 Found 167 hits with Last Name = 'chang' and Initial = 'ac'
Found 167 hits with Last Name = 'chang' and Initial = 'ac' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50007344
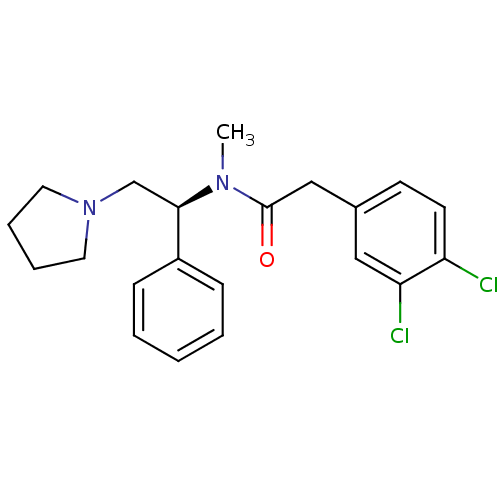
((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093965
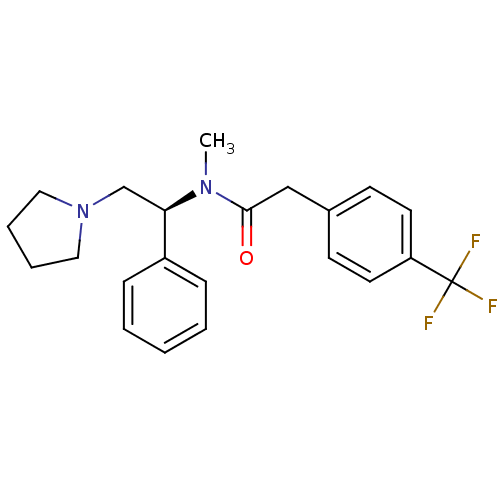
(CHEMBL86324 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-9-11-19(12-10-17)22(23,24)25)20(16-27-13-5-6-14-27)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093964
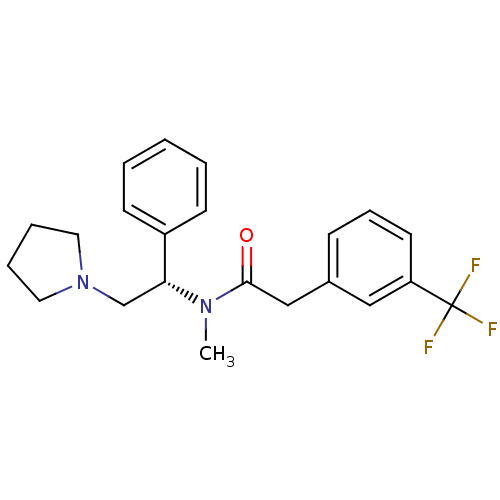
(CHEMBL313484 | N-Methyl-N-((S)-1-phenyl-2-pyrrolid...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-8-7-11-19(14-17)22(23,24)25)20(16-27-12-5-6-13-27)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093969
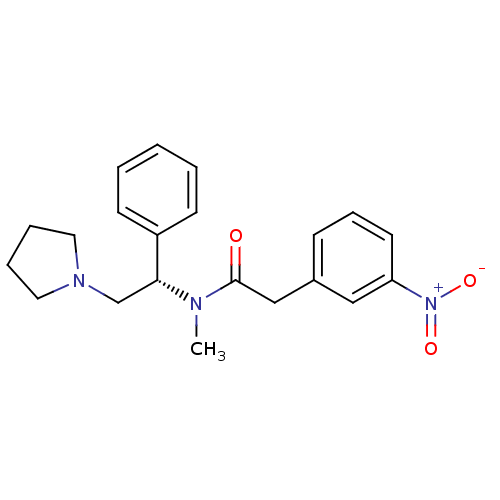
(CHEMBL82919 | N-Methyl-2-(3-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-17-8-7-11-19(14-17)24(26)27)20(16-23-12-5-6-13-23)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093958
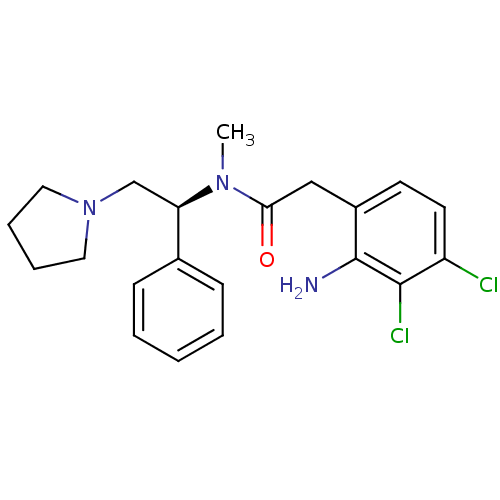
(2-(2-Amino-3,4-dichloro-phenyl)-N-methyl-N-((S)-1-...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1N Show InChI InChI=1S/C21H25Cl2N3O/c1-25(19(27)13-16-9-10-17(22)20(23)21(16)24)18(14-26-11-5-6-12-26)15-7-3-2-4-8-15/h2-4,7-10,18H,5-6,11-14,24H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093966
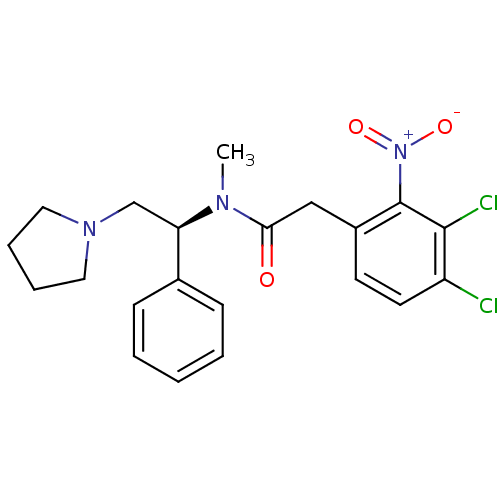
(2-(3,4-Dichloro-2-nitro-phenyl)-N-methyl-N-((S)-1-...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1[N+]([O-])=O Show InChI InChI=1S/C21H23Cl2N3O3/c1-24(18(14-25-11-5-6-12-25)15-7-3-2-4-8-15)19(27)13-16-9-10-17(22)20(23)21(16)26(28)29/h2-4,7-10,18H,5-6,11-14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093970
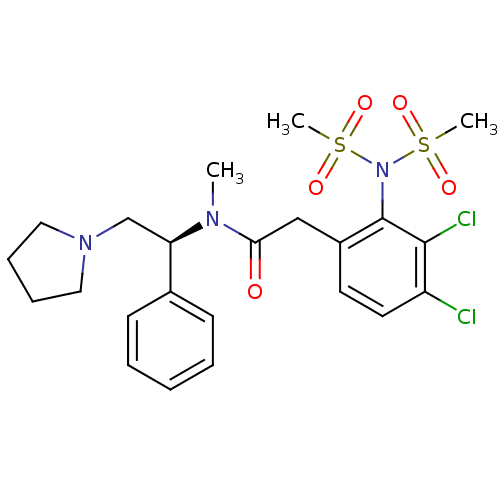
(2-(3,4-Dichloro-2-dimethanesulfonylamino-phenyl)-N...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H29Cl2N3O5S2/c1-26(20(16-27-13-7-8-14-27)17-9-5-4-6-10-17)21(29)15-18-11-12-19(24)22(25)23(18)28(34(2,30)31)35(3,32)33/h4-6,9-12,20H,7-8,13-16H2,1-3H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093962
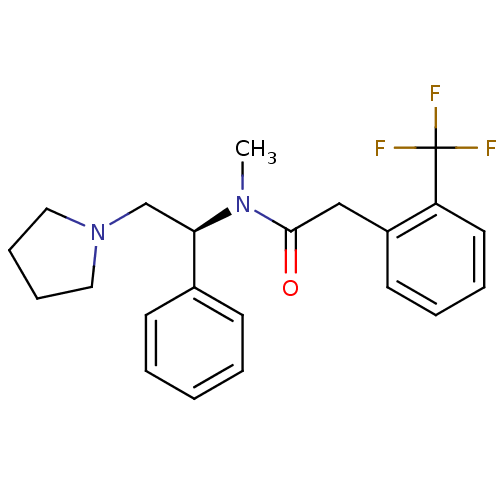
(CHEMBL87306 | N-Methyl-N-((S)-1-phenyl-2-pyrrolidi...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1C(F)(F)F Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-18-11-5-6-12-19(18)22(23,24)25)20(16-27-13-7-8-14-27)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50369223

