 Found 180 hits with Last Name = 'chamberlain' and Initial = 'ts'
Found 180 hits with Last Name = 'chamberlain' and Initial = 'ts' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049025
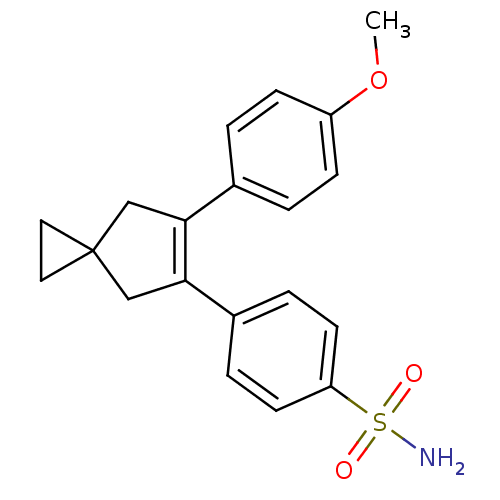
(4-[6-(4-Methoxy-phenyl)-spiro[2.4]hept-5-en-5-yl]-...)Show SMILES COc1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C20H21NO3S/c1-24-16-6-2-14(3-7-16)18-12-20(10-11-20)13-19(18)15-4-8-17(9-5-15)25(21,22)23/h2-9H,10-13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049011
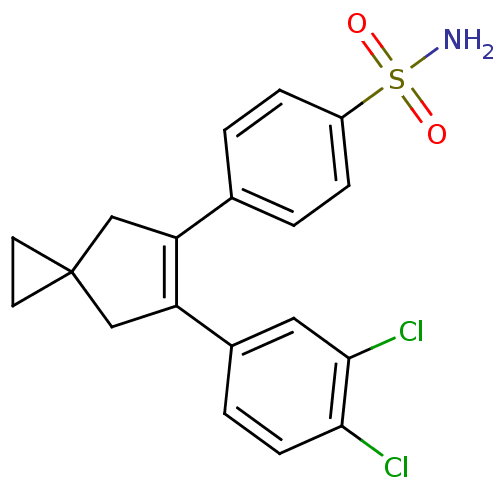
(4-[6-(3,4-Dichloro-phenyl)-spiro[2.4]hept-5-en-5-y...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)c(Cl)c1 |t:11| Show InChI InChI=1S/C19H17Cl2NO2S/c20-17-6-3-13(9-18(17)21)16-11-19(7-8-19)10-15(16)12-1-4-14(5-2-12)25(22,23)24/h1-6,9H,7-8,10-11H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049014

(4-[6-(4-Chloro-phenyl)-spiro[2.4]hept-5-en-5-yl]-b...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C19H18ClNO2S/c20-15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)24(21,22)23/h1-8H,9-12H2,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049042

(4-[6-(4-Trifluoromethyl-phenyl)-spiro[2.4]hept-5-e...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)C(F)(F)F |t:11| Show InChI InChI=1S/C20H18F3NO2S/c21-20(22,23)15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)27(24,25)26/h1-8H,9-12H2,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049038
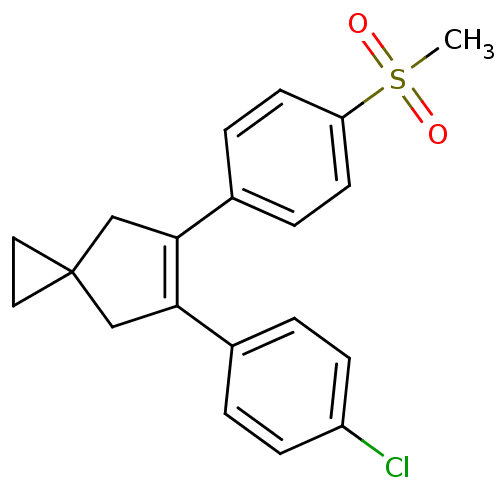
(5-(4-Chloro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C20H19ClO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049030
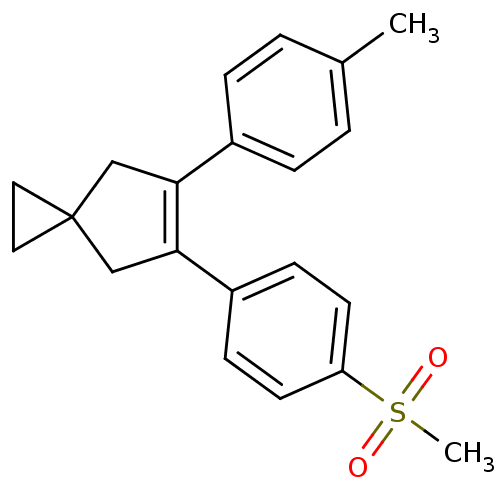
(5-(4-Methanesulfonyl-phenyl)-6-p-tolyl-spiro[2.4]h...)Show SMILES Cc1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:8| Show InChI InChI=1S/C21H22O2S/c1-15-3-5-16(6-4-15)19-13-21(11-12-21)14-20(19)17-7-9-18(10-8-17)24(2,22)23/h3-10H,11-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049033
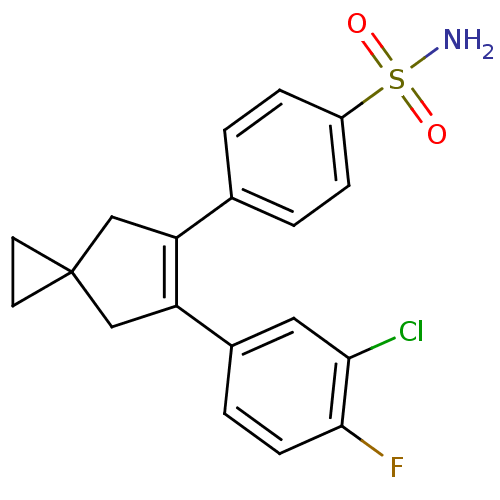
(4-[6-(3-Chloro-4-fluoro-phenyl)-spiro[2.4]hept-5-e...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)c(Cl)c1 |t:11| Show InChI InChI=1S/C19H17ClFNO2S/c20-17-9-13(3-6-18(17)21)16-11-19(7-8-19)10-15(16)12-1-4-14(5-2-12)25(22,23)24/h1-6,9H,7-8,10-11H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049037
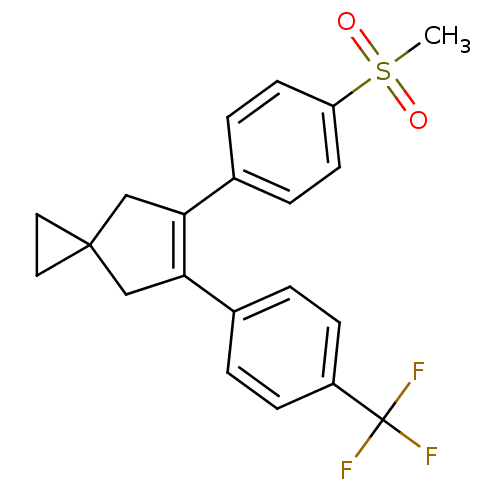
(5-(4-Methanesulfonyl-phenyl)-6-(4-trifluoromethyl-...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)C(F)(F)F |t:11| Show InChI InChI=1S/C21H19F3O2S/c1-27(25,26)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(7-3-14)21(22,23)24/h2-9H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049040
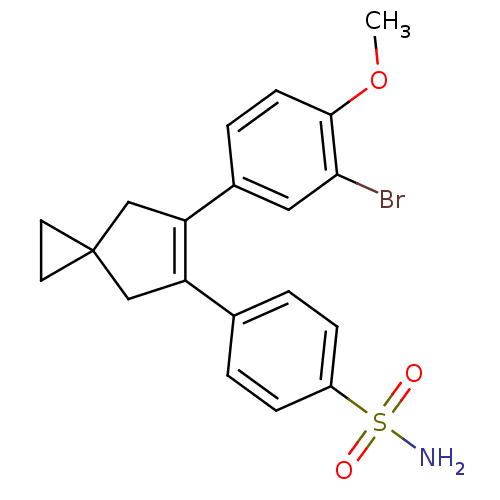
(4-[6-(3-Bromo-4-methoxy-phenyl)-spiro[2.4]hept-5-e...)Show SMILES COc1ccc(cc1Br)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C20H20BrNO3S/c1-25-19-7-4-14(10-18(19)21)17-12-20(8-9-20)11-16(17)13-2-5-15(6-3-13)26(22,23)24/h2-7,10H,8-9,11-12H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049029

