 Found 100 hits with Last Name = 'chabrier' and Initial = 'pe'
Found 100 hits with Last Name = 'chabrier' and Initial = 'pe' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21283

(3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...)Show SMILES CN1CCCCC1Cn1cc(C(=O)c2cc(ccc2I)[N+]([O-])=O)c2ccccc12 Show InChI InChI=1S/C22H22IN3O3/c1-24-11-5-4-6-16(24)13-25-14-19(17-7-2-3-8-21(17)25)22(27)18-12-15(26(28)29)9-10-20(18)23/h2-3,7-10,12,14,16H,4-6,11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089965
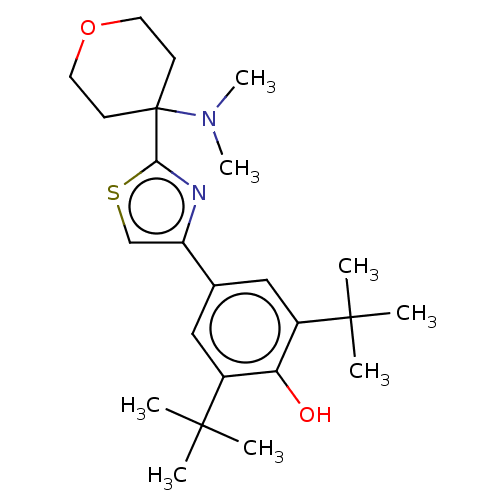
(CHEMBL3581222)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O2S/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089958
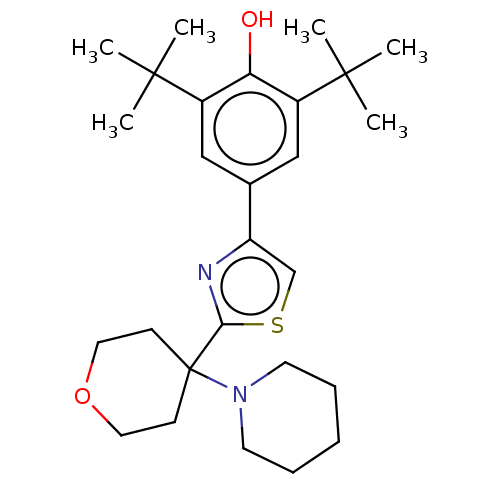
(CHEMBL3581226)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(CCOCC1)N1CCCCC1 Show InChI InChI=1S/C27H40N2O2S/c1-25(2,3)20-16-19(17-21(23(20)30)26(4,5)6)22-18-32-24(28-22)27(10-14-31-15-11-27)29-12-8-7-9-13-29/h16-18,30H,7-15H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089956
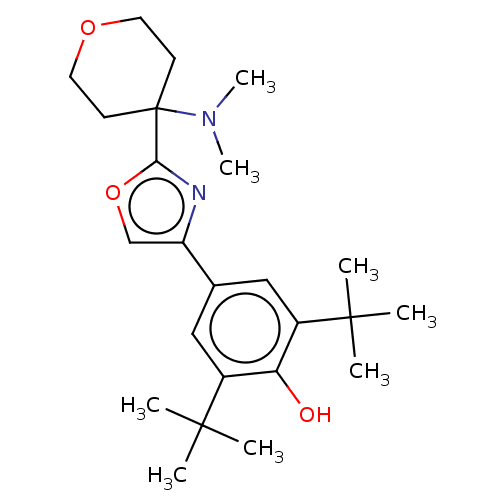
(CHEMBL3581228)Show SMILES CN(C)C1(CCOCC1)c1nc(co1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O3/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089959
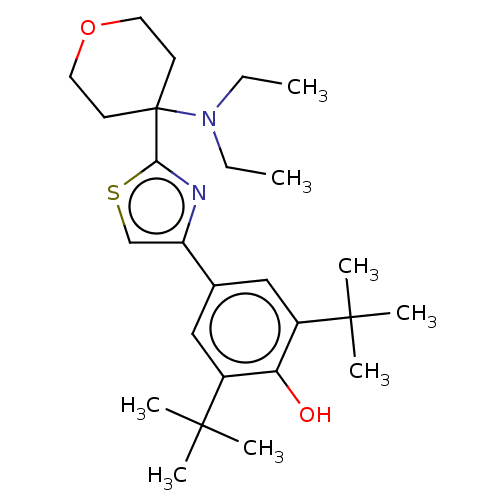
(CHEMBL3581225)Show SMILES CCN(CC)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C26H40N2O2S/c1-9-28(10-2)26(11-13-30-14-12-26)23-27-21(17-31-23)18-15-19(24(3,4)5)22(29)20(16-18)25(6,7)8/h15-17,29H,9-14H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089968
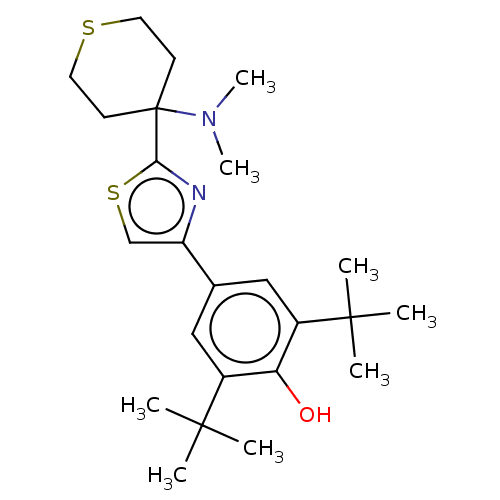
(CHEMBL3581220)Show SMILES CN(C)C1(CCSCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS2/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089970
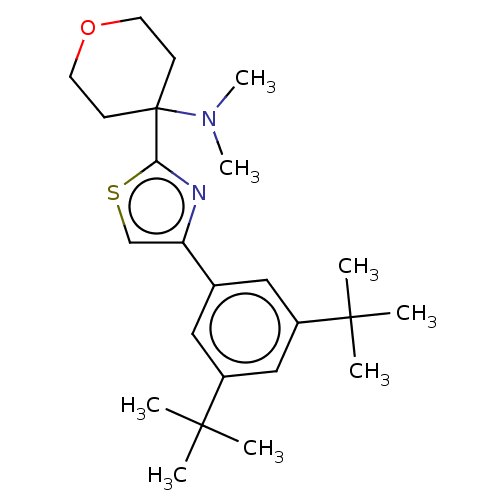
(CHEMBL3581229)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(cc(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS/c1-22(2,3)18-13-17(14-19(15-18)23(4,5)6)20-16-28-21(25-20)24(26(7)8)9-11-27-12-10-24/h13-16H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089963
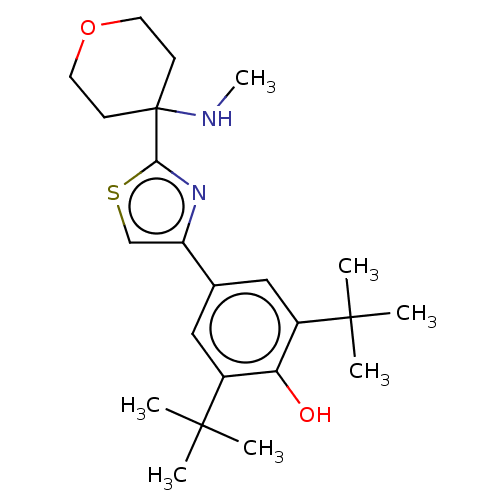
(CHEMBL3581224)Show SMILES CNC1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C23H34N2O2S/c1-21(2,3)16-12-15(13-17(19(16)26)22(4,5)6)18-14-28-20(25-18)23(24-7)8-10-27-11-9-23/h12-14,24,26H,8-11H2,1-7H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089957
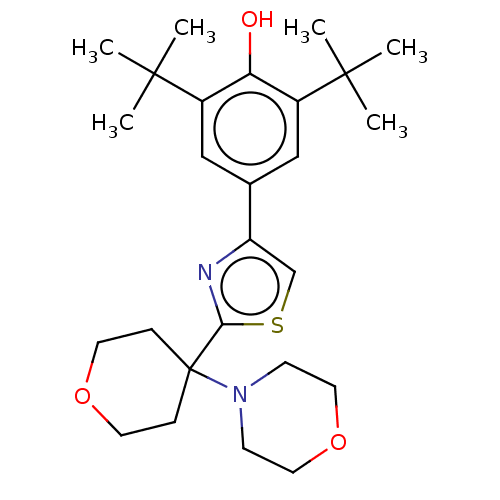
(CHEMBL3581227)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(CCOCC1)N1CCOCC1 Show InChI InChI=1S/C26H38N2O3S/c1-24(2,3)19-15-18(16-20(22(19)29)25(4,5)6)21-17-32-23(27-21)26(7-11-30-12-8-26)28-9-13-31-14-10-28/h15-17,29H,7-14H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089956