(CHEMBL1907788)Show SMILES [#6]-[#7](-[#6@H](-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-1)-c1cccc(-[#7]-[#6](=O)-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c1)-[#6](=O)-[#6]-c1ccc(Cl)c(Cl)c1 |r,w:21.21,c:24,27| Show InChI InChI=1S/C44H39Cl2N5O7S/c1-50(41(55)18-25-7-14-35(45)36(46)17-25)37(24-51-15-2-3-16-51)26-5-4-6-27(19-26)48-40(54)23-47-44(59)49-28-8-11-31(34(20-28)43(56)57)42-32-12-9-29(52)21-38(32)58-39-22-30(53)10-13-33(39)42/h4-14,17,19-22,37,52-53H,2-3,15-16,18,23-24H2,1H3,(H,47,59)(H,48,54)(H,56,57)/t37-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor kappa 1 in guinea pig brain |
J Med Chem 39: 1729-35 (1996)
Article DOI: 10.1021/jm950813b
BindingDB Entry DOI: 10.7270/Q27S7PFG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50054439
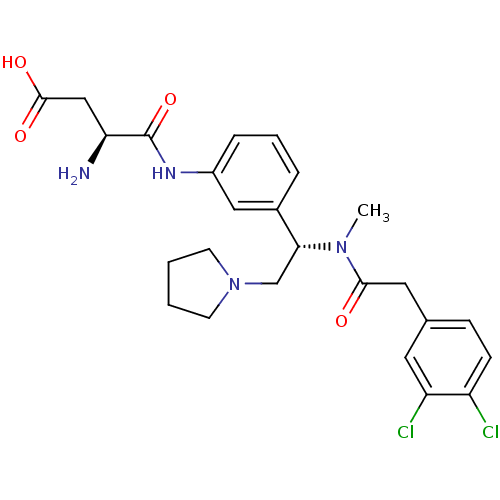
((S)-3-Amino-N-[3-((S)-1-{[2-(3,4-dichloro-phenyl)-...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NC(=O)[C@@H](N)CC(O)=O)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H30Cl2N4O4/c1-30(23(32)12-16-7-8-19(26)20(27)11-16)22(15-31-9-2-3-10-31)17-5-4-6-18(13-17)29-25(35)21(28)14-24(33)34/h4-8,11,13,21-22H,2-3,9-10,12,14-15,28H2,1H3,(H,29,35)(H,33,34)/t21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity and selectivity by competitive inhibition of radioligands on opoid kappa receptor in guinea pig brain me... |
J Med Chem 39: 4478-82 (1996)
Article DOI: 10.1021/jm960459x
BindingDB Entry DOI: 10.7270/Q2N878WW |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093968
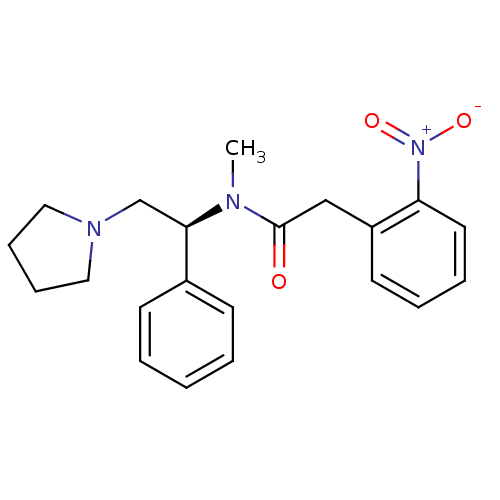
(CHEMBL87207 | N-Methyl-2-(2-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-18-11-5-6-12-19(18)24(26)27)20(16-23-13-7-8-14-23)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093971
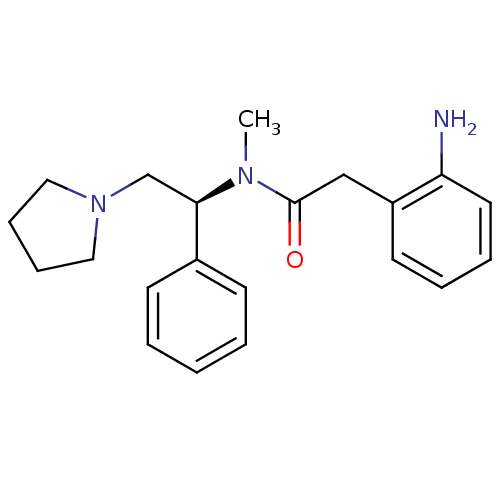
(2-(2-Amino-phenyl)-N-methyl-N-((S)-1-phenyl-2-pyrr...)Show InChI InChI=1S/C21H27N3O/c1-23(21(25)15-18-11-5-6-12-19(18)22)20(16-24-13-7-8-14-24)17-9-3-2-4-10-17/h2-6,9-12,20H,7-8,13-16,22H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50369224
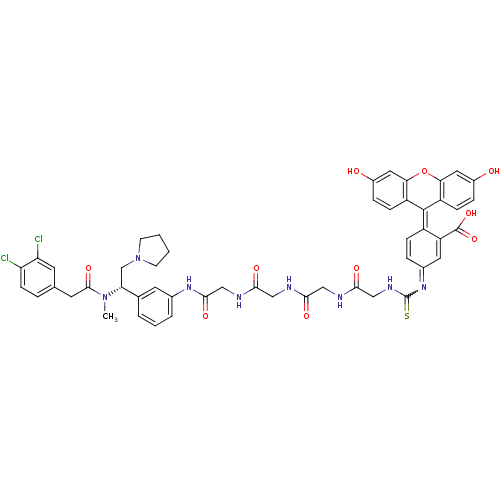
(CHEMBL1907787)Show SMILES [#6]-[#7](-[#6@H](-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-1)-c1cccc(-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c1)-[#6](=O)-[#6]-c1ccc(Cl)c(Cl)c1 |r,w:33.33,c:36,39| Show InChI InChI=1S/C50H48Cl2N8O10S/c1-59(47(67)18-28-7-14-38(51)39(52)17-28)40(27-60-15-2-3-16-60)29-5-4-6-30(19-29)57-46(66)26-55-44(64)24-53-43(63)23-54-45(65)25-56-50(71)58-31-8-11-34(37(20-31)49(68)69)48-35-12-9-32(61)21-41(35)70-42-22-33(62)10-13-36(42)48/h4-14,17,19-22,40,61-62H,2-3,15-16,18,23-27H2,1H3,(H,53,63)(H,54,65)(H,55,64)(H,56,71)(H,57,66)(H,68,69)/t40-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor kappa 1 in guinea pig brain |
J Med Chem 39: 1729-35 (1996)
Article DOI: 10.1021/jm950813b
BindingDB Entry DOI: 10.7270/Q27S7PFG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093967
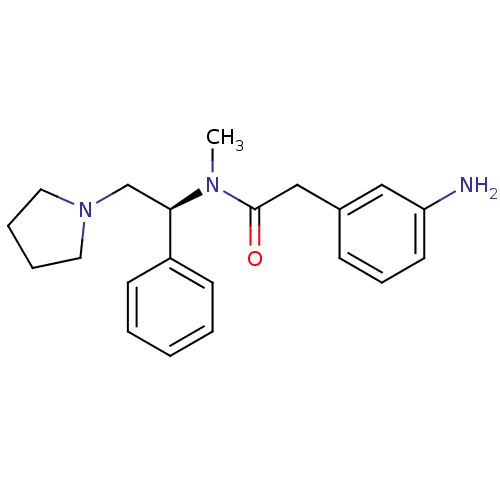
(2-(3-Amino-phenyl)-N-methyl-N-((S)-1-phenyl-2-pyrr...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(N)c1 Show InChI InChI=1S/C21H27N3O/c1-23(21(25)15-17-8-7-11-19(22)14-17)20(16-24-12-5-6-13-24)18-9-3-2-4-10-18/h2-4,7-11,14,20H,5-6,12-13,15-16,22H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093963
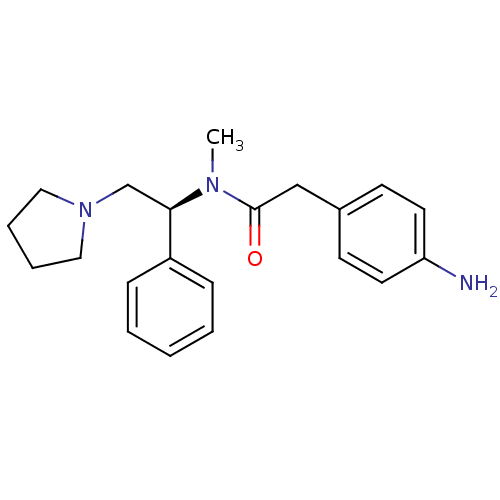
(2-(4-Amino-phenyl)-N-methyl-N-((S)-1-phenyl-2-pyrr...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(N)cc1 Show InChI InChI=1S/C21H27N3O/c1-23(21(25)15-17-9-11-19(22)12-10-17)20(16-24-13-5-6-14-24)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16,22H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093972