(4-[6-(3-Chloro-4-methoxy-phenyl)-spiro[2.4]hept-5-...)Show SMILES COc1ccc(cc1Cl)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C20H20ClNO3S/c1-25-19-7-4-14(10-18(19)21)17-12-20(8-9-20)11-16(17)13-2-5-15(6-3-13)26(22,23)24/h2-7,10H,8-9,11-12H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049023
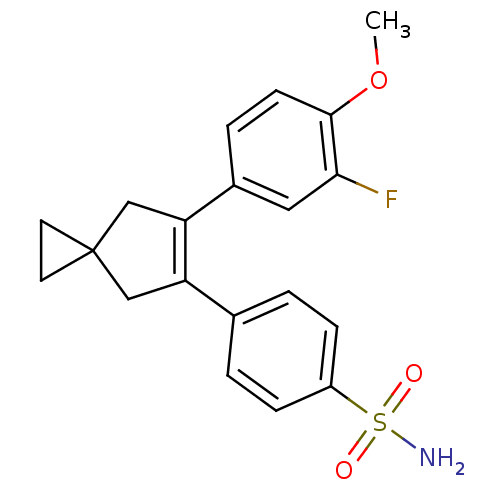
(4-[6-(3-Fluoro-4-methoxy-phenyl)-spiro[2.4]hept-5-...)Show SMILES COc1ccc(cc1F)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:10| Show InChI InChI=1S/C20H20FNO3S/c1-25-19-7-4-14(10-18(19)21)17-12-20(8-9-20)11-16(17)13-2-5-15(6-3-13)26(22,23)24/h2-7,10H,8-9,11-12H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049028
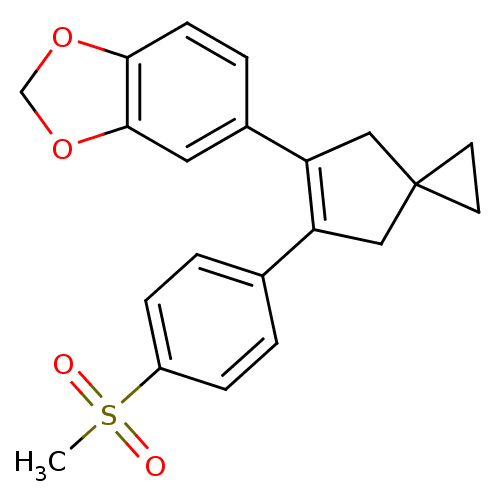
(5-[6-(4-Methanesulfonyl-phenyl)-spiro[2.4]hept-5-e...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc2OCOc2c1 |t:11| Show InChI InChI=1S/C21H20O4S/c1-26(22,23)16-5-2-14(3-6-16)17-11-21(8-9-21)12-18(17)15-4-7-19-20(10-15)25-13-24-19/h2-7,10H,8-9,11-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029614
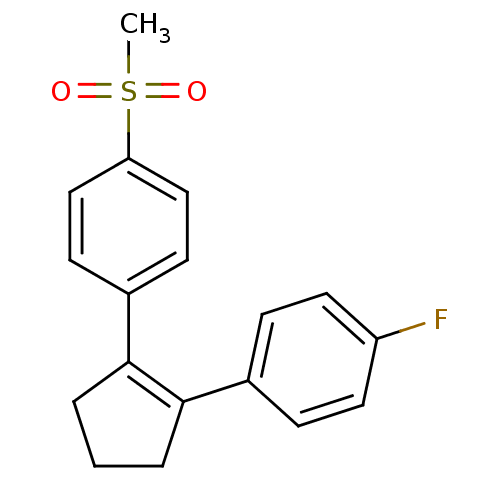
((SC-57666)1-[2-(4-fluorophenyl)-1-cyclopentenyl]-4...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C18H17FO2S/c1-22(20,21)16-11-7-14(8-12-16)18-4-2-3-17(18)13-5-9-15(19)10-6-13/h5-12H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049026

