 Found 3051 hits with Last Name = 'chang' and Initial = 'y'
Found 3051 hits with Last Name = 'chang' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | -57.6 | n/a | n/a | 5.10 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
J Med Chem 50: 5049-52 (2007)
Article DOI: 10.1021/jm070231h
BindingDB Entry DOI: 10.7270/Q29Z935Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM31817

(GRL-02031 | methyl-2-pyrrolidinone, 19b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22+,24+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 wild type protease using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometry |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM31817

(GRL-02031 | methyl-2-pyrrolidinone, 19b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22+,24+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease I47V mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometry |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50107460
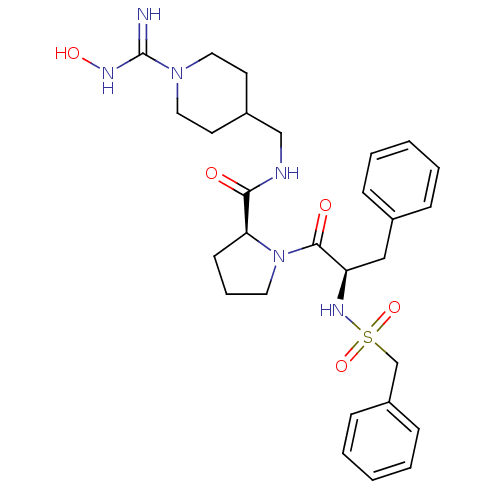
((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...)Show SMILES ONC(=N)N1CCC(CNC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2ccccc2)NS(=O)(=O)Cc2ccccc2)CC1 Show InChI InChI=1S/C28H38N6O5S/c29-28(31-37)33-16-13-22(14-17-33)19-30-26(35)25-12-7-15-34(25)27(36)24(18-21-8-3-1-4-9-21)32-40(38,39)20-23-10-5-2-6-11-23/h1-6,8-11,22,24-25,32,37H,7,12-20H2,(H2,29,31)(H,30,35)/t24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Competitive kinetic for thrombin inhibition Ki was determined |
Bioorg Med Chem Lett 12: 45-9 (2001)
BindingDB Entry DOI: 10.7270/Q2GX4C3P |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11542
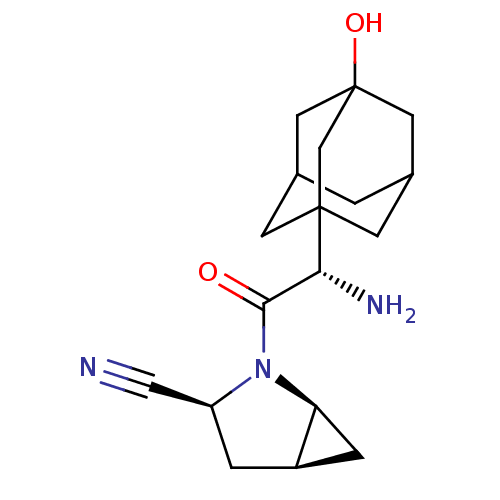
((1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(O)(C3)C1)C2 |r,TLB:15:16:21:20.13.14,THB:17:16:13:20.21.18,17:18:16.15.22:13,19:18:16.15.22:13,15:14:16.22.17:21| Show InChI InChI=1S/C18H25N3O2/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17/h10-15,23H,1-7,9,20H2/t10?,11?,12-,13+,14+,15-,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... |
J Med Chem 48: 5025-37 (2005)
Article DOI: 10.1021/jm050261p
BindingDB Entry DOI: 10.7270/Q2FN14DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
HIV-1 protease
(Human immunodeficiency virus) | BDBM31817