(CHEMBL87986 | N-Methyl-2-(4-nitro-phenyl)-N-((S)-1...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H25N3O3/c1-22(21(25)15-17-9-11-19(12-10-17)24(26)27)20(16-23-13-5-6-14-23)18-7-3-2-4-8-18/h2-4,7-12,20H,5-6,13-16H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50054440
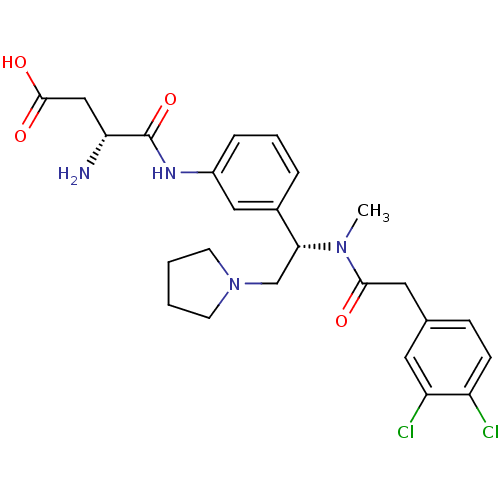
((R)-3-Amino-N-[3-((S)-1-{[2-(3,4-dichloro-phenyl)-...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NC(=O)[C@H](N)CC(O)=O)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H30Cl2N4O4/c1-30(23(32)12-16-7-8-19(26)20(27)11-16)22(15-31-9-2-3-10-31)17-5-4-6-18(13-17)29-25(35)21(28)14-24(33)34/h4-8,11,13,21-22H,2-3,9-10,12,14-15,28H2,1H3,(H,29,35)(H,33,34)/t21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity and selectivity by competitive inhibition of radioligands on Opioid receptor kappa 1 in guinea pig brain... |
J Med Chem 39: 4478-82 (1996)
Article DOI: 10.1021/jm960459x
BindingDB Entry DOI: 10.7270/Q2N878WW |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093961
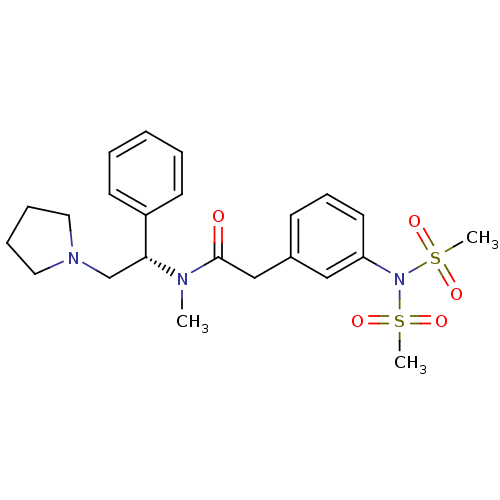
(2-(3-Dimethanesulfonylamino-phenyl)-N-methyl-N-((S...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1cccc(c1)N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H31N3O5S2/c1-24(22(18-25-14-7-8-15-25)20-11-5-4-6-12-20)23(27)17-19-10-9-13-21(16-19)26(32(2,28)29)33(3,30)31/h4-6,9-13,16,22H,7-8,14-15,17-18H2,1-3H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093960
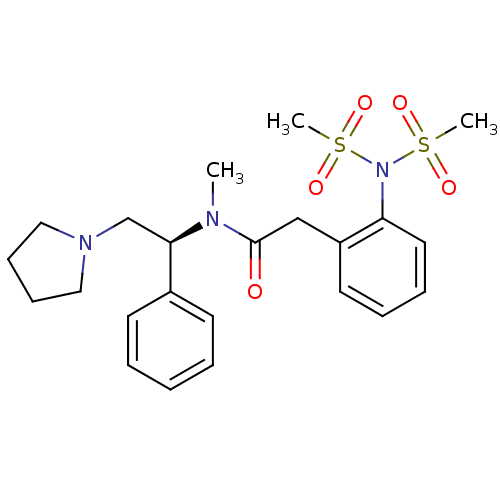
(2-(2-Dimethanesulfonylamino-phenyl)-N-methyl-N-((S...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccccc1N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H31N3O5S2/c1-24(22(18-25-15-9-10-16-25)19-11-5-4-6-12-19)23(27)17-20-13-7-8-14-21(20)26(32(2,28)29)33(3,30)31/h4-8,11-14,22H,9-10,15-18H2,1-3H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50369223

