

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085666 ((2S,3S,4R,5R)-5-{6-(2,2-Diphenyl-ethylamino)-2-[2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085674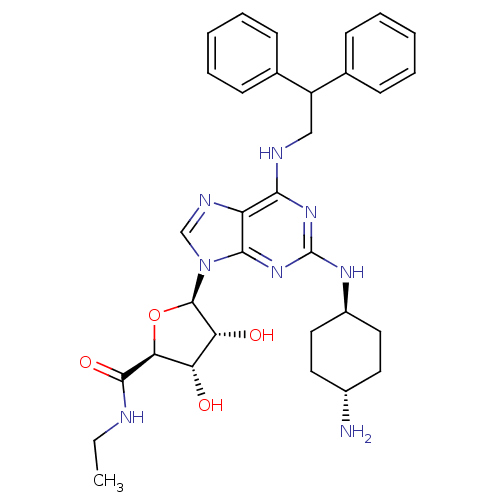 ((2S,3S,4R,5R)-5-[2-(4-Amino-cyclohexylamino)-6-(2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085671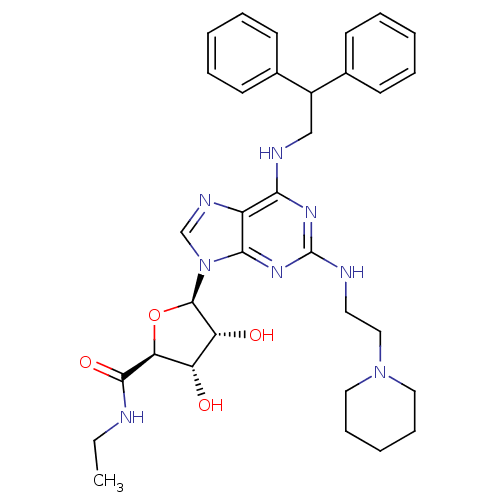 ((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-(2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085668 ((2S,3S,4R,5R)-5-[6-Amino-2-((1R,2R)-2-hydroxy-cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085662 ((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-((1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085661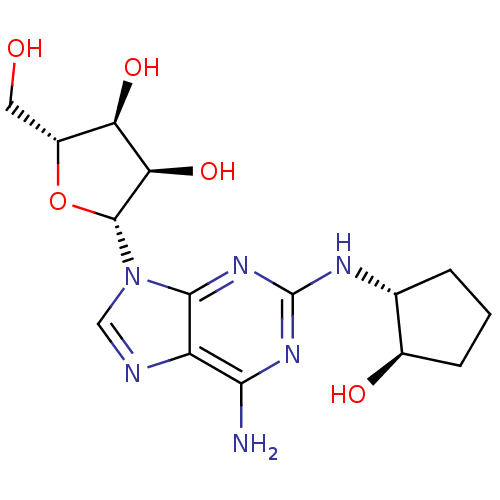 ((2R,3R,4S,5R)-2-[6-Amino-2-((1R,2R)-2-hydroxy-cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085664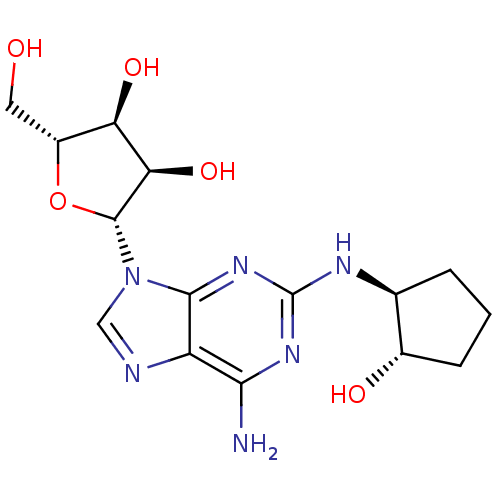 ((2R,3R,4S,5R)-2-[6-Amino-2-((1S,2S)-2-hydroxy-cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085663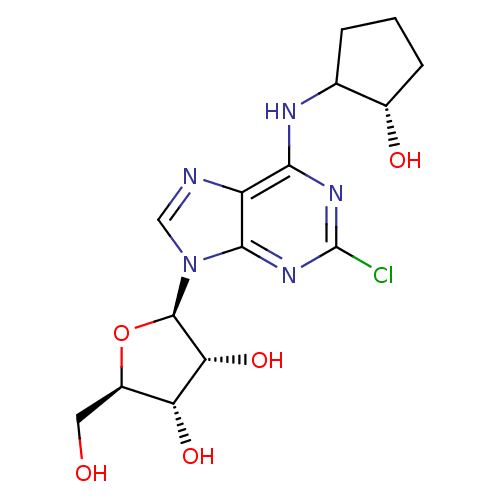 ((2R,3R,4S,5R)-2-[2-Chloro-6-((R)-(S)-2-hydroxy-cyc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085670 ((2S,3S,4R,5R)-5-(6-Amino-2-cyclopentylamino-purin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085672 ((2R,3R,4S,5R)-2-(6-Amino-2-cyclopentylamino-purin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate synthase (Escherichia coli (strain K12)) | BDBM50280172 (1,3,4-Trihydroxy-5-phosphonomethyl-cyclohexanecarb...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against calf thymus ribonucleotide reductase | Bioorg Med Chem Lett 2: 1349-1352 (1992) Article DOI: 10.1016/S0960-894X(00)80510-7 BindingDB Entry DOI: 10.7270/Q2MW2H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085665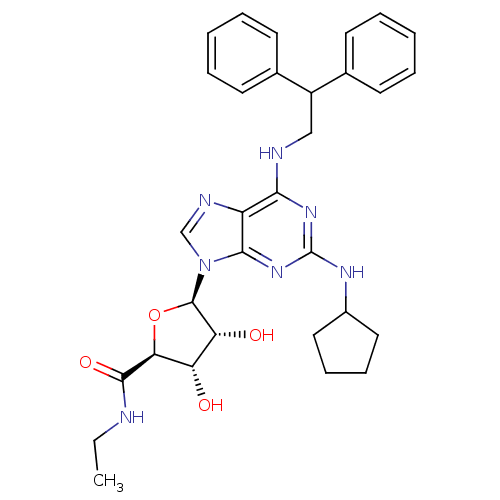 ((2S,3S,4R,5R)-5-[2-Cyclopentylamino-6-(2,2-dipheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50085667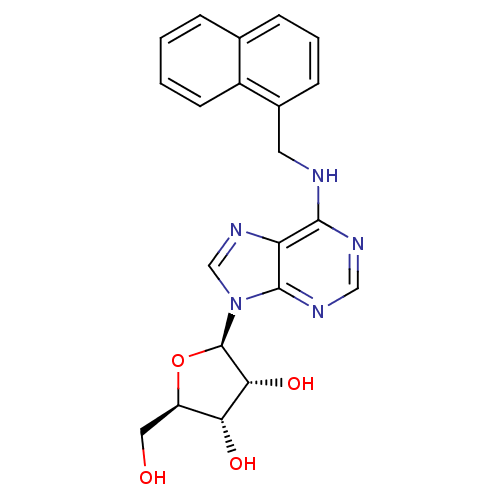 ((2R,3S,4R,5R)-2-Hydroxymethyl-5-{6-[(naphthalen-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Agonistic activity against Adenosine A2A receptor on rat striatal membranes | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50033666 (2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50085667 ((2R,3S,4R,5R)-2-Hydroxymethyl-5-{6-[(naphthalen-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Agonistic activity against Adenosine A1 receptor on rat whole-brain membranes | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085669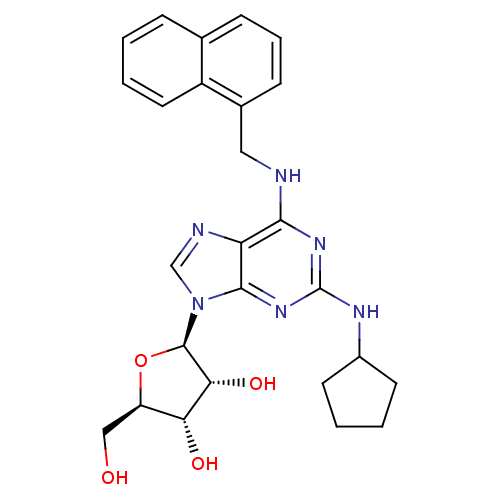 ((2R,3R,4S,5R)-2-{2-Cyclopentylamino-6-[(naphthalen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085673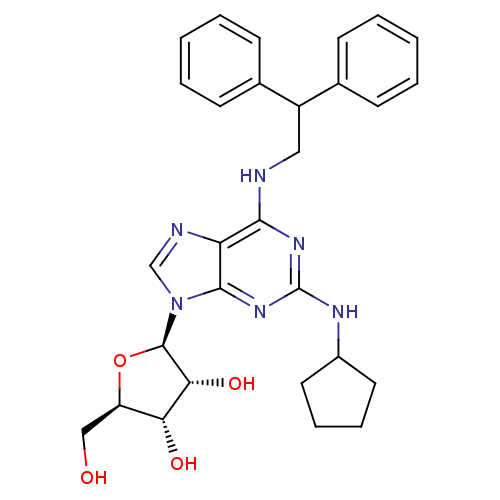 ((2R,3R,4S,5R)-2-[2-Cyclopentylamino-6-(2,2-dipheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085660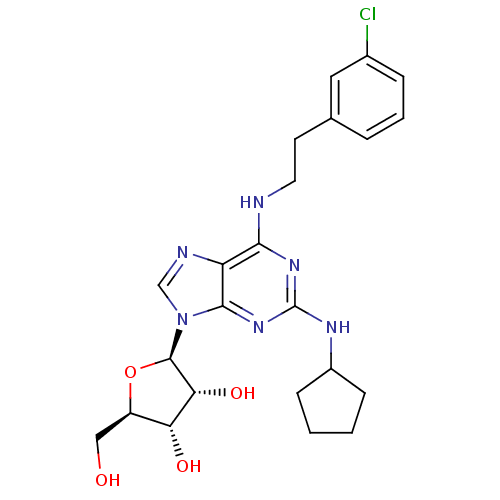 ((2R,3R,4S,5R)-2-{6-[2-(3-Chloro-phenyl)-ethylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049967 (CHEMBL174603 | [4-(5-Amino-7-oxo-6,7-dihydro-[1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049963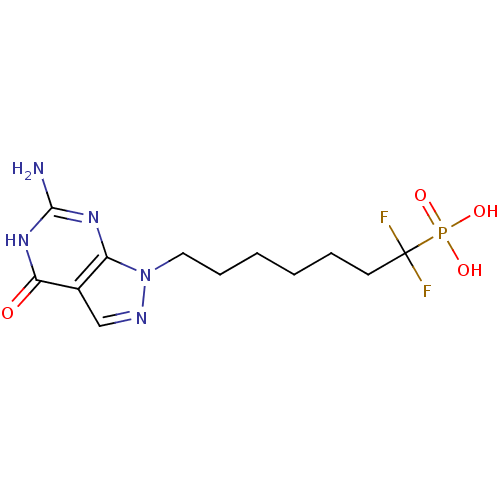 (CHEMBL368924 | [7-(6-Amino-4-oxo-4,5-dihydro-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049969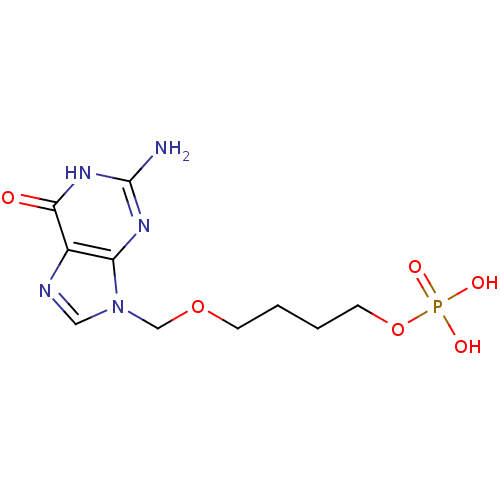 (CHEMBL172316 | Phosphoric acid mono-[4-(2-amino-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049966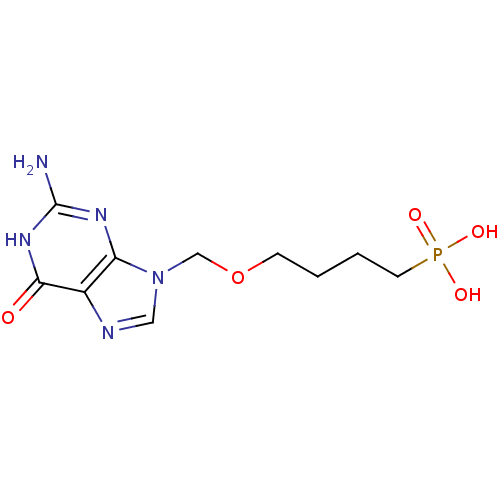 (CHEMBL177948 | [4-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049971 (CHEMBL177190 | [7-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049954 (CHEMBL369052 | [5-(5-Amino-7-oxo-6,7-dihydro-[1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085670 ((2S,3S,4R,5R)-5-(6-Amino-2-cyclopentylamino-purin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085664 ((2R,3R,4S,5R)-2-[6-Amino-2-((1S,2S)-2-hydroxy-cycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085675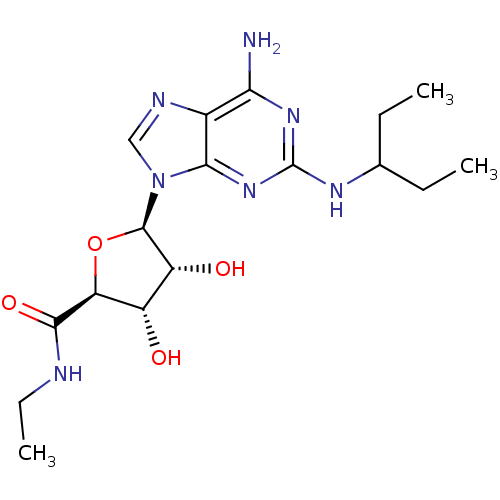 ((2S,3S,4R,5R)-5-[6-Amino-2-(1-ethyl-propylamino)-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049959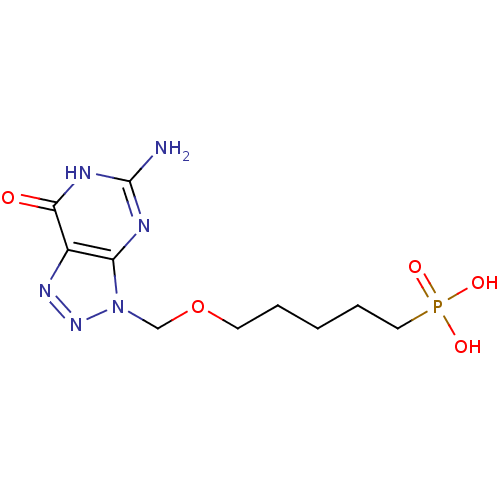 (CHEMBL173142 | [5-(5-Amino-7-oxo-6,7-dihydro-[1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049972 (CHEMBL176448 | [5-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085663 ((2R,3R,4S,5R)-2-[2-Chloro-6-((R)-(S)-2-hydroxy-cyc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049957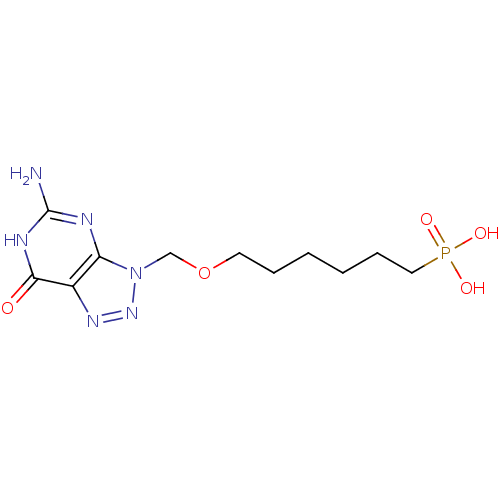 (CHEMBL366963 | [6-(5-Amino-7-oxo-6,7-dihydro-[1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate synthase (Escherichia coli (strain K12)) | BDBM50028881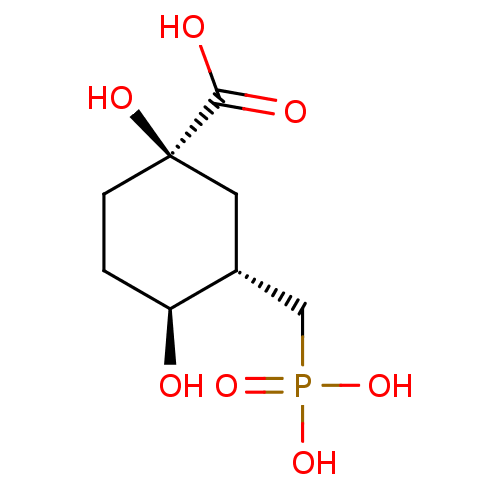 ((1R,3S,4S)-1,4-Dihydroxy-3-phosphonomethyl-cyclohe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Association rate constant of the compound was determined against 3-dehydroquinate synthase | Bioorg Med Chem Lett 2: 1349-1352 (1992) Article DOI: 10.1016/S0960-894X(00)80510-7 BindingDB Entry DOI: 10.7270/Q2MW2H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049968 (CHEMBL177945 | [6-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049955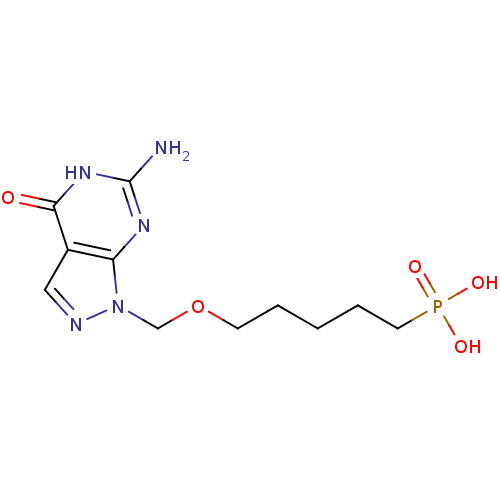 (CHEMBL175362 | [5-(6-Amino-4-oxo-4,5-dihydro-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50143282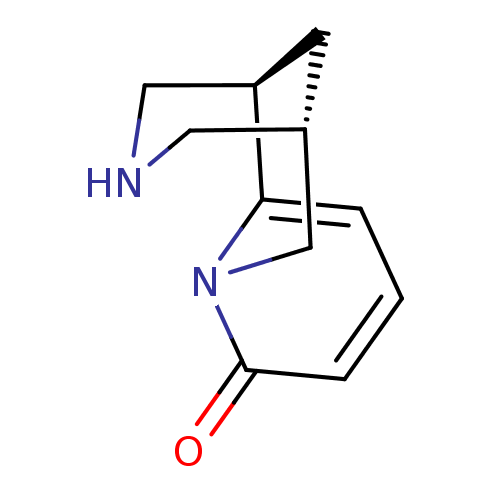 ((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha1 beta gamma delta of electroplax | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085668 ((2S,3S,4R,5R)-5-[6-Amino-2-((1R,2R)-2-hydroxy-cycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085671 ((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-(2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50166908 (5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha-7 subunit in rat GH4C1 cells | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049960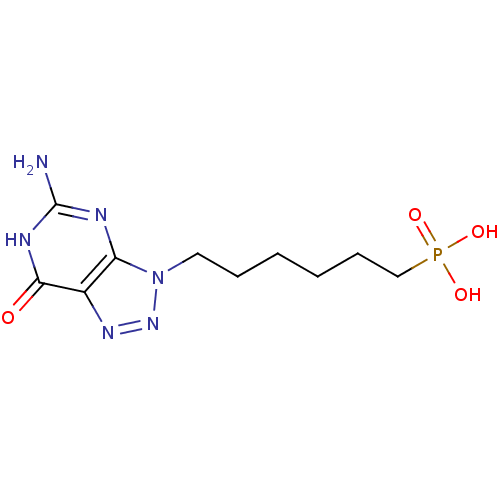 (CHEMBL368064 | [6-(5-Amino-7-oxo-6,7-dihydro-[1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085674 ((2S,3S,4R,5R)-5-[2-(4-Amino-cyclohexylamino)-6-(2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50166909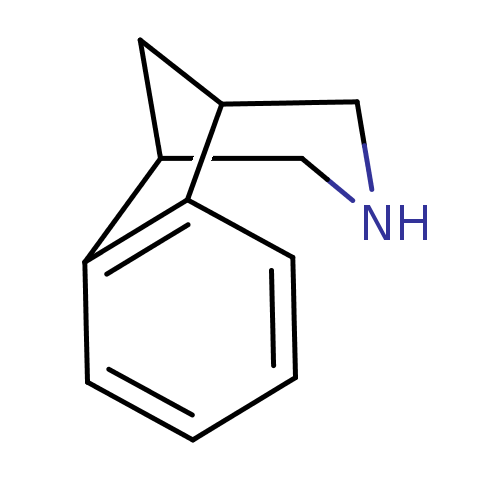 (10-Aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),3,5-trien...