(GRL-02031 | methyl-2-pyrrolidinone, 19b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22+,24+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82A mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometry |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM31817

(GRL-02031 | methyl-2-pyrrolidinone, 19b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22+,24+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease L76V mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometry |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11541
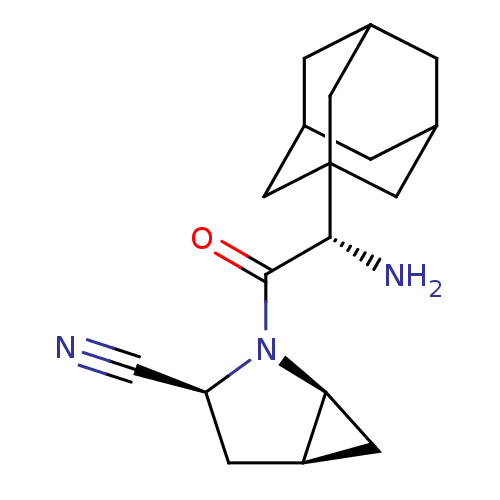
((1S,3S,5S)-2-[(2S)-2-(adamantan-1-yl)-2-aminoacety...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(C3)C1)C2 |r,TLB:15:16:20:19.13.14,THB:17:16:13:19.20.18,17:18:16.15.21:13,15:14:16.21.17:20| Show InChI InChI=1S/C18H25N3O/c19-9-14-4-13-5-15(13)21(14)17(22)16(20)18-6-10-1-11(7-18)3-12(2-10)8-18/h10-16H,1-8,20H2/t10?,11?,12?,13-,14+,15+,16-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... |
J Med Chem 48: 5025-37 (2005)
Article DOI: 10.1021/jm050261p
BindingDB Entry DOI: 10.7270/Q2FN14DM |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18216
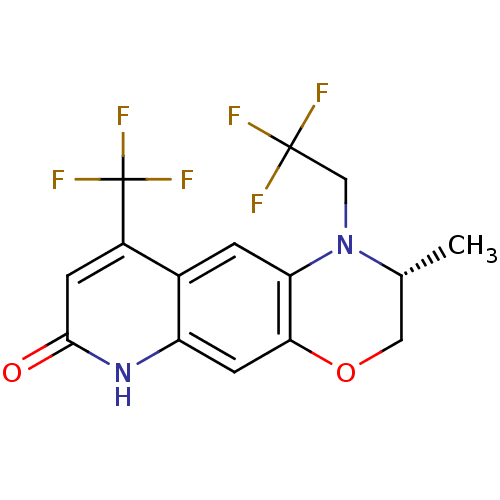
((2R)-2-methyl-1-(2,2,2-trifluoroethyl)-9-(trifluor...)Show SMILES C[C@@H]1COc2cc3[nH]c(=O)cc(c3cc2N1CC(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C15H12F6N2O2/c1-7-5-25-12-4-10-8(2-11(12)23(7)6-14(16,17)18)9(15(19,20)21)3-13(24)22-10/h2-4,7H,5-6H2,1H3,(H,22,24)/t7-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM31817

(GRL-02031 | methyl-2-pyrrolidinone, 19b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22+,24+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease N88D mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate after 5 mins by spectrophotometry |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50237710
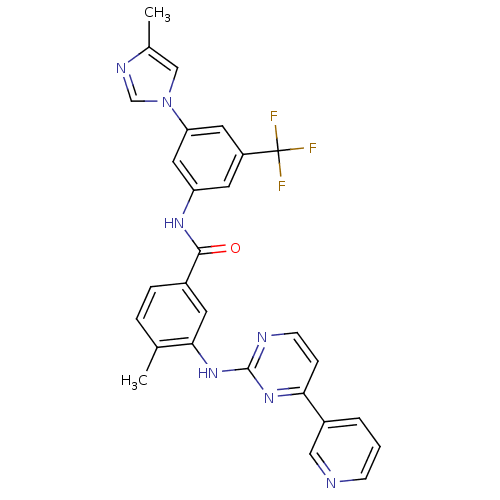
(4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 103: 3153-8 (2006)
Article DOI: 10.1073/pnas.0511292103
BindingDB Entry DOI: 10.7270/Q21Z430B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50377413

