorg Med Chem 16: 6353-63 (2008) Article DOI: 10.1016/j.bmc.2008.05.010 BindingDB Entry DOI: 10.7270/Q2B56NJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478750 (CHEMBL471691) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant wild type reverse transcriptase | Bioorg Med Chem 16: 4173-85 (2008) Article DOI: 10.1016/j.bmc.2007.12.046 BindingDB Entry DOI: 10.7270/Q2WW7MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168060 (CHEMBL195108 | N-[2-(4-Iodo-phenylthiocarbamoyloxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103R) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168052 ((4-Iodo-phenyl)-thiocarbamic acid 2-(1,3-dioxo-1,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103R) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168060 (CHEMBL195108 | N-[2-(4-Iodo-phenylthiocarbamoyloxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168052 ((4-Iodo-phenyl)-thiocarbamic acid 2-(1,3-dioxo-1,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168052 ((4-Iodo-phenyl)-thiocarbamic acid 2-(1,3-dioxo-1,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103N+Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103R) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103N+Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168060 (CHEMBL195108 | N-[2-(4-Iodo-phenylthiocarbamoyloxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103N+Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168060 (CHEMBL195108 | N-[2-(4-Iodo-phenylthiocarbamoyloxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103R) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Rattus norvegicus (Rat)-RAT) | BDBM50446782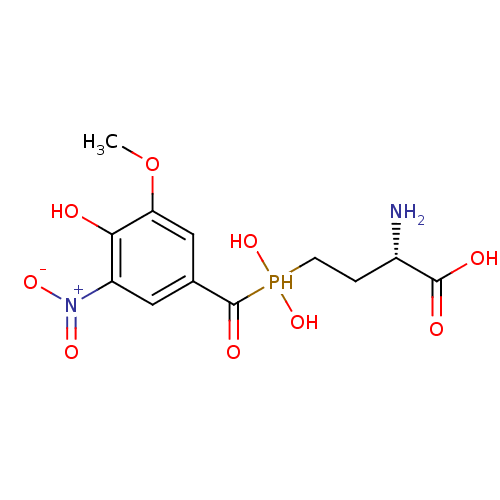 (CHEMBL3114672) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie et de Biochimie Pharmacologiques et Toxicologiques , CNRS UMR 8601, Université Paris Descartes, Sorbonne Paris Cité , 45 rue des Saints-Pères , 75270 Paris Cedex 06 , France. Curated by ChEMBL | Assay Description Antagonist activity at rat GluN1a/GluN2A receptors expressed in Xenopus laevis oocytes assessed as inhibition of L-glutamate/glucine induced current ... | J Med Chem 61: 1969-1989 (2018) Article DOI: 10.1021/acs.jmedchem.7b01438 BindingDB Entry DOI: 10.7270/Q2DZ0BR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2B (RAT-Mus musculus) | BDBM50446782 (CHEMBL3114672) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie et de Biochimie Pharmacologiques et Toxicologiques , CNRS UMR 8601, Université Paris Descartes, Sorbonne Paris Cité , 45 rue des Saints-Pères , 75270 Paris Cedex 06 , France. Curated by ChEMBL | Assay Description Antagonist activity at rat GluN1a/mouse GluN2B receptors expressed in Xenopus laevis oocytes assessed as inhibition of L-glutamate/glucine induced cu... | J Med Chem 61: 1969-1989 (2018) Article DOI: 10.1021/acs.jmedchem.7b01438 BindingDB