(CHEMBL1907788)Show SMILES [#6]-[#7](-[#6@H](-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-1)-c1cccc(-[#7]-[#6](=O)-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c1)-[#6](=O)-[#6]-c1ccc(Cl)c(Cl)c1 |r,w:21.21,c:24,27| Show InChI InChI=1S/C44H39Cl2N5O7S/c1-50(41(55)18-25-7-14-35(45)36(46)17-25)37(24-51-15-2-3-16-51)26-5-4-6-27(19-26)48-40(54)23-47-44(59)49-28-8-11-31(34(20-28)43(56)57)42-32-12-9-29(52)21-38(32)58-39-22-30(53)10-13-33(39)42/h4-14,17,19-22,37,52-53H,2-3,15-16,18,23-24H2,1H3,(H,47,59)(H,48,54)(H,56,57)/t37-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity towards delta-1 opioid receptor in guinea pig brain |
J Med Chem 39: 1729-35 (1996)
Article DOI: 10.1021/jm950813b
BindingDB Entry DOI: 10.7270/Q27S7PFG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50006781

((2S,3S)-methyl 8-methyl-3-p-tolyl-8-aza-bicyclo[3....)Show SMILES COC(=O)[C@@H]1C2CC[C@H](C[C@@H]1c1ccc(C)cc1)N2C |TLB:19:18:4.10.9:6.7,THB:2:4:18:6.7,11:10:18:6.7| Show InChI InChI=1S/C17H23NO2/c1-11-4-6-12(7-5-11)14-10-13-8-9-15(18(13)2)16(14)17(19)20-3/h4-7,13-16H,8-10H2,1-3H3/t13-,14-,15?,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HTT |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50177110