(CHEMBL3581228)Show SMILES CN(C)C1(CCOCC1)c1nc(co1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O3/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 817 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089965

(CHEMBL3581222)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O2S/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 857 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21283

(3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...)Show SMILES CN1CCCCC1Cn1cc(C(=O)c2cc(ccc2I)[N+]([O-])=O)c2ccccc12 Show InChI InChI=1S/C22H22IN3O3/c1-24-11-5-4-6-16(24)13-25-14-19(17-7-2-3-8-21(17)25)22(27)18-12-15(26(28)29)9-10-20(18)23/h2-3,7-10,12,14,16H,4-6,11,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089958

(CHEMBL3581226)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(CCOCC1)N1CCCCC1 Show InChI InChI=1S/C27H40N2O2S/c1-25(2,3)20-16-19(17-21(23(20)30)26(4,5)6)22-18-32-24(28-22)27(10-14-31-15-11-27)29-12-8-7-9-13-29/h16-18,30H,7-15H2,1-6H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089963

(CHEMBL3581224)Show SMILES CNC1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C23H34N2O2S/c1-21(2,3)16-12-15(13-17(19(16)26)22(4,5)6)18-14-28-20(25-18)23(24-7)8-10-27-11-9-23/h12-14,24,26H,8-11H2,1-7H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089968

(CHEMBL3581220)Show SMILES CN(C)C1(CCSCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS2/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089964
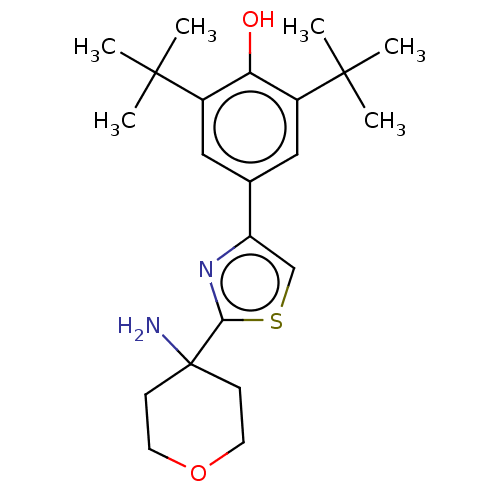
(CHEMBL3581223)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(N)CCOCC1 Show InChI InChI=1S/C22H32N2O2S/c1-20(2,3)15-11-14(12-16(18(15)25)21(4,5)6)17-13-27-19(24-17)22(23)7-9-26-10-8-22/h11-13,25H,7-10,23H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089959

(CHEMBL3581225)Show SMILES CCN(CC)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C26H40N2O2S/c1-9-28(10-2)26(11-13-30-14-12-26)23-27-21(17-31-23)18-15-19(24(3,4)5)22(29)20(16-18)25(6,7)8/h15-17,29H,9-14H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089970

(CHEMBL3581229)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(cc(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS/c1-22(2,3)18-13-17(14-19(15-18)23(4,5)6)20-16-28-21(25-20)24(26(7)8)9-11-27-12-10-24/h13-16H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089966
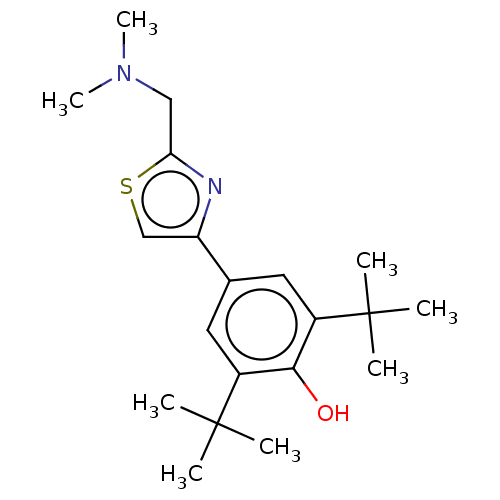
(CHEMBL3581221)Show SMILES CN(C)Cc1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C20H30N2OS/c1-19(2,3)14-9-13(10-15(18(14)23)20(4,5)6)16-12-24-17(21-16)11-22(7)8/h9-10,12,23H,11H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089964