Entry DOI: 10.7270/Q2DZ0BR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM82006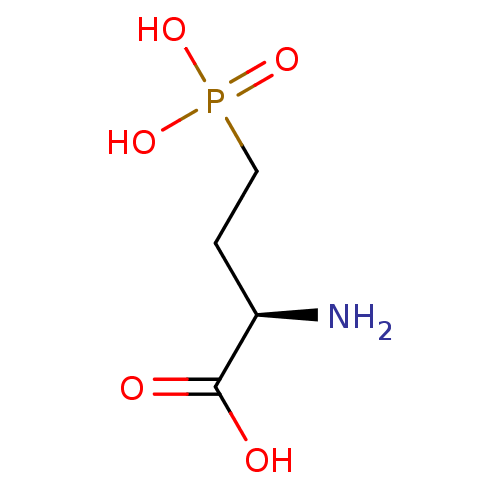 (D-AP4 | D-APB | US9212196, L-AP4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196906 (US9212196, Derivative 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196907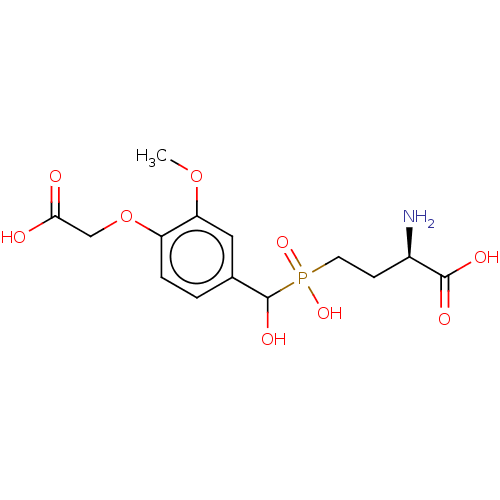 (US9212196, Derivative 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196908 (US9212196, Derivative 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196909 (US9212196, Derivative 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196910 (US9212196, Derivative 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196911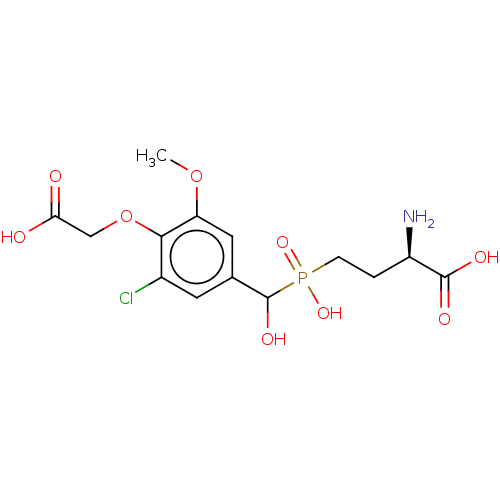 (US9212196, Derivative 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196912 (US9212196, Derivative 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196913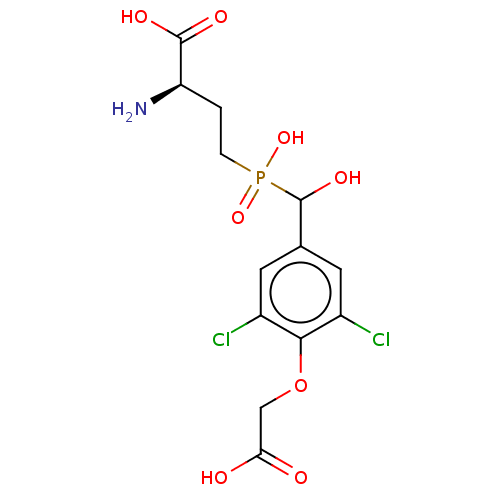 (US9212196, Derivative 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196914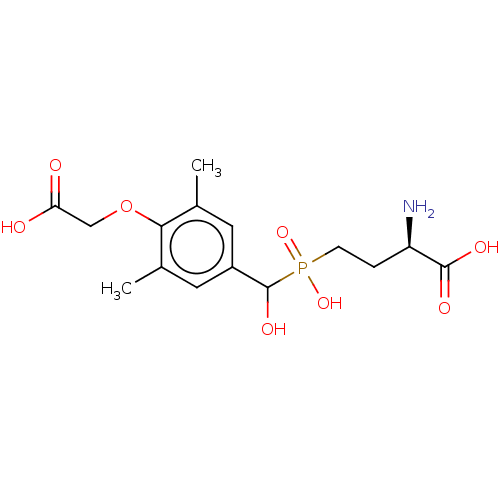 (US9212196, Derivative 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196915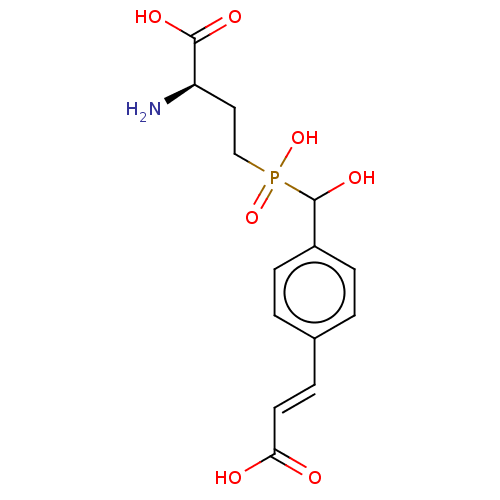 (US9212196, Derivative 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196916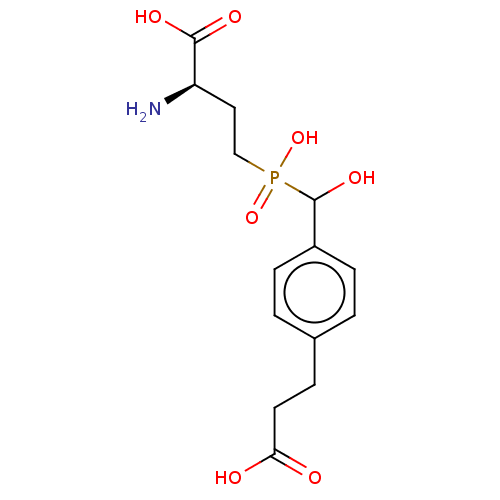 (US9212196, Derivative 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 980 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196917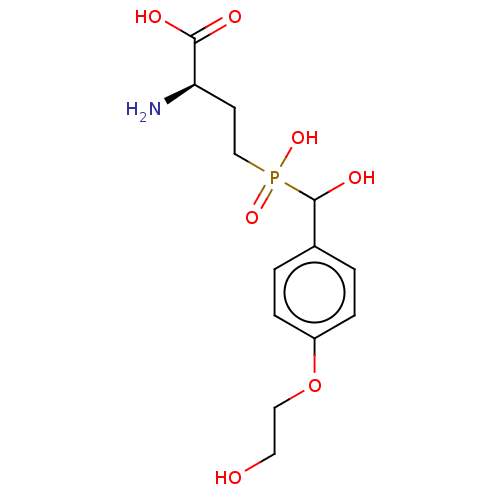 (US9212196, Derivative 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196918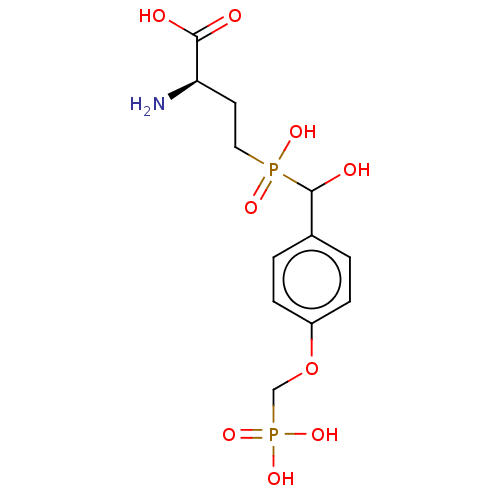 (US9212196, Derivative 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196919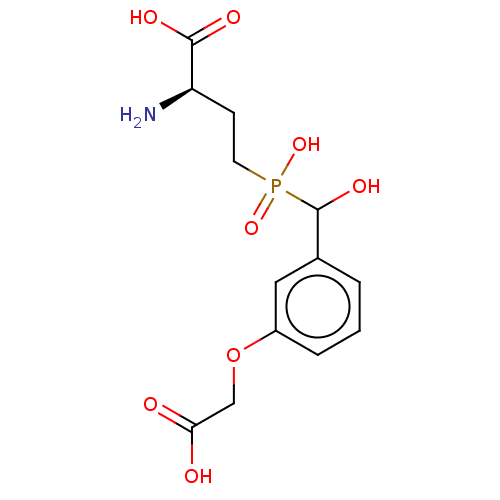 (US9212196, Derivative 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196920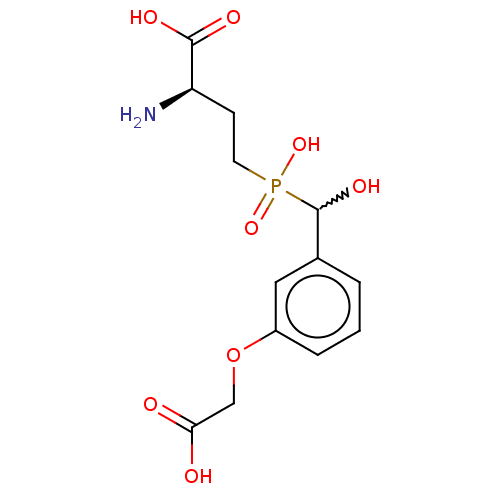 (US9212196, Derivative 16 | US9212196, Derivative 1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196920 (US9212196, Derivative 16 | US9212196, Derivative 1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196922 (US9212196, Derivative 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196923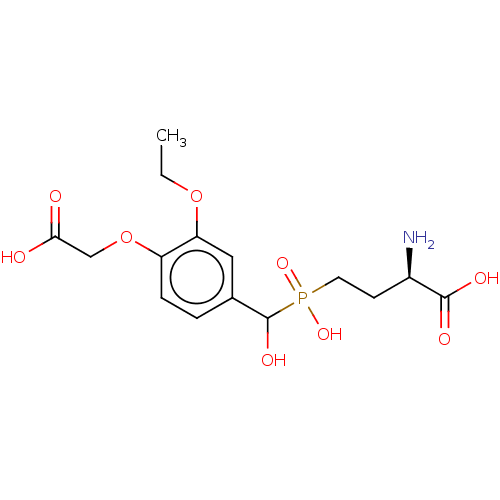 (US9212196, Derivative 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196924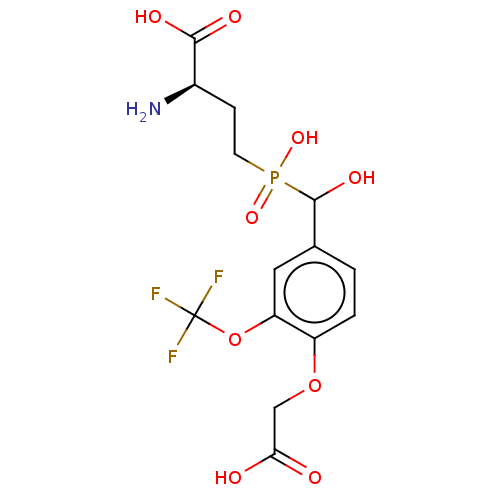 (US9212196, Derivative 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM196925 (US9212196, Derivative 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
UNIVERSITE PARIS DESCARTES; UNIVERSITE D'AUVERGNE US Patent | Assay Description Metabotropic glutamate receptors were transiently transfected in HEK293 cells by electroporation as described elsewhere (Brabet I. et al., 1998) and ... | US Patent US9212196 (2015) BindingDB Entry DOI: 10.7270/Q28G8JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 480 total ) | Next | Last >> |