(5-(4-Fluoro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=CC2(CC2)C=C1c1ccc(F)cc1 |c:17,t:11| Show InChI InChI=1S/C20H17FO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9,12-13H,10-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049010

(4-[6-(3,4-Difluoro-phenyl)-spiro[2.4]hept-5-en-5-y...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)c(F)c1 |t:11| Show InChI InChI=1S/C19H17F2NO2S/c20-17-6-3-13(9-18(17)21)16-11-19(7-8-19)10-15(16)12-1-4-14(5-2-12)25(22,23)24/h1-6,9H,7-8,10-11H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049024

(4-[6-(4-Fluoro-phenyl)-spiro[2.4]hept-5-en-5-yl]-b...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C19H18FNO2S/c20-15-5-1-13(2-6-15)17-11-19(9-10-19)12-18(17)14-3-7-16(8-4-14)24(21,22)23/h1-8H,9-12H2,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049018

(5-(3,4-Dichloro-phenyl)-6-(4-methanesulfonyl-pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(Cl)c(Cl)c1 |t:11| Show InChI InChI=1S/C20H18Cl2O2S/c1-25(23,24)15-5-2-13(3-6-15)16-11-20(8-9-20)12-17(16)14-4-7-18(21)19(22)10-14/h2-7,10H,8-9,11-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044356
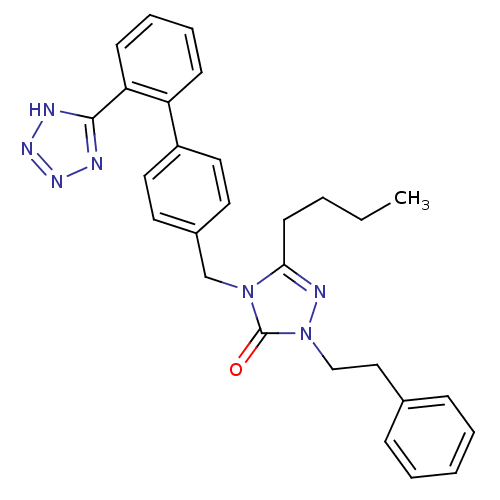
(5-Butyl-2-phenethyl-4-[2'-(1H-tetrazol-5-yl)-biphe...)Show SMILES CCCCc1nn(CCc2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H29N7O/c1-2-3-13-26-31-35(19-18-21-9-5-4-6-10-21)28(36)34(26)20-22-14-16-23(17-15-22)24-11-7-8-12-25(24)27-29-32-33-30-27/h4-12,14-17H,2-3,13,18-20H2,1H3,(H,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049013
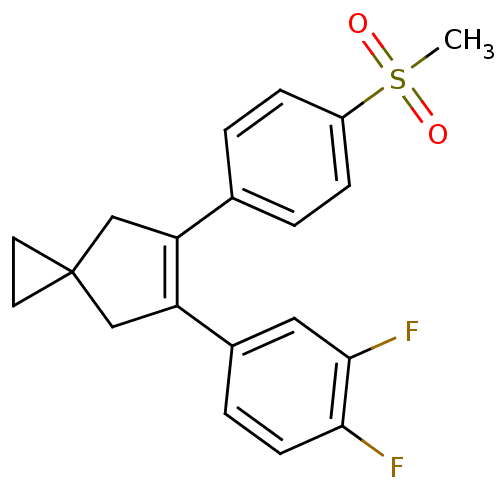
(5-(3,4-Difluoro-phenyl)-6-(4-methanesulfonyl-pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)c(F)c1 |t:11| Show InChI InChI=1S/C20H18F2O2S/c1-25(23,24)15-5-2-13(3-6-15)16-11-20(8-9-20)12-17(16)14-4-7-18(21)19(22)10-14/h2-7,10H,8-9,11-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044345

(5-Butyl-2-(2-hydroxy-2-phenyl-ethyl)-4-[2'-(1H-tet...)Show SMILES CCCCc1nn(CC(O)c2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H29N7O2/c1-2-3-13-26-31-35(19-25(36)22-9-5-4-6-10-22)28(37)34(26)18-20-14-16-21(17-15-20)23-11-7-8-12-24(23)27-29-32-33-30-27/h4-12,14-17,25,36H,2-3,13,18-19H2,1H3,(H,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049022

(6-(4-Fluoro-phenyl)-7-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CCC2)C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C21H21FO2S/c1-25(23,24)18-9-5-16(6-10-18)20-14-21(11-2-12-21)13-19(20)15-3-7-17(22)8-4-15/h3-10H,2,11-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049027
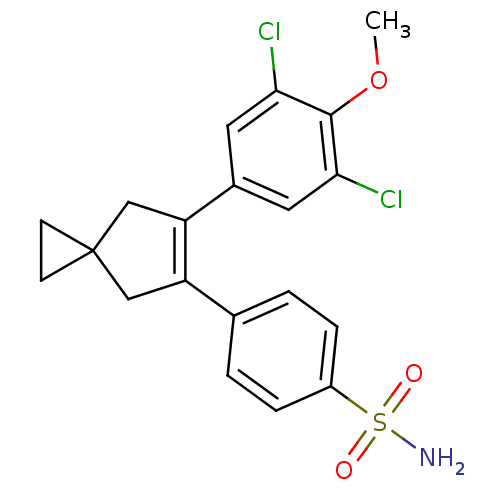
(4-[6-(3,5-Dichloro-4-methoxy-phenyl)-spiro[2.4]hep...)Show SMILES COc1c(Cl)cc(cc1Cl)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:11| Show InChI InChI=1S/C20H19Cl2NO3S/c1-26-19-17(21)8-13(9-18(19)22)16-11-20(6-7-20)10-15(16)12-2-4-14(5-3-12)27(23,24)25/h2-5,8-9H,6-7,10-11H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044334
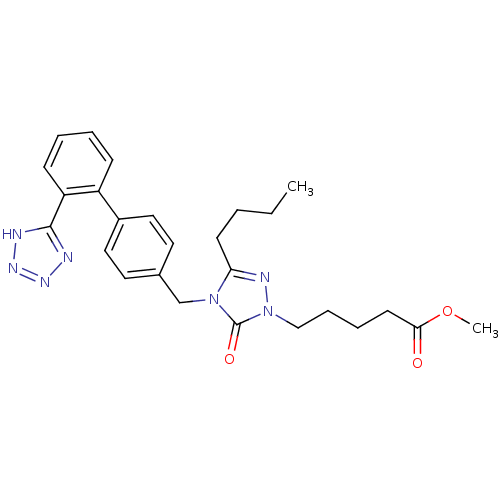
(5-{3-Butyl-5-oxo-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nn(CCCCC(=O)OC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C26H31N7O3/c1-3-4-11-23-29-33(17-8-7-12-24(34)36-2)26(35)32(23)18-19-13-15-20(16-14-19)21-9-5-6-10-22(21)25-27-30-31-28-25/h5-6,9-10,13-16H,3-4,7-8,11-12,17-18H2,1-2H3,(H,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049034
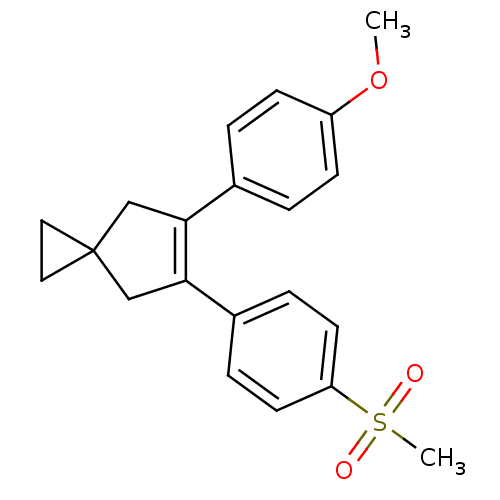
(5-(4-Methanesulfonyl-phenyl)-6-(4-methoxy-phenyl)-...)Show SMILES COc1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:9| Show InChI InChI=1S/C21H22O3S/c1-24-17-7-3-15(4-8-17)19-13-21(11-12-21)14-20(19)16-5-9-18(10-6-16)25(2,22)23/h3-10H,11-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50049025