(2-beta-(4-fluorophenyl)-3-beta-(4-methylphenyl)tro...)Show SMILES CN1C2CCC1[C@H]([C@H](C2)c1ccc(C)cc1)c1ccc(F)cc1 |r,TLB:16:6:4.3:1,THB:9:7:4.3:1| Show InChI InChI=1S/C21H24FN/c1-14-3-5-15(6-4-14)19-13-18-11-12-20(23(18)2)21(19)16-7-9-17(22)10-8-16/h3-10,18-21H,11-13H2,1-2H3/t18?,19-,20?,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HTT |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50093959
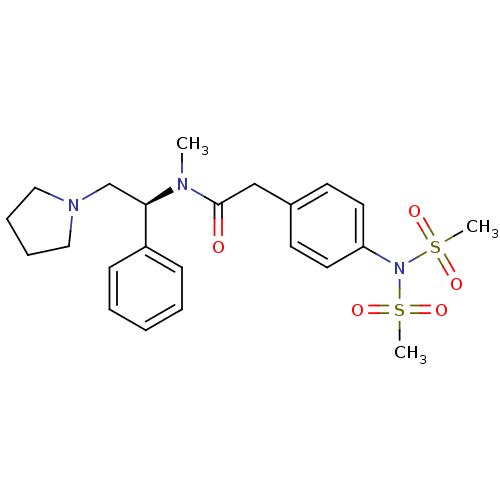
(2-(4-Dimethanesulfonylamino-phenyl)-N-methyl-N-((S...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(cc1)N(S(C)(=O)=O)S(C)(=O)=O Show InChI InChI=1S/C23H31N3O5S2/c1-24(22(18-25-15-7-8-16-25)20-9-5-4-6-10-20)23(27)17-19-11-13-21(14-12-19)26(32(2,28)29)33(3,30)31/h4-6,9-14,22H,7-8,15-18H2,1-3H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards Opioid receptor kappa 1 by displacement of bound [3H]U69,593 |
Bioorg Med Chem Lett 10: 2567-70 (2001)
BindingDB Entry DOI: 10.7270/Q26H4GNG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50369223

(CHEMBL1907788)Show SMILES [#6]-[#7](-[#6@H](-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-1)-c1cccc(-[#7]-[#6](=O)-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c1)-[#6](=O)-[#6]-c1ccc(Cl)c(Cl)c1 |r,w:21.21,c:24,27| Show InChI InChI=1S/C44H39Cl2N5O7S/c1-50(41(55)18-25-7-14-35(45)36(46)17-25)37(24-51-15-2-3-16-51)26-5-4-6-27(19-26)48-40(54)23-47-44(59)49-28-8-11-31(34(20-28)43(56)57)42-32-12-9-29(52)21-38(32)58-39-22-30(53)10-13-33(39)42/h4-14,17,19-22,37,52-53H,2-3,15-16,18,23-24H2,1H3,(H,47,59)(H,48,54)(H,56,57)/t37-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity towards delta-2 opioid receptor in guinea pig brain |
J Med Chem 39: 1729-35 (1996)
Article DOI: 10.1021/jm950813b
BindingDB Entry DOI: 10.7270/Q27S7PFG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50084717
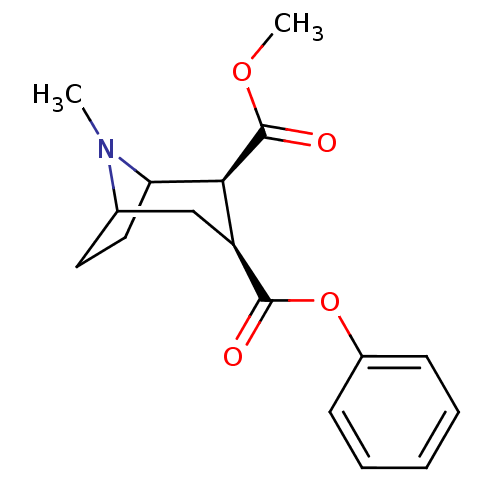
((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1C(=O)Oc1ccccc1)N2C |THB:2:4:20:6.7| Show InChI InChI=1S/C17H21NO4/c1-18-11-8-9-14(18)15(17(20)21-2)13(10-11)16(19)22-12-6-4-3-5-7-12/h3-7,11,13-15H,8-10H2,1-2H3/t11?,13-,14?,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HTT |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50177110

(2-beta-(4-fluorophenyl)-3-beta-(4-methylphenyl)tro...)Show SMILES CN1C2CCC1[C@H]([C@H](C2)c1ccc(C)cc1)c1ccc(F)cc1 |r,TLB:16:6:4.3:1,THB:9:7:4.3:1| Show InChI InChI=1S/C21H24FN/c1-14-3-5-15(6-4-14)19-13-18-11-12-20(23(18)2)21(19)16-7-9-17(22)10-8-16/h3-10,18-21H,11-13H2,1-2H3/t18?,19-,20?,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50369224

(CHEMBL1907787)Show SMILES [#6]-[#7](-[#6@H](-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-1)-c1cccc(-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c1)-[#6](=O)-[#6]-c1ccc(Cl)c(Cl)c1 |r,w:33.33,c:36,39| Show InChI InChI=1S/C50H48Cl2N8O10S/c1-59(47(67)18-28-7-14-38(51)39(52)17-28)40(27-60-15-2-3-16-60)29-5-4-6-30(19-29)57-46(66)26-55-44(64)24-53-43(63)23-54-45(65)25-56-50(71)58-31-8-11-34(37(20-31)49(68)69)48-35-12-9-32(61)21-41(35)70-42-22-33(62)10-13-36(42)48/h4-14,17,19-22,40,61-62H,2-3,15-16,18,23-27H2,1H3,(H,53,63)(H,54,65)(H,55,64)(H,56,71)(H,57,66)(H,68,69)/t40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity towards delta-1 opioid receptor in guinea pig brain |
J Med Chem 39: 1729-35 (1996)
Article DOI: 10.1021/jm950813b
BindingDB Entry DOI: 10.7270/Q27S7PFG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50177109