(CHEMBL257379)Show SMILES Cc1cccc(c1)[C@@H]1COc2cc3[nH]c(=O)cc(c3cc2N1CC(F)(F)F)C(F)(F)F Show InChI InChI=1S/C21H16F6N2O2/c1-11-3-2-4-12(5-11)17-9-31-18-8-15-13(6-16(18)29(17)10-20(22,23)24)14(21(25,26)27)7-19(30)28-15/h2-8,17H,9-10H2,1H3,(H,28,30)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against the binding of [3H]-spiperone to Dopamine receptor D2 in rat striatal membranes |
J Med Chem 30: 1631-5 (1987)
BindingDB Entry DOI: 10.7270/Q2FB51ZZ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50377414
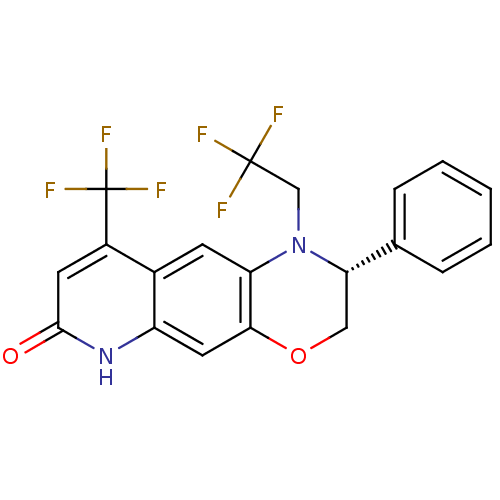
(CHEMBL402835)Show SMILES FC(F)(F)CN1[C@@H](COc2cc3[nH]c(=O)cc(c3cc12)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C20H14F6N2O2/c21-19(22,23)10-28-15-6-12-13(20(24,25)26)7-18(29)27-14(12)8-17(15)30-9-16(28)11-4-2-1-3-5-11/h1-8,16H,9-10H2,(H,27,29)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11530
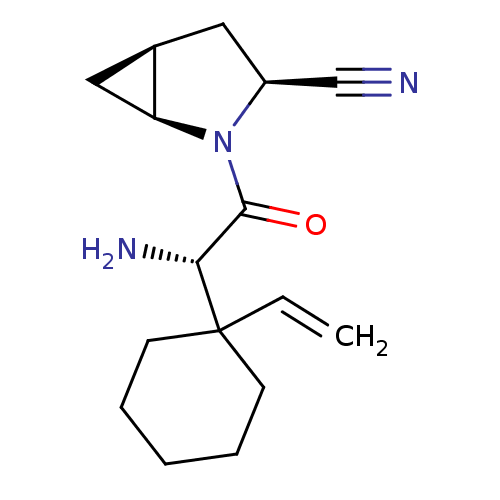
((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclohexyl)...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C1(CCCCC1)C=C |r| Show InChI InChI=1S/C16H23N3O/c1-2-16(6-4-3-5-7-16)14(18)15(20)19-12(10-17)8-11-9-13(11)19/h2,11-14H,1,3-9,18H2/t11-,12+,13+,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... |
J Med Chem 48: 5025-37 (2005)
Article DOI: 10.1021/jm050261p
BindingDB Entry DOI: 10.7270/Q2FN14DM |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18522
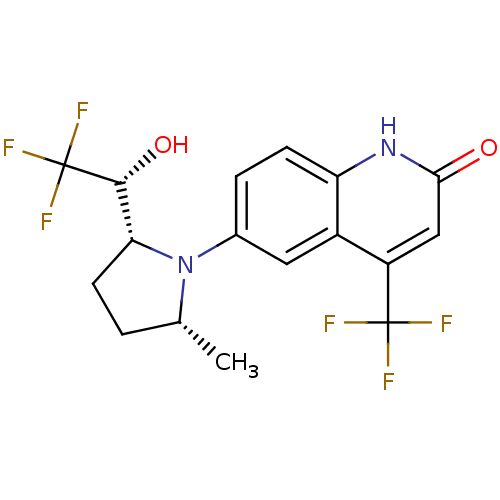
(6-(1-Pyrrolidine)quinolin-2(1H)-one, 6a | 6-[(2R,5...)Show SMILES C[C@@H]1CC[C@H]([C@@H](O)C(F)(F)F)N1c1ccc2[nH]c(=O)cc(c2c1)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O2/c1-8-2-5-13(15(27)17(21,22)23)25(8)9-3-4-12-10(6-9)11(16(18,19)20)7-14(26)24-12/h3-4,6-8,13,15,27H,2,5H2,1H3,(H,24,26)/t8-,13-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | -52.4 | n/a | n/a | 7.10 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
J Med Chem 50: 5049-52 (2007)
Article DOI: 10.1021/jm070231h
BindingDB Entry DOI: 10.7270/Q29Z935Z |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11544
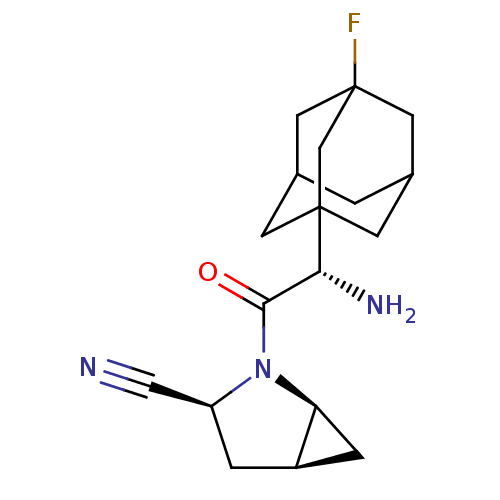
((1S,3S,5S)-2-[(2S)-2-amino-2-(3-fluoroadamantan-1-...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(CC(F)(C3)C1)C2 |r,TLB:15:16:14.20.13:21,THB:17:16:13:20.21.18,17:18:16.15.22:13,19:18:16.15.22:13,15:14:16.22.17:21| Show InChI InChI=1S/C18H24FN3O/c19-18-6-10-1-11(7-18)5-17(4-10,9-18)15(21)16(23)22-13(8-20)2-12-3-14(12)22/h10-15H,1-7,9,21H2/t10?,11?,12-,13+,14+,15-,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... |