(CHEMBL3581223)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(N)CCOCC1 Show InChI InChI=1S/C22H32N2O2S/c1-20(2,3)15-11-14(12-16(18(15)25)21(4,5)6)17-13-27-19(24-17)22(23)7-9-26-10-8-22/h11-13,25H,7-10,23H2,1-6H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1/1 catalytic subunit
(Homo sapiens (Human)) | BDBM50179750
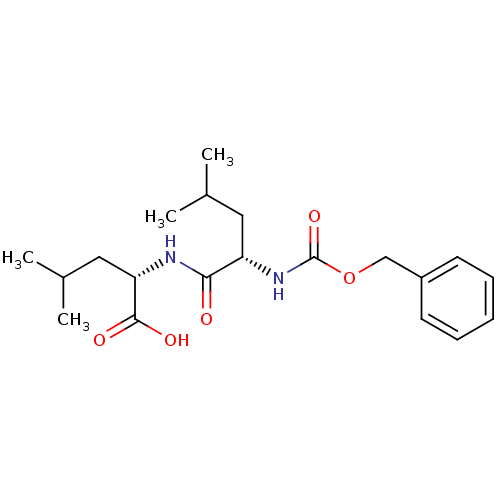
((S)-2-((S)-2-(benzyloxycarbonyl)-4-methylpentanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C(O)=O Show InChI InChI=1S/C20H30N2O5/c1-13(2)10-16(18(23)21-17(19(24)25)11-14(3)4)22-20(26)27-12-15-8-6-5-7-9-15/h5-9,13-14,16-17H,10-12H2,1-4H3,(H,21,23)(H,22,26)(H,24,25)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of isolated human calpain1 |
Bioorg Med Chem Lett 16: 1586-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.036
BindingDB Entry DOI: 10.7270/Q2T15367 |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1/1 catalytic subunit
(Homo sapiens (Human)) | BDBM50179749
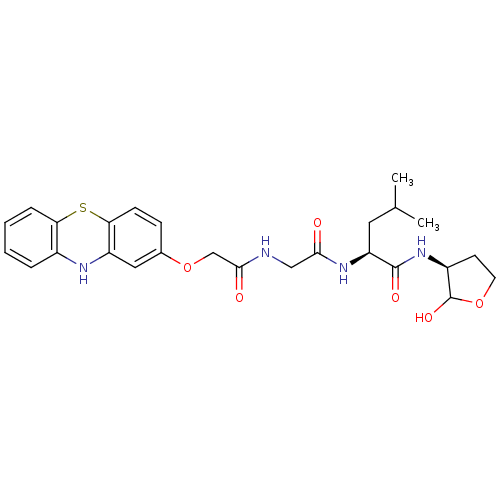
((S)-2-(2-(2-(10H-phenothiazin-2-yloxy)acetamido)ac...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)COc1ccc2Sc3ccccc3Nc2c1)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C26H32N4O6S/c1-15(2)11-20(25(33)30-18-9-10-35-26(18)34)29-23(31)13-27-24(32)14-36-16-7-8-22-19(12-16)28-17-5-3-4-6-21(17)37-22/h3-8,12,15,18,20,26,28,34H,9-11,13-14H2,1-2H3,(H,27,32)(H,29,31)(H,30,33)/t18-,20-,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of isolated human calpain1 |
Bioorg Med Chem Lett 16: 1586-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.036
BindingDB Entry DOI: 10.7270/Q2T15367 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223720
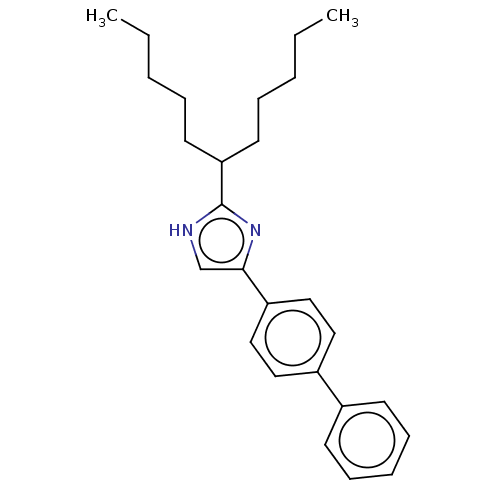
(CHEMBL332257)Show SMILES CCCCCC(CCCCC)c1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C26H34N2/c1-3-5-8-14-24(15-9-6-4-2)26-27-20-25(28-26)23-18-16-22(17-19-23)21-12-10-7-11-13-21/h7,10-13,16-20,24H,3-6,8-9,14-15H2,1-2H3,(H,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223731
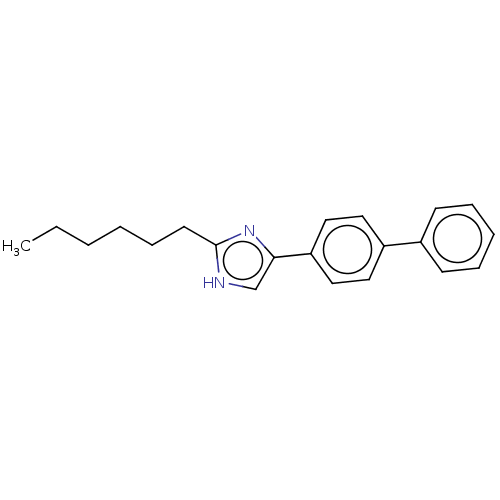
(CHEMBL118603)Show InChI InChI=1S/C21H24N2/c1-2-3-4-8-11-21-22-16-20(23-21)19-14-12-18(13-15-19)17-9-6-5-7-10-17/h5-7,9-10,12-16H,2-4,8,11H2,1H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223721
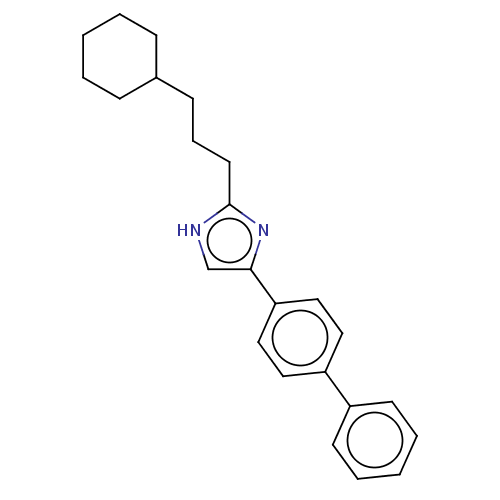
(CHEMBL331513)Show SMILES C(CC1CCCCC1)Cc1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C24H28N2/c1-3-8-19(9-4-1)10-7-13-24-25-18-23(26-24)22-16-14-21(15-17-22)20-11-5-2-6-12-20/h2,5-6,11-12,14-19H,1,3-4,7-10,13H2,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50149195
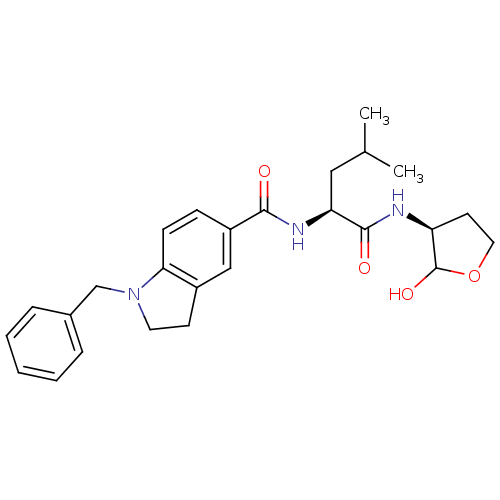