(4-[6-(4-Methoxy-phenyl)-spiro[2.4]hept-5-en-5-yl]-...)Show SMILES COc1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C20H21NO3S/c1-24-16-6-2-14(3-7-16)18-12-20(10-11-20)13-19(18)15-4-8-17(9-5-15)25(21,22)23/h2-9H,10-13H2,1H3,(H2,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against prostaglandin G/H synthase 1 (COX-1). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50285518
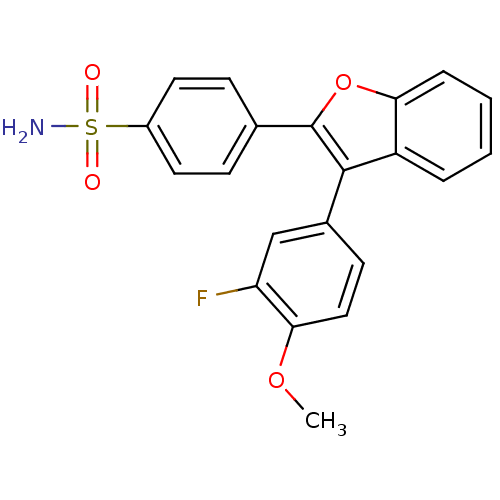
(4-[3-(3-Fluoro-4-methoxy-phenyl)-benzofuran-2-yl]-...)Show SMILES COc1ccc(cc1F)-c1c(oc2ccccc12)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C21H16FNO4S/c1-26-19-11-8-14(12-17(19)22)20-16-4-2-3-5-18(16)27-21(20)13-6-9-15(10-7-13)28(23,24)25/h2-12H,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant cyclooxygenase-2 enzyme |
Bioorg Med Chem Lett 5: 2377-2380 (1995)
Article DOI: 10.1016/0960-894X(95)00414-O
BindingDB Entry DOI: 10.7270/Q2X06705 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50285514
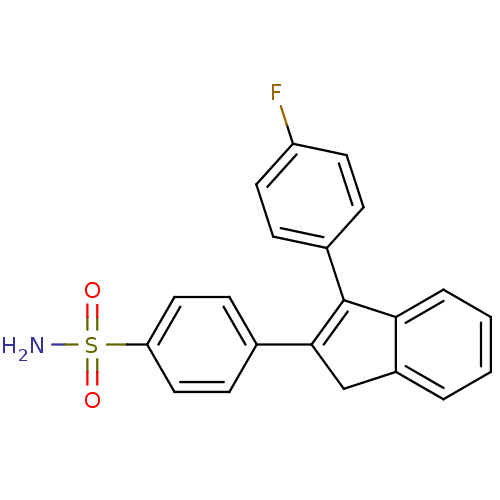
(4-[3-(4-Fluoro-phenyl)-1H-inden-2-yl]-benzenesulfo...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(c2ccccc2C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C21H16FNO2S/c22-17-9-5-15(6-10-17)21-19-4-2-1-3-16(19)13-20(21)14-7-11-18(12-8-14)26(23,24)25/h1-12H,13H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant cyclooxygenase-2 enzyme |
Bioorg Med Chem Lett 5: 2377-2380 (1995)
Article DOI: 10.1016/0960-894X(95)00414-O
BindingDB Entry DOI: 10.7270/Q2X06705 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044355

(2,5-Dibutyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...)Show SMILES CCCCc1nn(CCCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H29N7O/c1-3-5-11-22-27-31(16-6-4-2)24(32)30(22)17-18-12-14-19(15-13-18)20-9-7-8-10-21(20)23-25-28-29-26-23/h7-10,12-15H,3-6,11,16-17H2,1-2H3,(H,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125-I]-labeled angiotensin II from Angiotensin II receptor, type 1 of rat uterine membranes |
Bioorg Med Chem Lett 4: 2591-2596 (1994)
Article DOI: 10.1016/S0960-894X(01)80290-0
BindingDB Entry DOI: 10.7270/Q29Z94VB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044355