J Med Chem 48: 5025-37 (2005)
Article DOI: 10.1021/jm050261p
BindingDB Entry DOI: 10.7270/Q2FN14DM |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50237710

(4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 103: 3153-8 (2006)
Article DOI: 10.1073/pnas.0511292103
BindingDB Entry DOI: 10.7270/Q21Z430B |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM13530
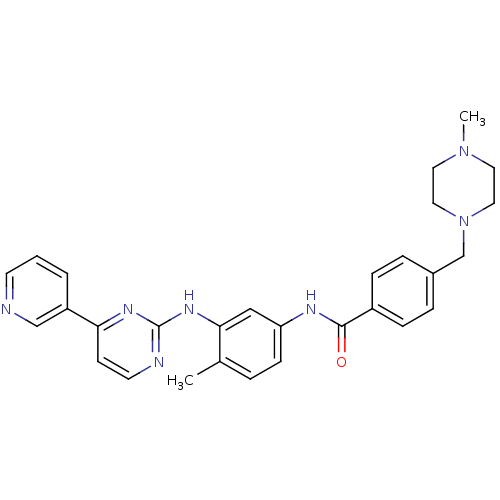
(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 103: 3153-8 (2006)
Article DOI: 10.1073/pnas.0511292103
BindingDB Entry DOI: 10.7270/Q21Z430B |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50237710

(4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 103: 3153-8 (2006)
Article DOI: 10.1073/pnas.0511292103
BindingDB Entry DOI: 10.7270/Q21Z430B |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50237710

(4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 103: 3153-8 (2006)
Article DOI: 10.1073/pnas.0511292103
BindingDB Entry DOI: 10.7270/Q21Z430B |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11543
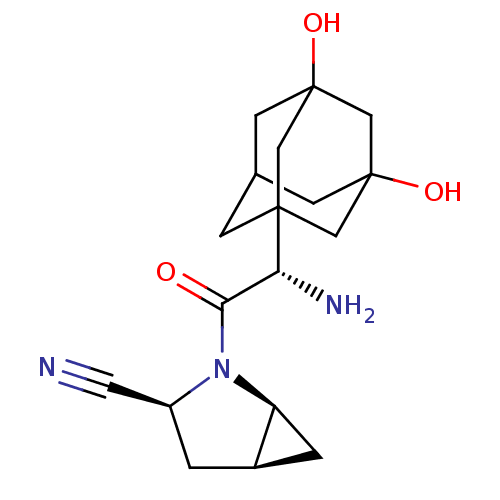
((1S,3S,5S)-2-[(2S)-2-amino-2-(3,5-dihydroxyadamant...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C12CC3CC(O)(CC(O)(C3)C1)C2 |r,TLB:21:14:23:18.22.19,21:19:14.15.13:23,20:19:14.15.13:23,17:16:14.13.21:22,15:16:14.13.21:22,THB:15:14:22:18.23.16| Show InChI InChI=1S/C18H25N3O3/c19-6-12-1-11-2-13(11)21(12)15(22)14(20)16-3-10-4-17(23,7-16)9-18(24,5-10)8-16/h10-14,23-24H,1-5,7-9,20H2/t10?,11-,12+,13+,14-,16?,17?,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... |
J Med Chem 48: 5025-37 (2005)
Article DOI: 10.1021/jm050261p
BindingDB Entry DOI: 10.7270/Q2FN14DM |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50377415
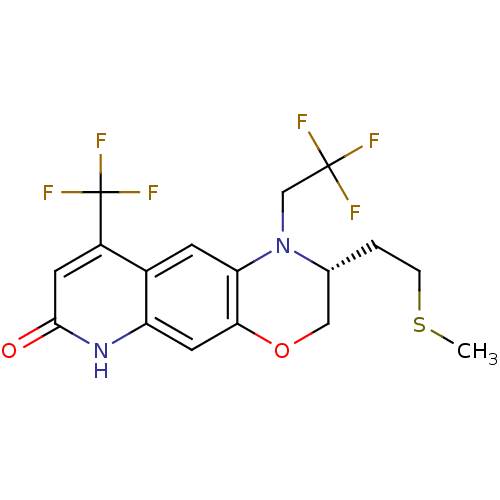
(CHEMBL404483)Show SMILES CSCC[C@@H]1COc2cc3[nH]c(=O)cc(c3cc2N1CC(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H16F6N2O2S/c1-28-3-2-9-7-27-14-6-12-10(4-13(14)25(9)8-16(18,19)20)11(17(21,22)23)5-15(26)24-12/h4-6,9H,2-3,7-8H2,1H3,(H,24,26)/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50377419
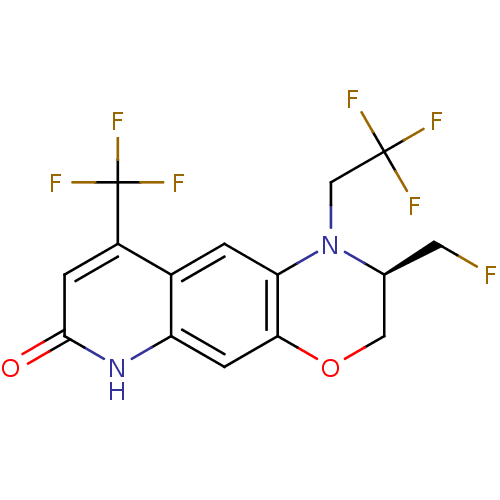