(1-Benzyl-2,3-dihydro-1H-indole-5-carboxylic acid [...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc2N(Cc3ccccc3)CCc2c1)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C26H33N3O4/c1-17(2)14-22(25(31)27-21-11-13-33-26(21)32)28-24(30)20-8-9-23-19(15-20)10-12-29(23)16-18-6-4-3-5-7-18/h3-9,15,17,21-22,26,32H,10-14,16H2,1-2H3,(H,27,31)(H,28,30)/t21-,22-,26?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory activity against on human calpain 1 |
Bioorg Med Chem Lett 14: 3825-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.105
BindingDB Entry DOI: 10.7270/Q2NK3DH6 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50223715

(CHEMBL3706732)Show SMILES CC(C)C[C@H](NC(=O)c1ccc2Sc3ccccc3Nc2c1)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C23H27N3O4S/c1-13(2)11-18(22(28)25-16-9-10-30-23(16)29)26-21(27)14-7-8-20-17(12-14)24-15-5-3-4-6-19(15)31-20/h3-8,12-13,16,18,23-24,29H,9-11H2,1-2H3,(H,25,28)(H,26,27)/t16-,18-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory activity against on human calpain 1 |
Bioorg Med Chem Lett 14: 3825-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.105
BindingDB Entry DOI: 10.7270/Q2NK3DH6 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223726
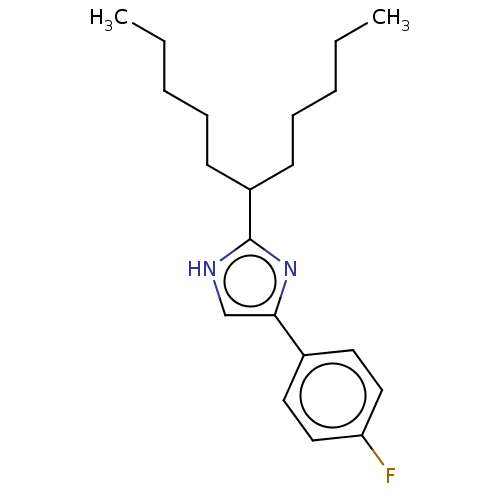
(CHEMBL117331)Show InChI InChI=1S/C20H29FN2/c1-3-5-7-9-17(10-8-6-4-2)20-22-15-19(23-20)16-11-13-18(21)14-12-16/h11-15,17H,3-10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1/1 catalytic subunit
(Homo sapiens (Human)) | BDBM50179745
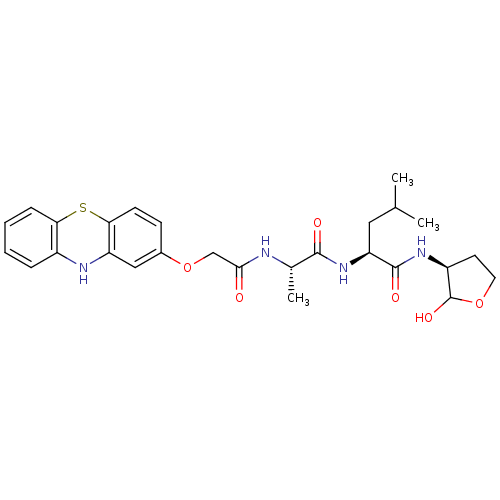
((S)-2-((S)-2-(2-(10H-phenothiazin-2-yloxy)acetamid...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)COc1ccc2Sc3ccccc3Nc2c1)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C27H34N4O6S/c1-15(2)12-21(26(34)30-19-10-11-36-27(19)35)31-25(33)16(3)28-24(32)14-37-17-8-9-23-20(13-17)29-18-6-4-5-7-22(18)38-23/h4-9,13,15-16,19,21,27,29,35H,10-12,14H2,1-3H3,(H,28,32)(H,30,34)(H,31,33)/t16-,19-,21-,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of isolated human calpain1 |
Bioorg Med Chem Lett 16: 1586-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.036
BindingDB Entry DOI: 10.7270/Q2T15367 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223735
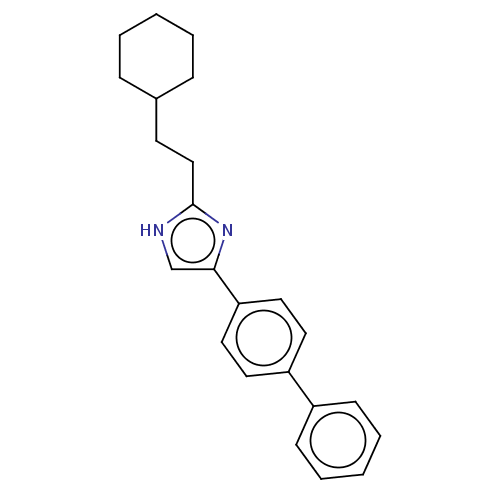
(CHEMBL119139)Show SMILES C(Cc1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1)C1CCCCC1 Show InChI InChI=1S/C23H26N2/c1-3-7-18(8-4-1)11-16-23-24-17-22(25-23)21-14-12-20(13-15-21)19-9-5-2-6-10-19/h2,5-6,9-10,12-15,17-18H,1,3-4,7-8,11,16H2,(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223734
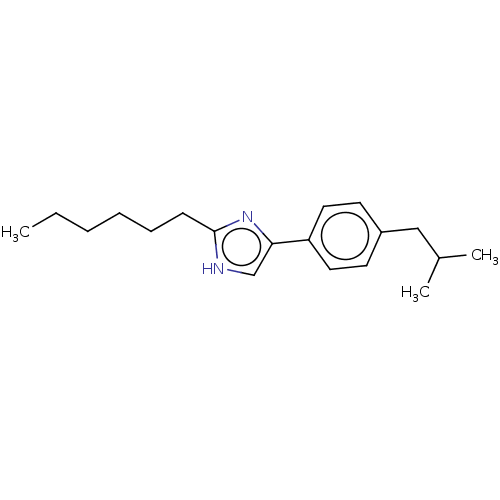
(CHEMBL331942)Show InChI InChI=1S/C19H28N2/c1-4-5-6-7-8-19-20-14-18(21-19)17-11-9-16(10-12-17)13-15(2)3/h9-12,14-15H,4-8,13H2,1-3H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223733