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha1 beta gamma delta of electroplax | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049961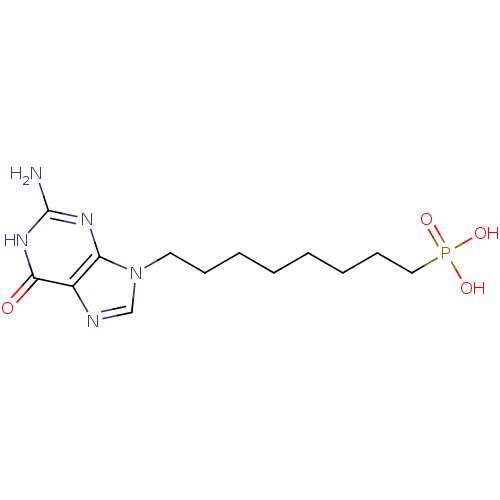 (CHEMBL354409 | [8-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085662 ((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-((1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50049962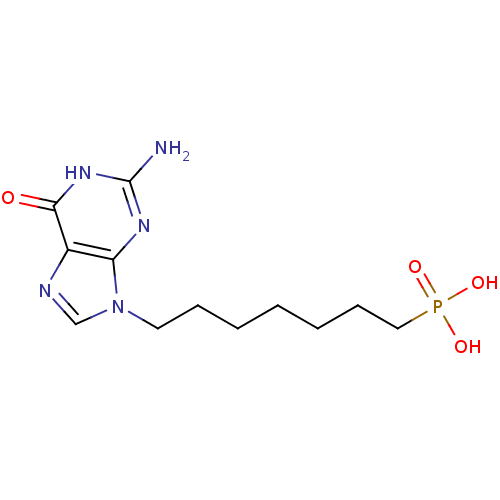 (CHEMBL172844 | [7-(2-Amino-6-oxo-1,6-dihydro-purin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Co. Curated by ChEMBL | Assay Description In vitro inhibition of purine nucleoside phosphorylase isolated from human erythrocytes. | J Med Chem 39: 949-56 (1996) Article DOI: 10.1021/jm950736k BindingDB Entry DOI: 10.7270/Q2SN082H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate synthase (Escherichia coli (strain K12)) | BDBM50028883 ((1S,3S,5R)-1,3-Dihydroxy-5-phosphonomethyl-cyclohe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Association rate constant of the compound was determined against 3-dehydroquinate synthase | Bioorg Med Chem Lett 2: 1349-1352 (1992) Article DOI: 10.1016/S0960-894X(00)80510-7 BindingDB Entry DOI: 10.7270/Q2MW2H21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085672 ((2R,3R,4S,5R)-2-(6-Amino-2-cyclopentylamino-purin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 549 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 492 total ) | Next | Last >> |