(CHEMBL429311)Show SMILES FC[C@H]1COc2cc3[nH]c(=O)cc(c3cc2N1CC(F)(F)F)C(F)(F)F Show InChI InChI=1S/C15H11F7N2O2/c16-4-7-5-26-12-3-10-8(1-11(12)24(7)6-14(17,18)19)9(15(20,21)22)2-13(25)23-10/h1-3,7H,4-6H2,(H,23,25)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 103: 3153-8 (2006)
Article DOI: 10.1073/pnas.0511292103
BindingDB Entry DOI: 10.7270/Q21Z430B |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11529
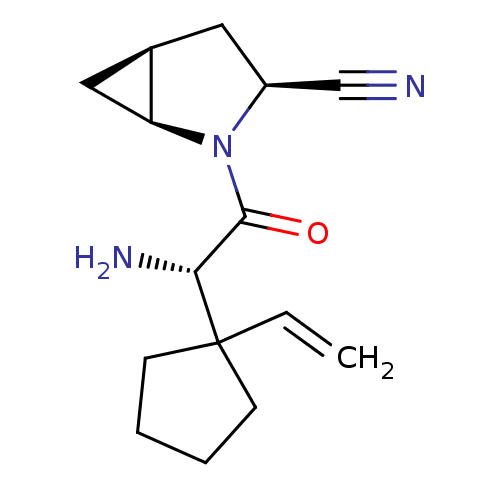
((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclopentyl...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C1(CCCC1)C=C |r| Show InChI InChI=1S/C15H21N3O/c1-2-15(5-3-4-6-15)13(17)14(19)18-11(9-16)7-10-8-12(10)18/h2,10-13H,1,3-8,17H2/t10-,11+,12+,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... |
J Med Chem 48: 5025-37 (2005)
Article DOI: 10.1021/jm050261p
BindingDB Entry DOI: 10.7270/Q2FN14DM |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50237710

(4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 103: 3153-8 (2006)
Article DOI: 10.1073/pnas.0511292103
BindingDB Entry DOI: 10.7270/Q21Z430B |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM50586388

(CHEMBL5081557)Show SMILES CS(=O)(=O)c1ccc(CNC(=O)c2cc3CCOC4(CCN(Cc5ccccc5Cl)CC4)c3s2)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora C kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18524
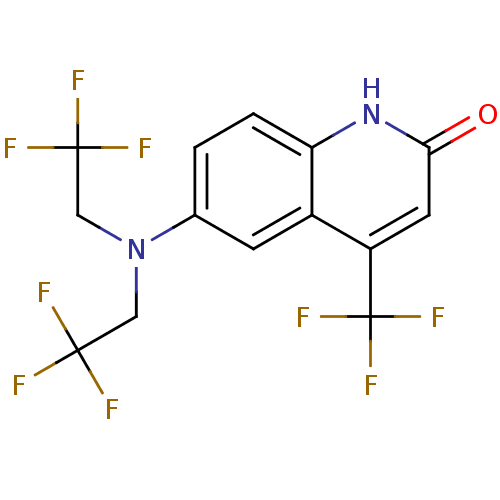
(6-[bis(2,2,2-trifluoroethyl)amino]-4-(trifluoromet...)Show SMILES FC(F)(F)CN(CC(F)(F)F)c1ccc2[nH]c(=O)cc(c2c1)C(F)(F)F Show InChI InChI=1S/C14H9F9N2O/c15-12(16,17)5-25(6-13(18,19)20)7-1-2-10-8(3-7)9(14(21,22)23)4-11(26)24-10/h1-4H,5-6H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4.60 | -49.5 | n/a | n/a | 0.200 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
J Med Chem 50: 5049-52 (2007)
Article DOI: 10.1021/jm070231h
BindingDB Entry DOI: 10.7270/Q29Z935Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of Aurora C kinase |
Bioorg Med Chem Lett 18: 1623-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.068
BindingDB Entry DOI: 10.7270/Q261115V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11535
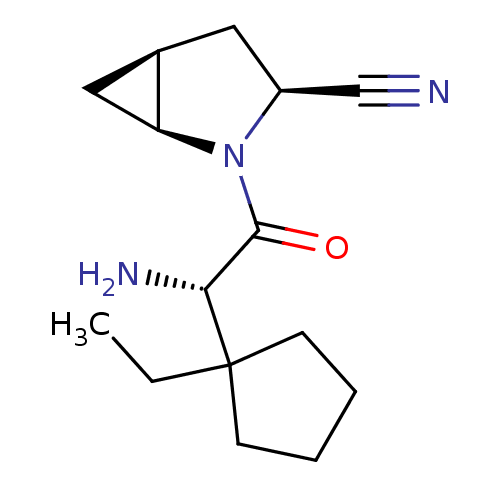
((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethylcyclopentyl)a...)Show SMILES CCC1(CCCC1)[C@H](N)C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N |r| Show InChI InChI=1S/C15H23N3O/c1-2-15(5-3-4-6-15)13(17)14(19)18-11(9-16)7-10-8-12(10)18/h10-13H,2-8,17H2,1H3/t10-,11+,12+,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... |