((R)-3-alpha-(methylphenyl)-2-alpha-phenyltropane |...)Show SMILES CN1C2CCC1[C@H]([C@H](C2)c1ccc(C)cc1)c1ccccc1 |r,TLB:16:6:4.3:1,THB:9:7:4.3:1| Show InChI InChI=1S/C21H25N/c1-15-8-10-16(11-9-15)19-14-18-12-13-20(22(18)2)21(19)17-6-4-3-5-7-17/h3-11,18-21H,12-14H2,1-2H3/t18?,19-,20?,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HTT |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50366564
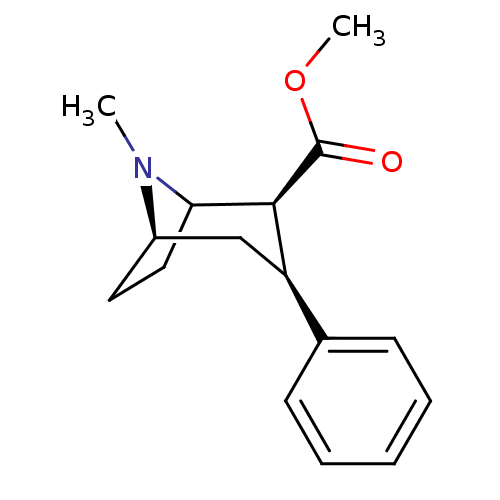
(CHEMBL1790051 | CHEMBL83729 | WIN-35065-2)Show SMILES COC(=O)[C@@H]1C2CC[C@H](C[C@@H]1c1ccccc1)N2C |r,TLB:11:10:17:6.7,THB:2:4:17:6.7| Show InChI InChI=1S/C16H21NO2/c1-17-12-8-9-14(17)15(16(18)19-2)13(10-12)11-6-4-3-5-7-11/h3-7,12-15H,8-10H2,1-2H3/t12-,13-,14?,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HTT |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50177107

(3-alpha-(4-methoxyphenyl)-2-alpha-(4-methylphenyl)...)Show SMILES COc1ccc(cc1)[C@@H]1C2CCC(C[C@@H]1c1ccc(C)cc1)N2C |r,TLB:15:14:10.11:22,THB:5:8:10.11:22| Show InChI InChI=1S/C22H27NO/c1-15-4-6-16(7-5-15)20-14-18-10-13-21(23(18)2)22(20)17-8-11-19(24-3)12-9-17/h4-9,11-12,18,20-22H,10,13-14H2,1-3H3/t18?,20-,21?,22+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50369224

(CHEMBL1907787)Show SMILES [#6]-[#7](-[#6@H](-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-1)-c1cccc(-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c1)-[#6](=O)-[#6]-c1ccc(Cl)c(Cl)c1 |r,w:33.33,c:36,39| Show InChI InChI=1S/C50H48Cl2N8O10S/c1-59(47(67)18-28-7-14-38(51)39(52)17-28)40(27-60-15-2-3-16-60)29-5-4-6-30(19-29)57-46(66)26-55-44(64)24-53-43(63)23-54-45(65)25-56-50(71)58-31-8-11-34(37(20-31)49(68)69)48-35-12-9-32(61)21-41(35)70-42-22-33(62)10-13-36(42)48/h4-14,17,19-22,40,61-62H,2-3,15-16,18,23-27H2,1H3,(H,53,63)(H,54,65)(H,55,64)(H,56,71)(H,57,66)(H,68,69)/t40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 264 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity towards delta-2 opioid receptor in guinea pig brain |
J Med Chem 39: 1729-35 (1996)
Article DOI: 10.1021/jm950813b
BindingDB Entry DOI: 10.7270/Q27S7PFG |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50177109

((R)-3-alpha-(methylphenyl)-2-alpha-phenyltropane |...)Show SMILES CN1C2CCC1[C@H]([C@H](C2)c1ccc(C)cc1)c1ccccc1 |r,TLB:16:6:4.3:1,THB:9:7:4.3:1| Show InChI InChI=1S/C21H25N/c1-15-8-10-16(11-9-15)19-14-18-12-13-20(22(18)2)21(19)17-6-4-3-5-7-17/h3-11,18-21H,12-14H2,1-2H3/t18?,19-,20?,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50054440

((R)-3-Amino-N-[3-((S)-1-{[2-(3,4-dichloro-phenyl)-...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NC(=O)[C@H](N)CC(O)=O)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H30Cl2N4O4/c1-30(23(32)12-16-7-8-19(26)20(27)11-16)22(15-31-9-2-3-10-31)17-5-4-6-18(13-17)29-25(35)21(28)14-24(33)34/h4-8,11,13,21-22H,2-3,9-10,12,14-15,28H2,1H3,(H,29,35)(H,33,34)/t21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity and selectivity by competitive inhibition of radioligands on opioid Mu receptor in guinea pig brain memb... |
J Med Chem 39: 4478-82 (1996)
Article DOI: 10.1021/jm960459x
BindingDB Entry DOI: 10.7270/Q2N878WW |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50369223

(CHEMBL1907788)Show SMILES [#6]-[#7](-[#6@H](-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-1)-c1cccc(-[#7]-[#6](=O)-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c1)-[#6](=O)-[#6]-c1ccc(Cl)c(Cl)c1 |r,w:21.21,c:24,27| Show InChI InChI=1S/C44H39Cl2N5O7S/c1-50(41(55)18-25-7-14-35(45)36(46)17-25)37(24-51-15-2-3-16-51)26-5-4-6-27(19-26)48-40(54)23-47-44(59)49-28-8-11-31(34(20-28)43(56)57)42-32-12-9-29(52)21-38(32)58-39-22-30(53)10-13-33(39)42/h4-14,17,19-22,37,52-53H,2-3,15-16,18,23-24H2,1H3,(H,47,59)(H,48,54)(H,56,57)/t37-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 in guinea pig brain |
J Med Chem 39: 1729-35 (1996)
Article DOI: 10.1021/jm950813b
BindingDB Entry DOI: 10.7270/Q27S7PFG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50177107