(2,5-Dibutyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...)Show SMILES CCCCc1nn(CCCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H29N7O/c1-3-5-11-22-27-31(16-6-4-2)24(32)30(22)17-18-12-14-19(15-13-18)20-9-7-8-10-21(20)23-25-28-29-26-23/h7-10,12-15H,3-6,11,16-17H2,1-2H3,(H,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044357
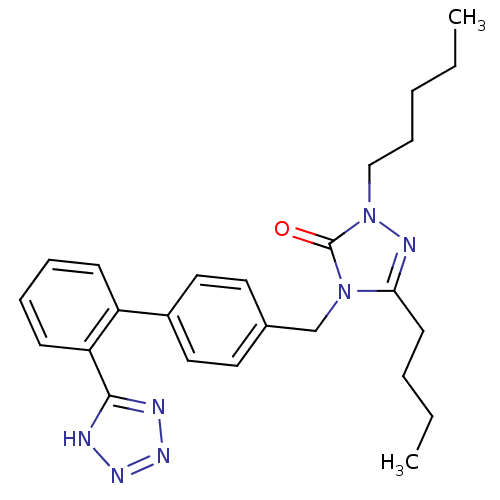
(5-Butyl-2-pentyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCCn1nc(CCCC)n(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c1=O Show InChI InChI=1S/C25H31N7O/c1-3-5-9-17-32-25(33)31(23(28-32)12-6-4-2)18-19-13-15-20(16-14-19)21-10-7-8-11-22(21)24-26-29-30-27-24/h7-8,10-11,13-16H,3-6,9,12,17-18H2,1-2H3,(H,26,27,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049031
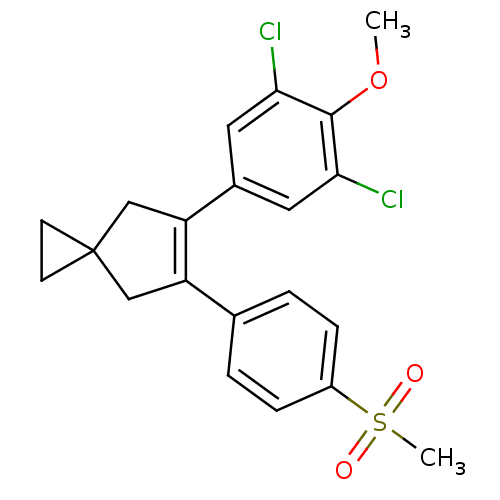
(5-(3,5-Dichloro-4-methoxy-phenyl)-6-(4-methanesulf...)Show SMILES COc1c(Cl)cc(cc1Cl)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:11| Show InChI InChI=1S/C21H20Cl2O3S/c1-26-20-18(22)9-14(10-19(20)23)17-12-21(7-8-21)11-16(17)13-3-5-15(6-4-13)27(2,24)25/h3-6,9-10H,7-8,11-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50046078
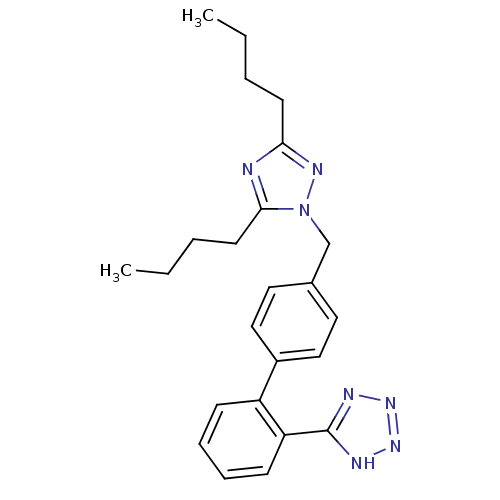
(5-[4'-(3,5-Dibutyl-[1,2,4]triazol-1-ylmethyl)-biph...)Show SMILES CCCCc1nc(CCCC)n(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)n1 Show InChI InChI=1S/C24H29N7/c1-3-5-11-22-25-23(12-6-4-2)31(28-22)17-18-13-15-19(16-14-18)20-9-7-8-10-21(20)24-26-29-30-27-24/h7-10,13-16H,3-6,11-12,17H2,1-2H3,(H,26,27,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle & Company
Curated by ChEMBL
| Assay Description
Activity against high affinity Angiotensin II receptor, type 1 was measured from the ability to inhibit [125I]-angiotensin II binding to rat uterine ... |
J Med Chem 36: 101-10 (1993)
BindingDB Entry DOI: 10.7270/Q2KH0MDW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049021

(5-(3-Chloro-4-fluoro-phenyl)-6-(4-methanesulfonyl-...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)c(Cl)c1 |t:11| Show InChI InChI=1S/C20H18ClFO2S/c1-25(23,24)15-5-2-13(3-6-15)16-11-20(8-9-20)12-17(16)14-4-7-19(22)18(21)10-14/h2-7,10H,8-9,11-12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50285514