J Med Chem 48: 5025-37 (2005)
Article DOI: 10.1021/jm050261p
BindingDB Entry DOI: 10.7270/Q2FN14DM |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50377411
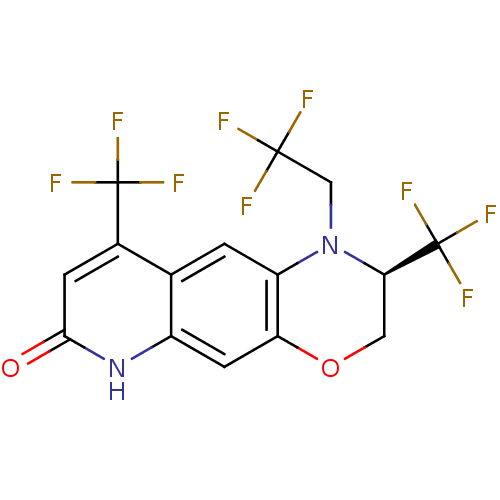
(CHEMBL401805)Show SMILES FC(F)(F)CN1[C@H](COc2cc3[nH]c(=O)cc(c3cc12)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C15H9F9N2O2/c16-13(17,18)5-26-9-1-6-7(14(19,20)21)2-12(27)25-8(6)3-10(9)28-4-11(26)15(22,23)24/h1-3,11H,4-5H2,(H,25,27)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM498074
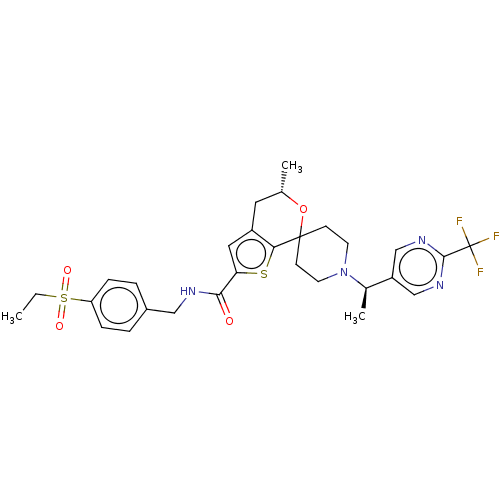
((5′S)óN-[4-(Ethylsulfonyl)benzyl]-5′-m...)Show SMILES CCS(=O)(=O)c1ccc(CNC(=O)c2cc3C[C@H](C)OC4(CCN(CC4)[C@H](C)c4cnc(nc4)C(F)(F)F)c3s2)cc1 |r| Show InChI InChI=1S/C29H33F3N4O4S2/c1-4-42(38,39)23-7-5-20(6-8-23)15-33-26(37)24-14-21-13-18(2)40-28(25(21)41-24)9-11-36(12-10-28)19(3)22-16-34-27(35-17-22)29(30,31)32/h5-8,14,16-19H,4,9-13,15H2,1-3H3,(H,33,37)/t18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-25-hydroxycholesterol from His-Flag-tagged human RORgammat LBD (309 to 508 residues) expressed in Escherichia coli measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01918
BindingDB Entry DOI: 10.7270/Q2B85D1S |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50377417
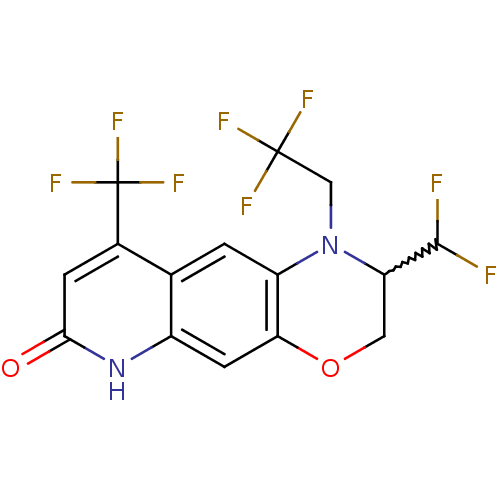
(CHEMBL256326)Show SMILES FC(F)C1COc2cc3[nH]c(=O)cc(c3cc2N1CC(F)(F)F)C(F)(F)F |w:3.2| Show InChI InChI=1S/C15H10F8N2O2/c16-13(17)10-4-27-11-3-8-6(1-9(11)25(10)5-14(18,19)20)7(15(21,22)23)2-12(26)24-8/h1-3,10,13H,4-5H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11533
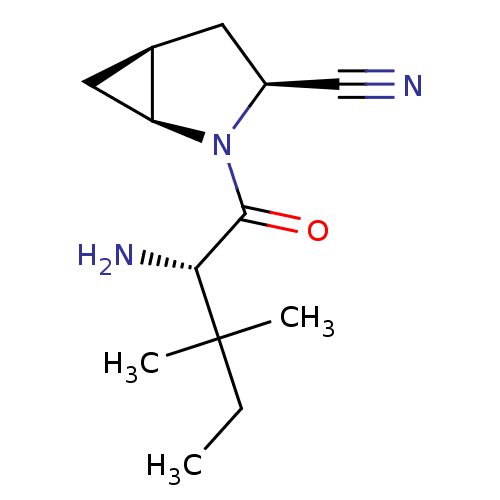
((1S,3S,5S)-2-[(2S)-2-amino-3,3-dimethylpentanoyl]-...)Show SMILES CCC(C)(C)[C@H](N)C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N |r| Show InChI InChI=1S/C13H21N3O/c1-4-13(2,3)11(15)12(17)16-9(7-14)5-8-6-10(8)16/h8-11H,4-6,15H2,1-3H3/t8-,9+,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | -46.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... |
J Med Chem 48: 5025-37 (2005)
Article DOI: 10.1021/jm050261p
BindingDB Entry DOI: 10.7270/Q2FN14DM |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11538
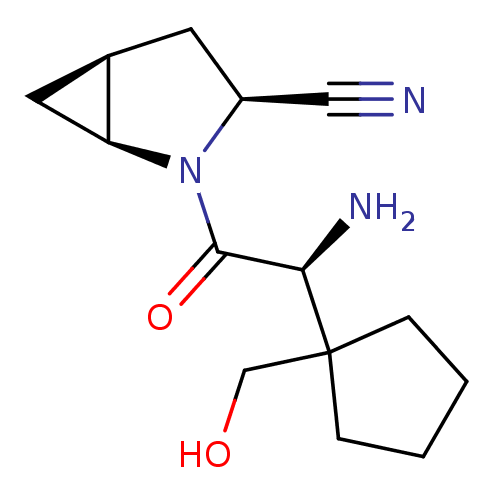