(3-alpha-(4-methoxyphenyl)-2-alpha-(4-methylphenyl)...)Show SMILES COc1ccc(cc1)[C@@H]1C2CCC(C[C@@H]1c1ccc(C)cc1)N2C |r,TLB:15:14:10.11:22,THB:5:8:10.11:22| Show InChI InChI=1S/C22H27NO/c1-15-4-6-16(7-5-15)20-14-18-10-13-21(23(18)2)22(20)17-8-11-19(24-3)12-9-17/h4-9,11-12,18,20-22H,10,13-14H2,1-3H3/t18?,20-,21?,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HTT |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50054439

((S)-3-Amino-N-[3-((S)-1-{[2-(3,4-dichloro-phenyl)-...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NC(=O)[C@@H](N)CC(O)=O)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H30Cl2N4O4/c1-30(23(32)12-16-7-8-19(26)20(27)11-16)22(15-31-9-2-3-10-31)17-5-4-6-18(13-17)29-25(35)21(28)14-24(33)34/h4-8,11,13,21-22H,2-3,9-10,12,14-15,28H2,1H3,(H,29,35)(H,33,34)/t21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 462 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity and selectivity by competitive inhibition of radioligands on opioid Mu receptor in guinea pig brain memb... |
J Med Chem 39: 4478-82 (1996)
Article DOI: 10.1021/jm960459x
BindingDB Entry DOI: 10.7270/Q2N878WW |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50366564

(CHEMBL1790051 | CHEMBL83729 | WIN-35065-2)Show SMILES COC(=O)[C@@H]1C2CC[C@H](C[C@@H]1c1ccccc1)N2C |r,TLB:11:10:17:6.7,THB:2:4:17:6.7| Show InChI InChI=1S/C16H21NO2/c1-17-12-8-9-14(17)15(16(18)19-2)13(10-12)11-6-4-3-5-7-11/h3-7,12-15H,8-10H2,1-2H3/t12-,13-,14?,15+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50177106

(2-alpha-3-alpha-diphenyltropane | 2-beta-3-beta-di...)Show SMILES CN1C2CCC1[C@H]([C@H](C2)c1ccccc1)c1ccccc1 |r,TLB:15:6:4.3:1,THB:9:7:4.3:1| Show InChI InChI=1S/C20H23N/c1-21-17-12-13-19(21)20(16-10-6-3-7-11-16)18(14-17)15-8-4-2-5-9-15/h2-11,17-20H,12-14H2,1H3/t17?,18-,19?,20+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50054440

((R)-3-Amino-N-[3-((S)-1-{[2-(3,4-dichloro-phenyl)-...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NC(=O)[C@H](N)CC(O)=O)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H30Cl2N4O4/c1-30(23(32)12-16-7-8-19(26)20(27)11-16)22(15-31-9-2-3-10-31)17-5-4-6-18(13-17)29-25(35)21(28)14-24(33)34/h4-8,11,13,21-22H,2-3,9-10,12,14-15,28H2,1H3,(H,29,35)(H,33,34)/t21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 573 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity and selectivity by competitive inhibition of radioligands on opioid delta receptor in guinea pig brain m... |
J Med Chem 39: 4478-82 (1996)
Article DOI: 10.1021/jm960459x
BindingDB Entry DOI: 10.7270/Q2N878WW |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50369224

(CHEMBL1907787)Show SMILES [#6]-[#7](-[#6@H](-[#6]-[#7]-1-[#6]-[#6]-[#6]-[#6]-1)-c1cccc(-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c1)-[#6](=O)-[#6]-c1ccc(Cl)c(Cl)c1 |r,w:33.33,c:36,39| Show InChI InChI=1S/C50H48Cl2N8O10S/c1-59(47(67)18-28-7-14-38(51)39(52)17-28)40(27-60-15-2-3-16-60)29-5-4-6-30(19-29)57-46(66)26-55-44(64)24-53-43(63)23-54-45(65)25-56-50(71)58-31-8-11-34(37(20-31)49(68)69)48-35-12-9-32(61)21-41(35)70-42-22-33(62)10-13-36(42)48/h4-14,17,19-22,40,61-62H,2-3,15-16,18,23-27H2,1H3,(H,53,63)(H,54,65)(H,55,64)(H,56,71)(H,57,66)(H,68,69)/t40-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity towards Opioid receptor mu 1 in guinea pig brain |
J Med Chem 39: 1729-35 (1996)
Article DOI: 10.1021/jm950813b
BindingDB Entry DOI: 10.7270/Q27S7PFG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50177108

(2-alpha,3-alpha-di(4-methylphenyl)tropane | 2-beta...)Show SMILES CN1C2CCC1[C@@H]([C@@H](C2)c1ccc(C)cc1)c1ccc(C)cc1 |r,TLB:16:6:4.3:1,THB:9:7:4.3:1| Show InChI InChI=1S/C22H27N/c1-15-4-8-17(9-5-15)20-14-19-12-13-21(23(19)3)22(20)18-10-6-16(2)7-11-18/h4-11,19-22H,12-14H2,1-3H3/t19?,20-,21?,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HTT |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50054439

((S)-3-Amino-N-[3-((S)-1-{[2-(3,4-dichloro-phenyl)-...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NC(=O)[C@@H](N)CC(O)=O)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H30Cl2N4O4/c1-30(23(32)12-16-7-8-19(26)20(27)11-16)22(15-31-9-2-3-10-31)17-5-4-6-18(13-17)29-25(35)21(28)14-24(33)34/h4-8,11,13,21-22H,2-3,9-10,12,14-15,28H2,1H3,(H,29,35)(H,33,34)/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Compound was tested for the binding affinity and selectivity by competitive inhibition of radioligands on opioid delta receptor in guinea pig brain m... |
J Med Chem 39: 4478-82 (1996)
Article DOI: 10.1021/jm960459x
BindingDB Entry DOI: 10.7270/Q2N878WW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50177104