(4-[3-(4-Fluoro-phenyl)-1H-inden-2-yl]-benzenesulfo...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(c2ccccc2C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C21H16FNO2S/c22-17-9-5-15(6-10-17)21-19-4-2-1-3-16(19)13-20(21)14-7-11-18(12-8-14)26(23,24)25/h1-12H,13H2,(H2,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant cyclooxygenase-1 enzyme |
Bioorg Med Chem Lett 5: 2377-2380 (1995)
Article DOI: 10.1016/0960-894X(95)00414-O
BindingDB Entry DOI: 10.7270/Q2X06705 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50285517
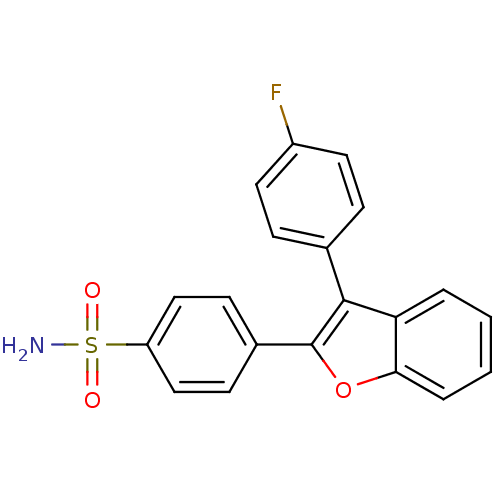
(4-[3-(4-Fluoro-phenyl)-benzofuran-2-yl]-benzenesul...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1oc2ccccc2c1-c1ccc(F)cc1 Show InChI InChI=1S/C20H14FNO3S/c21-15-9-5-13(6-10-15)19-17-3-1-2-4-18(17)25-20(19)14-7-11-16(12-8-14)26(22,23)24/h1-12H,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant cyclooxygenase-2 enzyme |
Bioorg Med Chem Lett 5: 2377-2380 (1995)
Article DOI: 10.1016/0960-894X(95)00414-O
BindingDB Entry DOI: 10.7270/Q2X06705 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044348
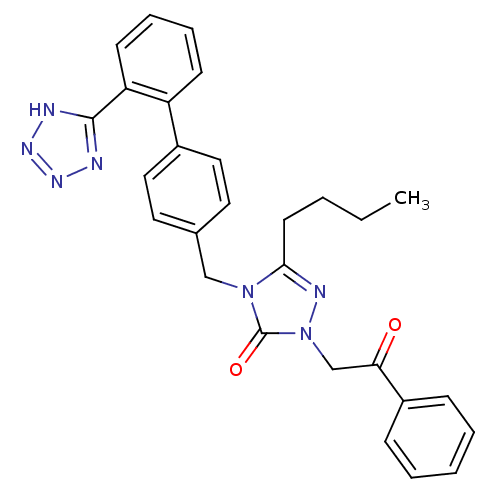
(5-Butyl-2-(2-oxo-2-phenyl-ethyl)-4-[2'-(1H-tetrazo...)Show SMILES CCCCc1nn(CC(=O)c2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H27N7O2/c1-2-3-13-26-31-35(19-25(36)22-9-5-4-6-10-22)28(37)34(26)18-20-14-16-21(17-15-20)23-11-7-8-12-24(23)27-29-32-33-30-27/h4-12,14-17H,2-3,13,18-19H2,1H3,(H,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049041
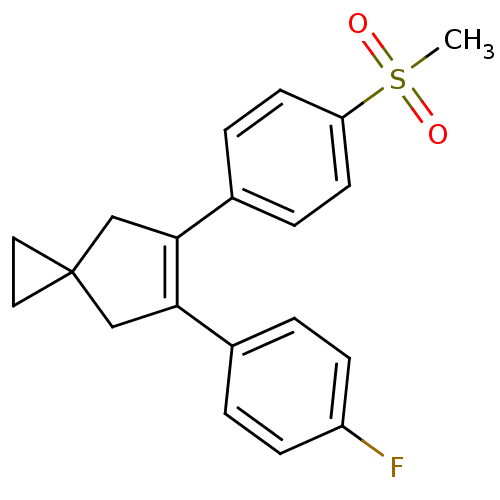
(5-(4-Fluoro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C20H19FO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044367
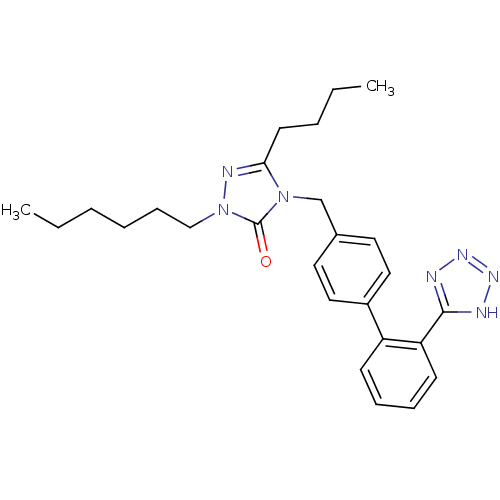
(5-Butyl-2-hexyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-...)Show SMILES CCCCCCn1nc(CCCC)n(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c1=O Show InChI InChI=1S/C26H33N7O/c1-3-5-7-10-18-33-26(34)32(24(29-33)13-6-4-2)19-20-14-16-21(17-15-20)22-11-8-9-12-23(22)25-27-30-31-28-25/h8-9,11-12,14-17H,3-7,10,13,18-19H2,1-2H3,(H,27,28,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044361