((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyc...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C1(CO)CCCC1 |r| Show InChI InChI=1S/C14H21N3O2/c15-7-10-5-9-6-11(9)17(10)13(19)12(16)14(8-18)3-1-2-4-14/h9-12,18H,1-6,8,16H2/t9-,10+,11+,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... |
J Med Chem 48: 5025-37 (2005)
Article DOI: 10.1021/jm050261p
BindingDB Entry DOI: 10.7270/Q2FN14DM |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18523
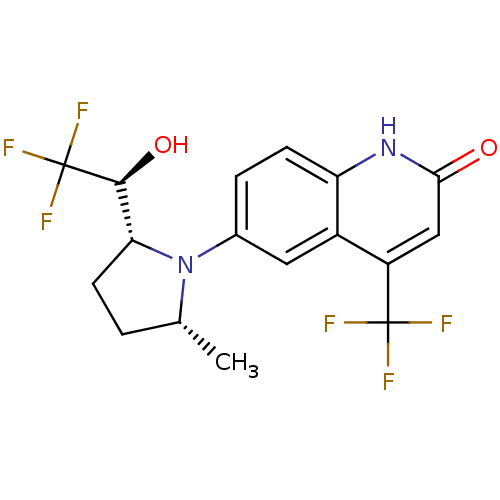
(6-(1-Pyrrolidine)quinolin-2(1H)-one, 6b | 6-[(2R,5...)Show SMILES C[C@@H]1CC[C@H]([C@H](O)C(F)(F)F)N1c1ccc2[nH]c(=O)cc(c2c1)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O2/c1-8-2-5-13(15(27)17(21,22)23)25(8)9-3-4-12-10(6-9)11(16(18,19)20)7-14(26)24-12/h3-4,6-8,13,15,27H,2,5H2,1H3,(H,24,26)/t8-,13-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | -48.2 | n/a | n/a | 8.40 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc.
| Assay Description
The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... |
J Med Chem 50: 5049-52 (2007)
Article DOI: 10.1021/jm070231h
BindingDB Entry DOI: 10.7270/Q29Z935Z |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11539
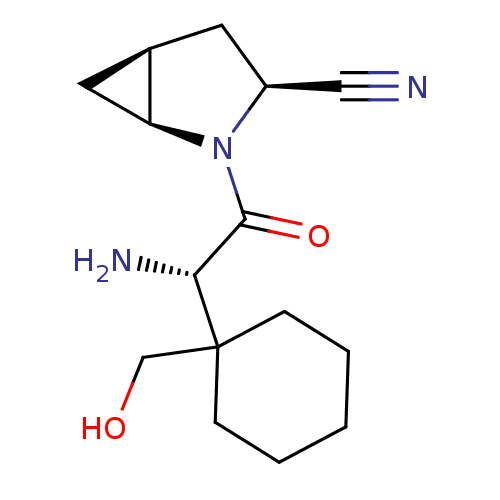
((1S,3S,5S)-2-[(2S)-2-amino-2-[1-(hydroxymethyl)cyc...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C1(CO)CCCCC1 |r| Show InChI InChI=1S/C15H23N3O2/c16-8-11-6-10-7-12(10)18(11)14(20)13(17)15(9-19)4-2-1-3-5-15/h10-13,19H,1-7,9,17H2/t10-,11+,12+,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... |
J Med Chem 48: 5025-37 (2005)
Article DOI: 10.1021/jm050261p
BindingDB Entry DOI: 10.7270/Q2FN14DM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50107463
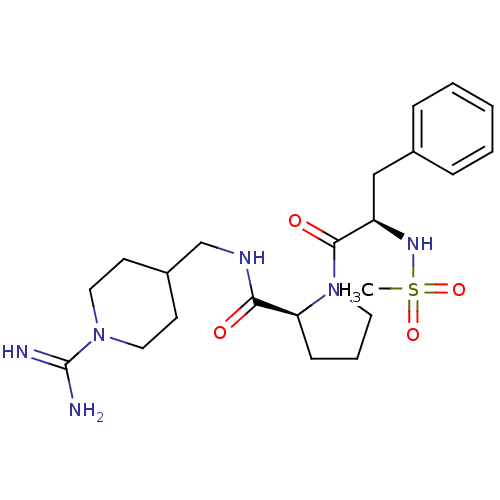
((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...)Show SMILES CS(=O)(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCC1CCN(CC1)C(N)=N Show InChI InChI=1S/C22H34N6O4S/c1-33(31,32)26-18(14-16-6-3-2-4-7-16)21(30)28-11-5-8-19(28)20(29)25-15-17-9-12-27(13-10-17)22(23)24/h2-4,6-7,17-19,26H,5,8-15H2,1H3,(H3,23,24)(H,25,29)/t18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Competitive kinetic for human alpha thrombin inhibition Ki was determined |
Bioorg Med Chem Lett 12: 45-9 (2001)
BindingDB Entry DOI: 10.7270/Q2GX4C3P |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50377410