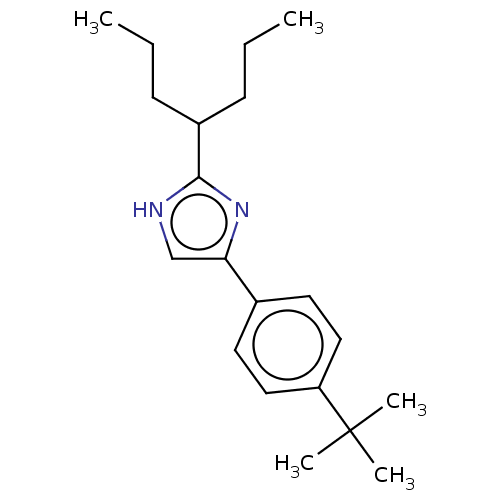
(CHEMBL120306)Show InChI InChI=1S/C20H30N2/c1-6-8-16(9-7-2)19-21-14-18(22-19)15-10-12-17(13-11-15)20(3,4)5/h10-14,16H,6-9H2,1-5H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1/1 catalytic subunit
(Homo sapiens (Human)) | BDBM50179747
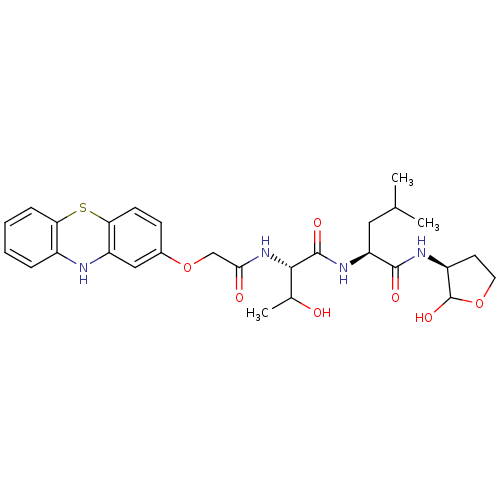
((S)-2-((2S,3S)-2-(2-(10H-phenothiazin-2-yloxy)acet...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)COc1ccc2Sc3ccccc3Nc2c1)C(C)O)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C28H36N4O7S/c1-15(2)12-21(26(35)30-19-10-11-38-28(19)37)31-27(36)25(16(3)33)32-24(34)14-39-17-8-9-23-20(13-17)29-18-6-4-5-7-22(18)40-23/h4-9,13,15-16,19,21,25,28-29,33,37H,10-12,14H2,1-3H3,(H,30,35)(H,31,36)(H,32,34)/t16?,19-,21-,25-,28?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of isolated human calpain1 |
Bioorg Med Chem Lett 16: 1586-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.036
BindingDB Entry DOI: 10.7270/Q2T15367 |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1/1 catalytic subunit
(Homo sapiens (Human)) | BDBM50179743
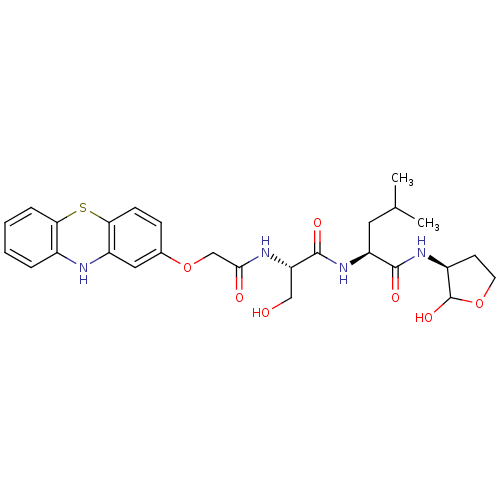
((S)-2-((S)-2-(2-(10H-phenothiazin-2-yloxy)acetamid...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)COc1ccc2Sc3ccccc3Nc2c1)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C27H34N4O7S/c1-15(2)11-20(25(34)30-18-9-10-37-27(18)36)31-26(35)21(13-32)29-24(33)14-38-16-7-8-23-19(12-16)28-17-5-3-4-6-22(17)39-23/h3-8,12,15,18,20-21,27-28,32,36H,9-11,13-14H2,1-2H3,(H,29,33)(H,30,34)(H,31,35)/t18-,20-,21-,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of isolated human calpain1 |
Bioorg Med Chem Lett 16: 1586-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.036
BindingDB Entry DOI: 10.7270/Q2T15367 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50065843
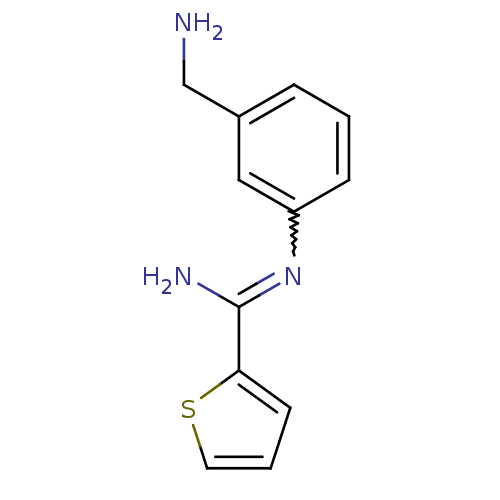
(CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...)Show InChI InChI=1S/C12H13N3S/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum |
Bioorg Med Chem Lett 13: 209-12 (2002)
BindingDB Entry DOI: 10.7270/Q2K9382B |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1/1 catalytic subunit
(Homo sapiens (Human)) | BDBM50179744
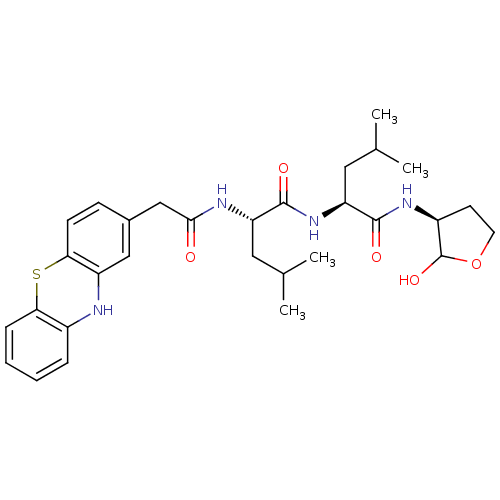
((S)-4-Methyl-2-[(S)-4-methyl-2-(2-10H-phenothiazin...)Show SMILES CC(C)C[C@H](NC(=O)Cc1ccc2Sc3ccccc3Nc2c1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C30H40N4O5S/c1-17(2)13-23(28(36)34-24(14-18(3)4)29(37)33-21-11-12-39-30(21)38)32-27(35)16-19-9-10-26-22(15-19)31-20-7-5-6-8-25(20)40-26/h5-10,15,17-18,21,23-24,30-31,38H,11-14,16H2,1-4H3,(H,32,35)(H,33,37)(H,34,36)/t21-,23-,24-,30?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of isolated human calpain1 |