(3-beta-(4'-methylphenyl)-2-beta-(3,4-dimethoxyphen...)Show SMILES COc1ccc(cc1OC)[C@@H]1C2CCC(C[C@@H]1c1ccc(C)cc1)N2C |r,TLB:17:16:12.13:24,THB:5:10:12.13:24| Show InChI InChI=1S/C23H29NO2/c1-15-5-7-16(8-6-15)19-14-18-10-11-20(24(18)2)23(19)17-9-12-21(25-3)22(13-17)26-4/h5-9,12-13,18-20,23H,10-11,14H2,1-4H3/t18?,19-,20?,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HTT |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50177111

(3-alpha-(4-methylphenyl)-2-beta-(3,4-dimethoxyphen...)Show SMILES COc1ccc(cc1OC)[C@@H]1C2CCC(C[C@H]1c1ccc(C)cc1)N2C |r,TLB:17:16:12.13:24,THB:5:10:12.13:24| Show InChI InChI=1S/C23H29NO2/c1-15-5-7-16(8-6-15)19-14-18-10-11-20(24(18)2)23(19)17-9-12-21(25-3)22(13-17)26-4/h5-9,12-13,18-20,23H,10-11,14H2,1-4H3/t18?,19-,20?,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HTT |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50177106

(2-alpha-3-alpha-diphenyltropane | 2-beta-3-beta-di...)Show SMILES CN1C2CCC1[C@H]([C@H](C2)c1ccccc1)c1ccccc1 |r,TLB:15:6:4.3:1,THB:9:7:4.3:1| Show InChI InChI=1S/C20H23N/c1-21-17-12-13-19(21)20(16-10-6-3-7-11-16)18(14-17)15-8-4-2-5-9-15/h2-11,17-20H,12-14H2,1H3/t17?,18-,19?,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HTT |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50006781

((2S,3S)-methyl 8-methyl-3-p-tolyl-8-aza-bicyclo[3....)Show SMILES COC(=O)[C@@H]1C2CC[C@H](C[C@@H]1c1ccc(C)cc1)N2C |TLB:19:18:4.10.9:6.7,THB:2:4:18:6.7,11:10:18:6.7| Show InChI InChI=1S/C17H23NO2/c1-11-4-6-12(7-5-11)14-10-13-8-9-15(18(13)2)16(14)17(19)20-3/h4-7,13-16H,8-10H2,1-3H3/t13-,14-,15?,16+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50006781

((2S,3S)-methyl 8-methyl-3-p-tolyl-8-aza-bicyclo[3....)Show SMILES COC(=O)[C@@H]1C2CC[C@H](C[C@@H]1c1ccc(C)cc1)N2C |TLB:19:18:4.10.9:6.7,THB:2:4:18:6.7,11:10:18:6.7| Show InChI InChI=1S/C17H23NO2/c1-11-4-6-12(7-5-11)14-10-13-8-9-15(18(13)2)16(14)17(19)20-3/h4-7,13-16H,8-10H2,1-3H3/t13-,14-,15?,16+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50084717

((+)-(1R,2R,3S,5S)-methyl 3-(benzoyloxy)-8-methyl-8...)Show SMILES COC(=O)[C@@H]1C2CCC(C[C@@H]1C(=O)Oc1ccccc1)N2C |THB:2:4:20:6.7| Show InChI InChI=1S/C17H21NO4/c1-18-11-8-9-14(18)15(17(20)21-2)13(10-11)16(19)22-12-6-4-3-5-7-12/h3-7,11,13-15H,8-10H2,1-2H3/t11?,13-,14?,15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
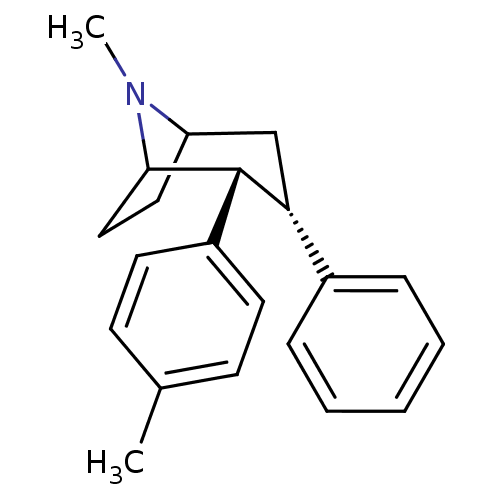
(Homo sapiens (Human)) | BDBM50177105
(3-alpha-phenyl-2-alpha-(4-methylphenyl)tropane | 3...)Show SMILES CN1C2CCC1[C@H]([C@H](C2)c1ccccc1)c1ccc(C)cc1 |r,TLB:15:6:4.3:1,THB:9:7:4.3:1| Show InChI InChI=1S/C21H25N/c1-15-8-10-17(11-9-15)21-19(16-6-4-3-5-7-16)14-18-12-13-20(21)22(18)2/h3-11,18-21H,12-14H2,1-2H3/t18?,19-,20?,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from NET |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50177105

(3-alpha-phenyl-2-alpha-(4-methylphenyl)tropane | 3...)Show SMILES CN1C2CCC1[C@H]([C@H](C2)c1ccccc1)c1ccc(C)cc1 |r,TLB:15:6:4.3:1,THB:9:7:4.3:1| Show InChI InChI=1S/C21H25N/c1-15-8-10-17(11-9-15)21-19(16-6-4-3-5-7-16)14-18-12-13-20(21)22(18)2/h3-11,18-21H,12-14H2,1-2H3/t18?,19-,20?,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from 5HTT |
J Med Chem 48: 7437-44 (2005)
Article DOI: 10.1021/jm0582423
BindingDB Entry DOI: 10.7270/Q2B56KJX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data