(CHEMBL257988)Show SMILES FC(F)(F)CN1[C@@H](COc2cc3[nH]c(=O)cc(c3cc12)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C15H9F9N2O2/c16-13(17,18)5-26-9-1-6-7(14(19,20)21)2-12(27)25-8(6)3-10(9)28-4-11(26)15(22,23)24/h1-3,11H,4-5H2,(H,25,27)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
Protein AF-9
(Homo sapiens) | BDBM50459727

(CHEMBL4206834)Show SMILES CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)N[C@@H](CN)C(=O)N[C@@H](CC1CCCC1)C(=O)N1CCC[C@H]1c1nc(cn1CC1CCCCC1)-c1ccccc1 |r| Show InChI InChI=1S/C48H66N8O5/c1-32(2)42(53-45(58)41-25-15-27-56(41)47(60)36-22-10-5-11-23-36)46(59)52-38(29-49)44(57)51-37(28-33-16-12-13-17-33)48(61)55-26-14-24-40(55)43-50-39(35-20-8-4-9-21-35)31-54(43)30-34-18-6-3-7-19-34/h4-5,8-11,20-23,31-34,37-38,40-42H,3,6-7,12-19,24-30,49H2,1-2H3,(H,51,57)(H,52,59)(H,53,58)/t37-,38-,40-,41-,42-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of N-Flu-DOT1L from MBP-tagged AF9 (unknown origin) (487 to 568 residues) expressed in Escherichia coli BL21(DE3) measured after 3 hrs b... |
ACS Med Chem Lett 9: 895-900 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00175
BindingDB Entry DOI: 10.7270/Q2DV1NHX |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50377425

(CHEMBL402039)Show SMILES COC[C@@H]1COc2cc3[nH]c(=O)cc(c3cc2N1CC(F)(F)F)C(F)(F)F Show InChI InChI=1S/C16H14F6N2O3/c1-26-5-8-6-27-13-4-11-9(2-12(13)24(8)7-15(17,18)19)10(16(20,21)22)3-14(25)23-11/h2-4,8H,5-7H2,1H3,(H,23,25)/t8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human Androgen receptor in human MDA-MB-453 cells |
Bioorg Med Chem Lett 18: 2967-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.062
BindingDB Entry DOI: 10.7270/Q2R2128X |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11532
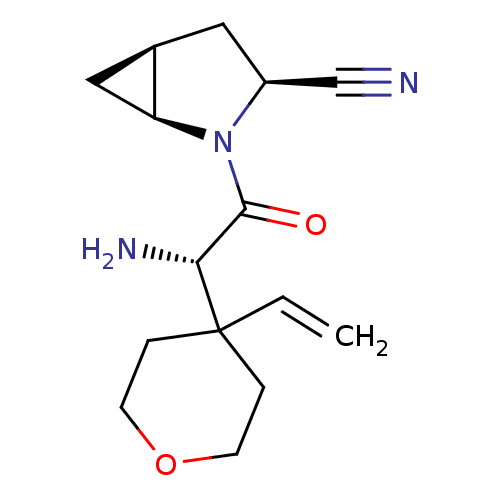
((1S,3S,5S)-2-[(2S)-2-amino-2-(4-ethenyloxan-4-yl)a...)Show SMILES N[C@H](C(=O)N1[C@H]2C[C@H]2C[C@H]1C#N)C1(CCOCC1)C=C |r| Show InChI InChI=1S/C15H21N3O2/c1-2-15(3-5-20-6-4-15)13(17)14(19)18-11(9-16)7-10-8-12(10)18/h2,10-13H,1,3-8,17H2/t10-,11+,12+,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... |
J Med Chem 48: 5025-37 (2005)
Article DOI: 10.1021/jm050261p
BindingDB Entry DOI: 10.7270/Q2FN14DM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data