(CHEMBL67764 | {3-Butyl-5-oxo-4-[2'-(1H-tetrazol-5-...)Show SMILES CCCCc1nn(CC(=O)OCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H27N7O3/c1-3-5-10-21-27-31(16-22(32)34-4-2)24(33)30(21)15-17-11-13-18(14-12-17)19-8-6-7-9-20(19)23-25-28-29-26-23/h6-9,11-14H,3-5,10,15-16H2,1-2H3,(H,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044338
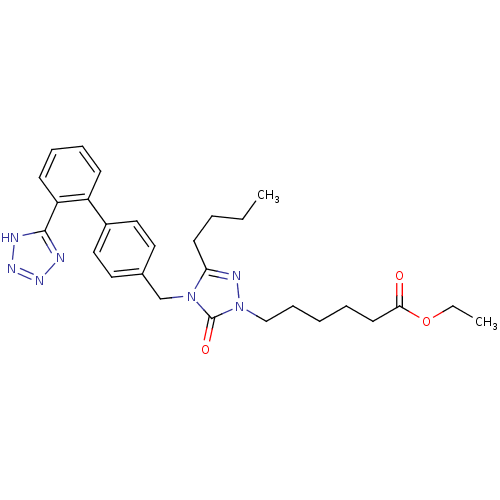
(6-{3-Butyl-5-oxo-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nn(CCCCCC(=O)OCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C28H35N7O3/c1-3-5-13-25-31-35(19-10-6-7-14-26(36)38-4-2)28(37)34(25)20-21-15-17-22(18-16-21)23-11-8-9-12-24(23)27-29-32-33-30-27/h8-9,11-12,15-18H,3-7,10,13-14,19-20H2,1-2H3,(H,29,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044366
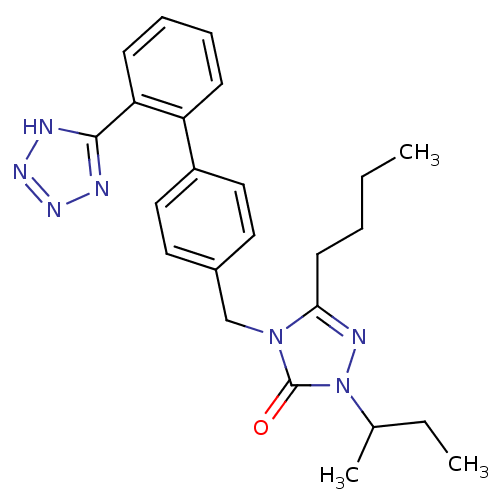
(5-Butyl-2-sec-butyl-4-[2'-(1H-tetrazol-5-yl)-biphe...)Show SMILES CCCCc1nn(C(C)CC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H29N7O/c1-4-6-11-22-27-31(17(3)5-2)24(32)30(22)16-18-12-14-19(15-13-18)20-9-7-8-10-21(20)23-25-28-29-26-23/h7-10,12-15,17H,4-6,11,16H2,1-3H3,(H,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049035
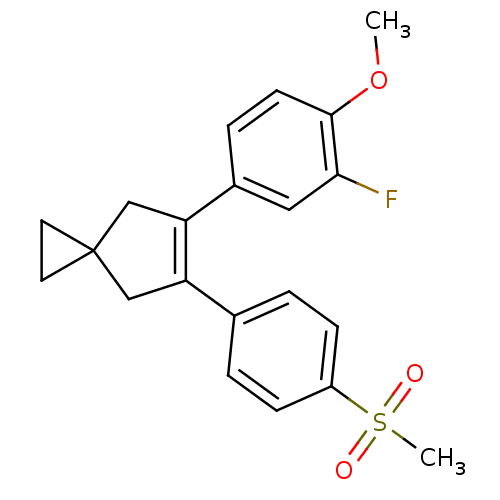
(5-(3-Fluoro-4-methoxy-phenyl)-6-(4-methanesulfonyl...)Show SMILES COc1ccc(cc1F)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:10| Show InChI InChI=1S/C21H21FO3S/c1-25-20-8-5-15(11-19(20)22)18-13-21(9-10-21)12-17(18)14-3-6-16(7-4-14)26(2,23)24/h3-8,11H,9-10,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50285516
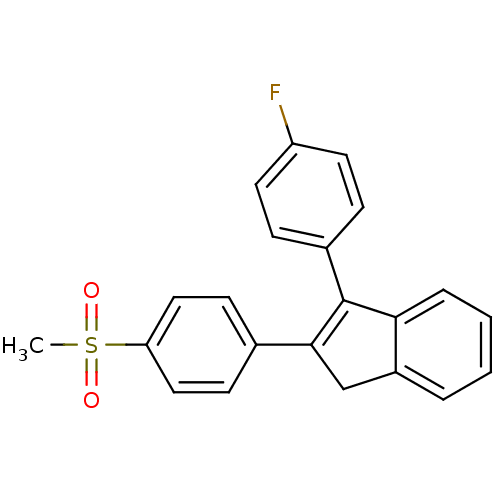
(3-(4-Fluoro-phenyl)-2-(4-methanesulfonyl-phenyl)-1...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(c2ccccc2C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C22H17FO2S/c1-26(24,25)19-12-8-15(9-13-19)21-14-17-4-2-3-5-20(17)22(21)16-6-10-18(23)11-7-16/h2-13H,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant cyclooxygenase-2 enzyme |
Bioorg Med Chem Lett 5: 2377-2380 (1995)
Article DOI: 10.1016/0960-894X(95)00414-O
BindingDB Entry DOI: 10.7270/Q2X06705 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50283548
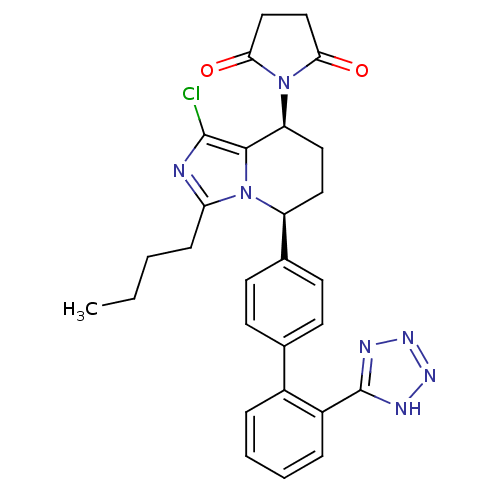
(1-{(5S,8S)-3-Butyl-1-chloro-5-[2'-(2H-tetrazol-5-y...)Show SMILES CCCCc1nc(Cl)c2[C@H](CC[C@@H](c3ccc(cc3)-c3ccccc3-c3nnn[nH]3)n12)N1C(=O)CCC1=O Show InChI InChI=1S/C28H28ClN7O2/c1-2-3-8-23-30-27(29)26-22(36-24(37)15-16-25(36)38)14-13-21(35(23)26)18-11-9-17(10-12-18)19-6-4-5-7-20(19)28-31-33-34-32-28/h4-7,9-12,21-22H,2-3,8,13-16H2,1H3,(H,31,32,33,34)/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125-I]-labeled angiotensin II binding to AT1 receptor in rat uterine membranes |
Bioorg Med Chem Lett 4: 2591-2596 (1994)
Article DOI: 10.1016/S0960-894X(01)80290-0
BindingDB Entry DOI: 10.7270/Q29Z94VB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044369