Bioorg Med Chem Lett 16: 1586-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.036
BindingDB Entry DOI: 10.7270/Q2T15367 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223722
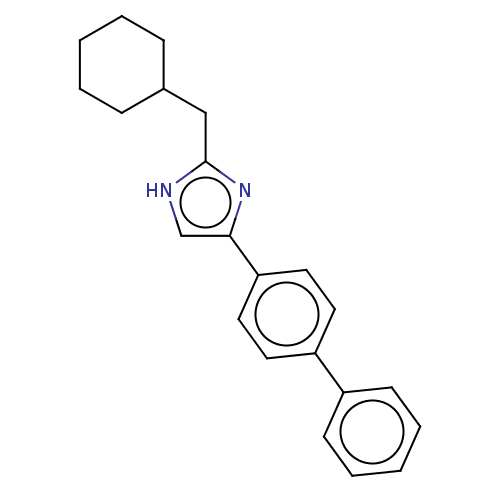
(CHEMBL334104)Show SMILES C(C1CCCCC1)c1nc(c[nH]1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H24N2/c1-3-7-17(8-4-1)15-22-23-16-21(24-22)20-13-11-19(12-14-20)18-9-5-2-6-10-18/h2,5-6,9-14,16-17H,1,3-4,7-8,15H2,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1/1 catalytic subunit
(Homo sapiens (Human)) | BDBM50179741
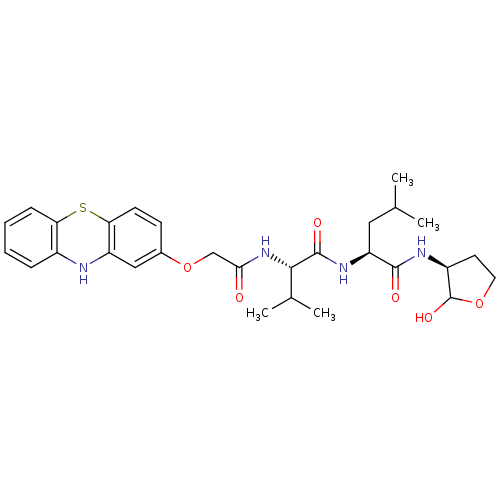
((S)-2-((S)-2-(2-(10H-phenothiazin-2-yloxy)acetamid...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)COc1ccc2Sc3ccccc3Nc2c1)C(C)C)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C29H38N4O6S/c1-16(2)13-22(27(35)31-20-11-12-38-29(20)37)32-28(36)26(17(3)4)33-25(34)15-39-18-9-10-24-21(14-18)30-19-7-5-6-8-23(19)40-24/h5-10,14,16-17,20,22,26,29-30,37H,11-13,15H2,1-4H3,(H,31,35)(H,32,36)(H,33,34)/t20-,22-,26-,29?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of isolated human calpain1 |
Bioorg Med Chem Lett 16: 1586-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.036
BindingDB Entry DOI: 10.7270/Q2T15367 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50122299
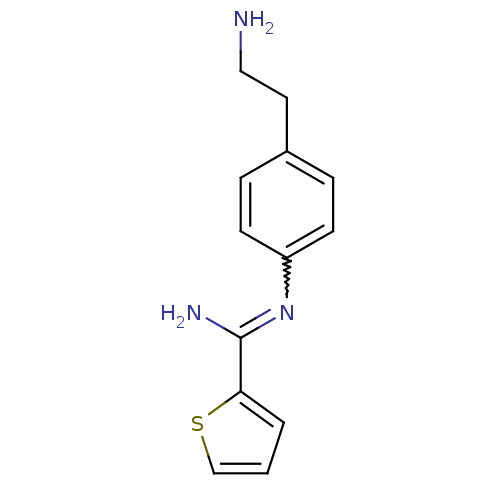
(CHEMBL538500 | N-[4-(2-Amino-ethyl)-phenyl]-thioph...)Show InChI InChI=1S/C13H15N3S/c14-8-7-10-3-5-11(6-4-10)16-13(15)12-2-1-9-17-12/h1-6,9H,7-8,14H2,(H2,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum |
Bioorg Med Chem Lett 13: 209-12 (2002)
BindingDB Entry DOI: 10.7270/Q2K9382B |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50223717

(CHEMBL3706727)Show SMILES CC(C)C[C@H](NC(=O)c1cccc2Sc3ccccc3Nc12)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C23H27N3O4S/c1-13(2)12-17(22(28)25-16-10-11-30-23(16)29)26-21(27)14-6-5-9-19-20(14)24-15-7-3-4-8-18(15)31-19/h3-9,13,16-17,23-24,29H,10-12H2,1-2H3,(H,25,28)(H,26,27)/t16-,17-,23?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory activity against on calpain in C6 glial cells |
Bioorg Med Chem Lett 14: 3825-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.105
BindingDB Entry DOI: 10.7270/Q2NK3DH6 |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1/1 catalytic subunit
(Homo sapiens (Human)) | BDBM50179738
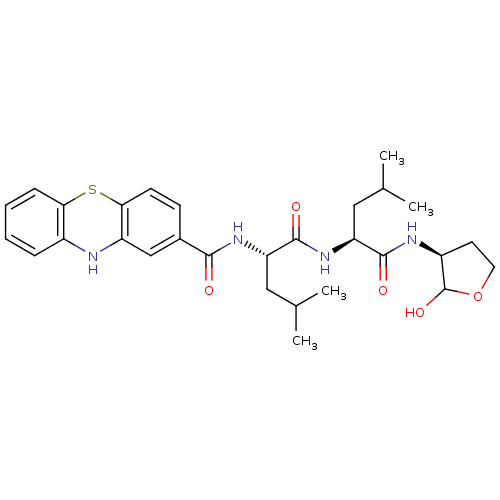
(CHEMBL203244 | N-((S)-1-((S)-1-((3S)-2-hydroxy-tet...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)c1ccc2Sc3ccccc3Nc2c1)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C29H38N4O5S/c1-16(2)13-22(28(36)33-23(14-17(3)4)27(35)31-20-11-12-38-29(20)37)32-26(34)18-9-10-25-21(15-18)30-19-7-5-6-8-24(19)39-25/h5-10,15-17,20,22-23,29-30,37H,11-14H2,1-4H3,(H,31,35)(H,32,34)(H,33,36)/t20-,22-,23-,29?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of isolated human calpain1 |
Bioorg Med Chem Lett 16: 1586-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.036
BindingDB Entry DOI: 10.7270/Q2T15367 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223723
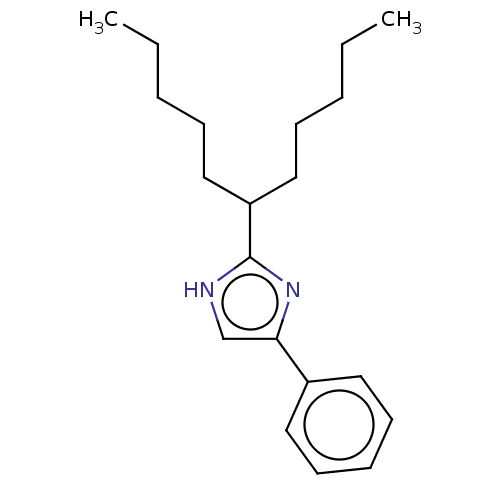
(CHEMBL119494)Show InChI InChI=1S/C20H30N2/c1-3-5-8-14-18(15-9-6-4-2)20-21-16-19(22-20)17-12-10-7-11-13-17/h7,10-13,16,18H,3-6,8-9,14-15H2,1-2H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1/1 catalytic subunit
(Homo sapiens (Human)) | BDBM50179746
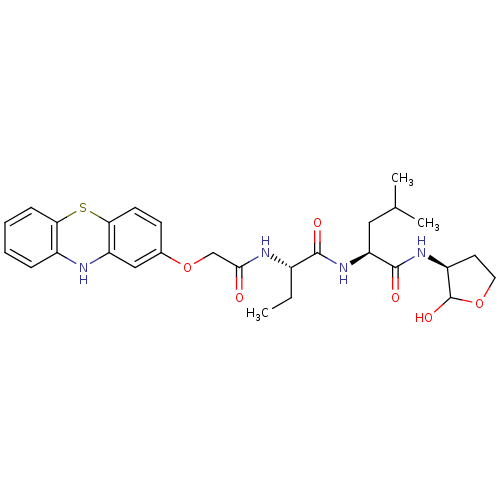
((S)-2-((S)-2-(2-(10H-phenothiazin-2-yloxy)acetamid...)Show SMILES CC[C@H](NC(=O)COc1ccc2Sc3ccccc3Nc2c1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C28H36N4O6S/c1-4-18(26(34)32-22(13-16(2)3)27(35)31-20-11-12-37-28(20)36)30-25(33)15-38-17-9-10-24-21(14-17)29-19-7-5-6-8-23(19)39-24/h5-10,14,16,18,20,22,28-29,36H,4,11-13,15H2,1-3H3,(H,30,33)(H,31,35)(H,32,34)/t18-,20-,22-,28?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of isolated human calpain1 |
Bioorg Med Chem Lett 16: 1586-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.036
BindingDB Entry DOI: 10.7270/Q2T15367 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223725
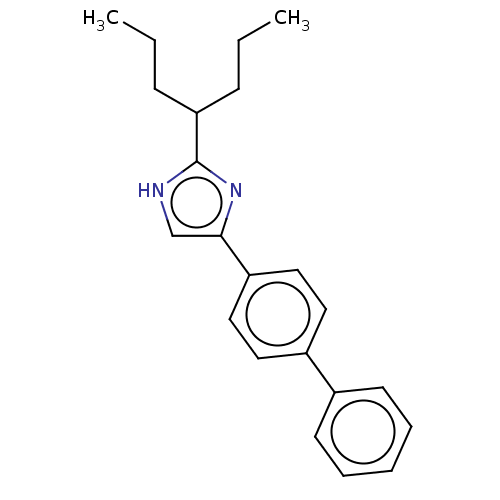
(CHEMBL118682)Show InChI InChI=1S/C22H26N2/c1-3-8-20(9-4-2)22-23-16-21(24-22)19-14-12-18(13-15-19)17-10-6-5-7-11-17/h5-7,10-16,20H,3-4,8-9H2,1-2H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1/1 catalytic subunit
(Homo sapiens (Human)) | BDBM50179739
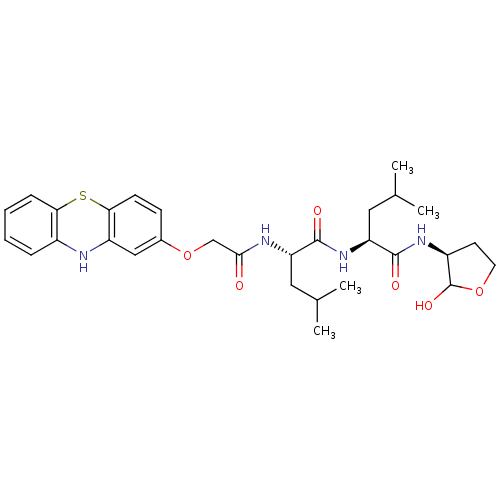
((S)-4-Methyl-2-{(S)-4-methyl-2-[2-(10H-phenothiazi...)Show SMILES CC(C)C[C@H](NC(=O)COc1ccc2Sc3ccccc3Nc2c1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C30H40N4O6S/c1-17(2)13-23(28(36)34-24(14-18(3)4)29(37)33-21-11-12-39-30(21)38)32-27(35)16-40-19-9-10-26-22(15-19)31-20-7-5-6-8-25(20)41-26/h5-10,15,17-18,21,23-24,30-31,38H,11-14,16H2,1-4H3,(H,32,35)(H,33,37)(H,34,36)/t21-,23-,24-,30?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of isolated human calpain1 |
Bioorg Med Chem Lett 16: 1586-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.036
BindingDB Entry DOI: 10.7270/Q2T15367 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50122300
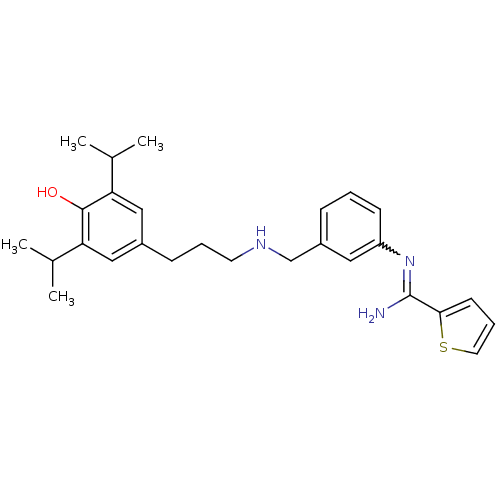