(5-{3-Butyl-5-oxo-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nn(CCCCC(O)=O)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H29N7O3/c1-2-3-10-22-28-32(16-7-6-11-23(33)34)25(35)31(22)17-18-12-14-19(15-13-18)20-8-4-5-9-21(20)24-26-29-30-27-24/h4-5,8-9,12-15H,2-3,6-7,10-11,16-17H2,1H3,(H,33,34)(H,26,27,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044365
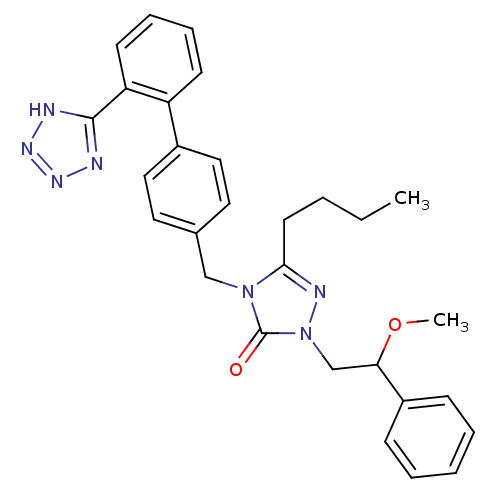
(5-Butyl-2-(2-methoxy-2-phenyl-ethyl)-4-[2'-(2H-tet...)Show SMILES CCCCc1nn(CC(OC)c2ccccc2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H31N7O2/c1-3-4-14-27-32-36(20-26(38-2)23-10-6-5-7-11-23)29(37)35(27)19-21-15-17-22(18-16-21)24-12-8-9-13-25(24)28-30-33-34-31-28/h5-13,15-18,26H,3-4,14,19-20H2,1-2H3,(H,30,31,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049032
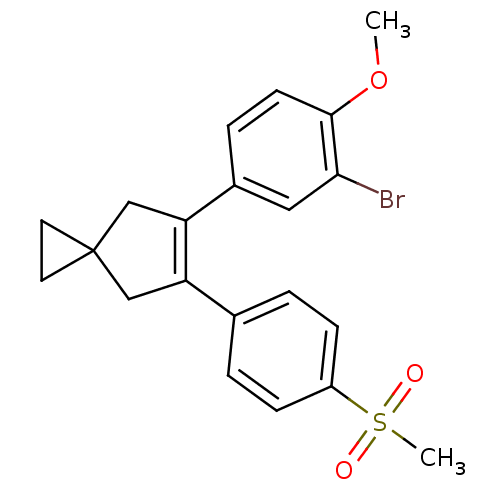
(5-(3-Bromo-4-methoxy-phenyl)-6-(4-methanesulfonyl-...)Show SMILES COc1ccc(cc1Br)C1=C(CC2(CC2)C1)c1ccc(cc1)S(C)(=O)=O |t:10| Show InChI InChI=1S/C21H21BrO3S/c1-25-20-8-5-15(11-19(20)22)18-13-21(9-10-21)12-17(18)14-3-6-16(7-4-14)26(2,23)24/h3-8,11H,9-10,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against prostaglandin G/H synthase 2 (COX-2). |
J Med Chem 39: 253-66 (1996)
Article DOI: 10.1021/jm950664x
BindingDB Entry DOI: 10.7270/Q2XG9Q6M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50283551
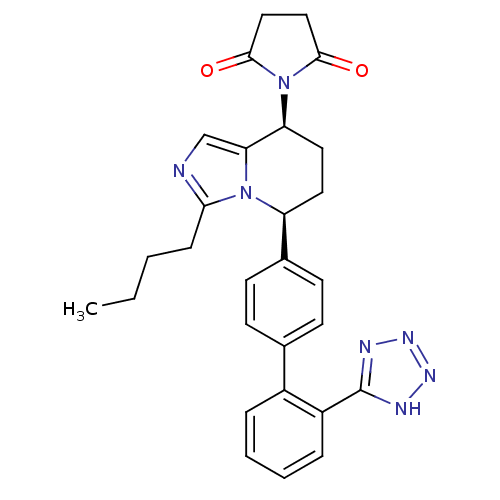
(1-{(5S,8S)-3-Butyl-5-[2'-(2H-tetrazol-5-yl)-biphen...)Show SMILES CCCCc1ncc2[C@H](CC[C@@H](c3ccc(cc3)-c3ccccc3-c3nnn[nH]3)n12)N1C(=O)CCC1=O Show InChI InChI=1S/C28H29N7O2/c1-2-3-8-25-29-17-24-23(35-26(36)15-16-27(35)37)14-13-22(34(24)25)19-11-9-18(10-12-19)20-6-4-5-7-21(20)28-30-32-33-31-28/h4-7,9-12,17,22-23H,2-3,8,13-16H2,1H3,(H,30,31,32,33)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125-I]-labeled angiotensin II binding to AT1 receptor in rat uterine membranes |
Bioorg Med Chem Lett 4: 2591-2596 (1994)
Article DOI: 10.1016/S0960-894X(01)80290-0
BindingDB Entry DOI: 10.7270/Q29Z94VB |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044346
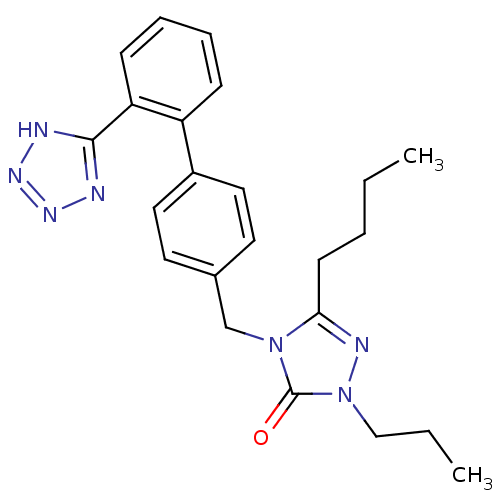
(5-Butyl-2-propyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nn(CCC)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H27N7O/c1-3-5-10-21-26-30(15-4-2)23(31)29(21)16-17-11-13-18(14-12-17)19-8-6-7-9-20(19)22-24-27-28-25-22/h6-9,11-14H,3-5,10,15-16H2,1-2H3,(H,24,25,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50044335
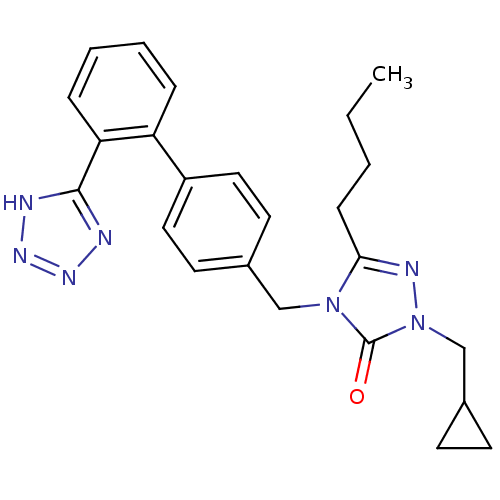
(5-Butyl-2-cyclopropylmethyl-4-[2'-(1H-tetrazol-5-y...)Show SMILES CCCCc1nn(CC2CC2)c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H27N7O/c1-2-3-8-22-27-31(16-18-9-10-18)24(32)30(22)15-17-11-13-19(14-12-17)20-6-4-5-7-21(20)23-25-28-29-26-23/h4-7,11-14,18H,2-3,8-10,15-16H2,1H3,(H,25,26,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle R&D
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-angiotensin-II binding to the angiotensin II receptor type 1 in rat uterine membranes |
J Med Chem 36: 2172-81 (1993)
BindingDB Entry DOI: 10.7270/Q2C53JXM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data