(CHEMBL541300 | N-(3-{[3-(4-Hydroxy-3,5-diisopropyl...)Show SMILES CC(C)c1cc(CCCNCc2cccc(c2)N=C(N)c2cccs2)cc(C(C)C)c1O |w:17.17| Show InChI InChI=1S/C27H35N3OS/c1-18(2)23-15-20(16-24(19(3)4)26(23)31)9-6-12-29-17-21-8-5-10-22(14-21)30-27(28)25-11-7-13-32-25/h5,7-8,10-11,13-16,18-19,29,31H,6,9,12,17H2,1-4H3,(H2,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum |
Bioorg Med Chem Lett 13: 209-12 (2002)
BindingDB Entry DOI: 10.7270/Q2K9382B |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM196649
(US9212144, 4)Show InChI InChI=1S/C20H20ClN3S/c21-17-4-1-3-16(13-17)14-23-11-10-15-6-8-18(9-7-15)24-20(22)19-5-2-12-25-19/h1-9,12-13,23H,10-11,14H2,(H2,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| DrugBank
PDB
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum |
Bioorg Med Chem Lett 13: 209-12 (2002)
BindingDB Entry DOI: 10.7270/Q2K9382B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calpain small subunit 1/1 catalytic subunit
(Homo sapiens (Human)) | BDBM50179754
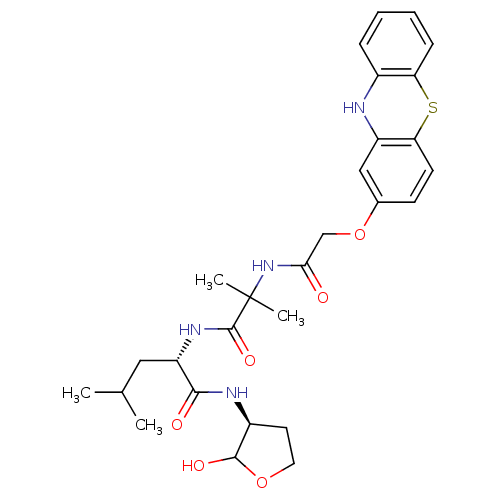
((S)-2-(2-(2-(10H-phenothiazin-2-yloxy)acetamido)-2...)Show SMILES CC(C)C[C@H](NC(=O)C(C)(C)NC(=O)COc1ccc2Sc3ccccc3Nc2c1)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C28H36N4O6S/c1-16(2)13-21(25(34)30-19-11-12-37-26(19)35)31-27(36)28(3,4)32-24(33)15-38-17-9-10-23-20(14-17)29-18-7-5-6-8-22(18)39-23/h5-10,14,16,19,21,26,29,35H,11-13,15H2,1-4H3,(H,30,34)(H,31,36)(H,32,33)/t19-,21-,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibition of isolated human calpain1 |
Bioorg Med Chem Lett 16: 1586-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.036
BindingDB Entry DOI: 10.7270/Q2T15367 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Rattus norvegicus) | BDBM50223729
(CHEMBL418863)Show InChI InChI=1S/C16H19FN2/c17-14-8-6-13(7-9-14)15-11-18-16(19-15)10-12-4-2-1-3-5-12/h6-9,11-12H,1-5,10H2,(H,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Concentration required to inhibit [3H]BTX binding to Sodium channel of rat brain |
Bioorg Med Chem Lett 14: 3521-3 (2004)
BindingDB Entry DOI: 10.7270/Q2474D2N |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50149199

(6-Hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic...)Show SMILES CC(C)C[C@H](NC(=O)C1(C)CCc2c(C)c(O)c(C)c(C)c2O1)C(=O)N[C@H]1CCOC1O Show InChI InChI=1S/C24H36N2O6/c1-12(2)11-18(21(28)25-17-8-10-31-22(17)29)26-23(30)24(6)9-7-16-15(5)19(27)13(3)14(4)20(16)32-24/h12,17-18,22,27,29H,7-11H2,1-6H3,(H,25,28)(H,26,30)/t17-,18-,22?,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour
Curated by ChEMBL
| Assay Description
Inhibitory activity against on human calpain 1 |
Bioorg Med Chem Lett 14: 3825-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.105
BindingDB Entry DOI: 10.7270/Q2NK3DH6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data