 Found 165 hits with Last Name = 'chapin' and Initial = 'd'
Found 165 hits with Last Name = 'chapin' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032162
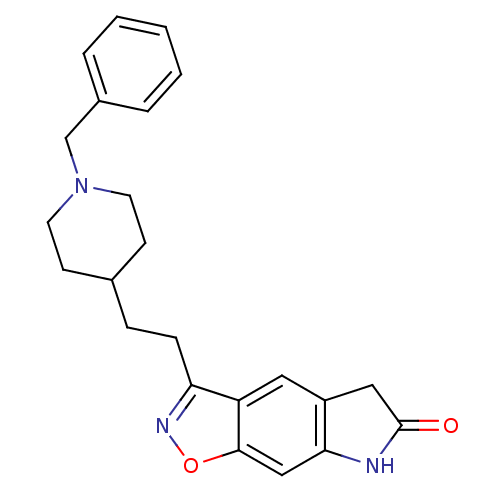
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,7-dihydro-...)Show SMILES O=C1Cc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-19-20(25-28-22(19)14-21(18)24-23)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31592
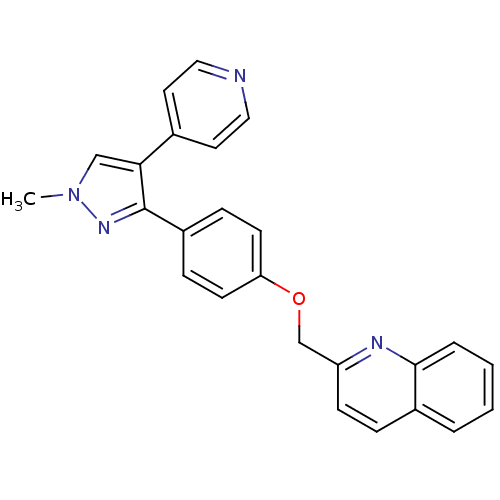
(PF-2545920 | US9138494, MP-10 | substituted pyraz...)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C25H20N4O/c1-29-16-23(18-12-14-26-15-13-18)25(28-29)20-7-10-22(11-8-20)30-17-21-9-6-19-4-2-3-5-24(19)27-21/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31591
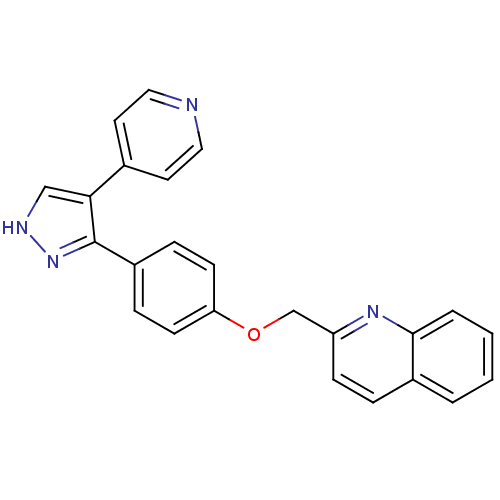
(pyrazole, 3)Show SMILES C(Oc1ccc(cc1)-c1n[nH]cc1-c1ccncc1)c1ccc2ccccc2n1 Show InChI InChI=1S/C24H18N4O/c1-2-4-23-18(3-1)5-8-20(27-23)16-29-21-9-6-19(7-10-21)24-22(15-26-28-24)17-11-13-25-14-12-17/h1-15H,16H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032161
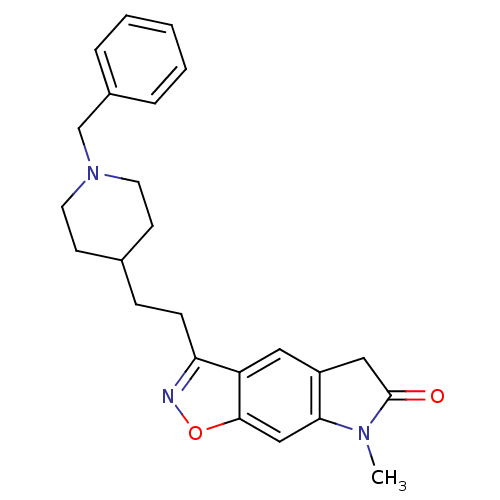
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-7-methyl-5,7...)Show SMILES CN1C(=O)Cc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc12 Show InChI InChI=1S/C24H27N3O2/c1-26-22-15-23-20(13-19(22)14-24(26)28)21(25-29-23)8-7-17-9-11-27(12-10-17)16-18-5-3-2-4-6-18/h2-6,13,15,17H,7-12,14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032164
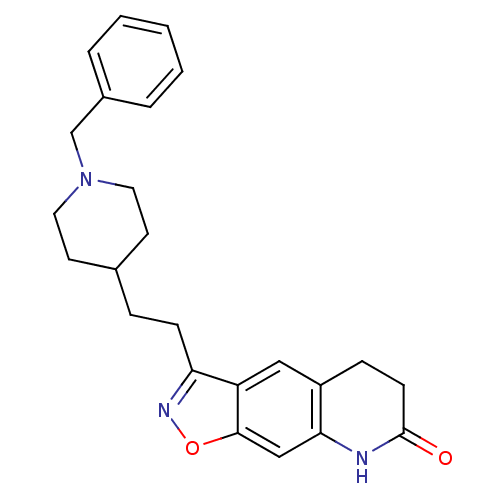
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dihydroiso...)Show SMILES O=C1CCc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C24H27N3O2/c28-24-9-7-19-14-20-21(26-29-23(20)15-22(19)25-24)8-6-17-10-12-27(13-11-17)16-18-4-2-1-3-5-18/h1-5,14-15,17H,6-13,16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31606
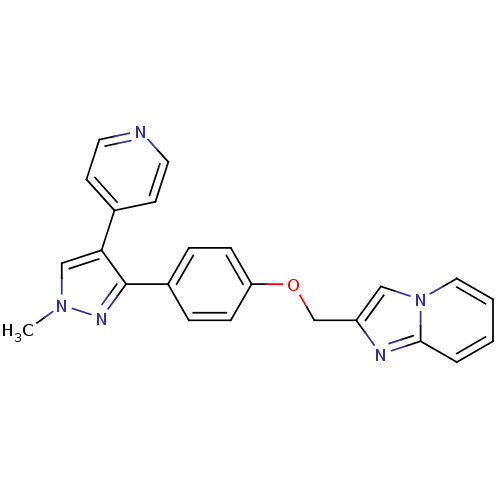
(methyl substituted pyrazole, 27)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2cn3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C23H19N5O/c1-27-15-21(17-9-11-24-12-10-17)23(26-27)18-5-7-20(8-6-18)29-16-19-14-28-13-3-2-4-22(28)25-19/h2-15H,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039721
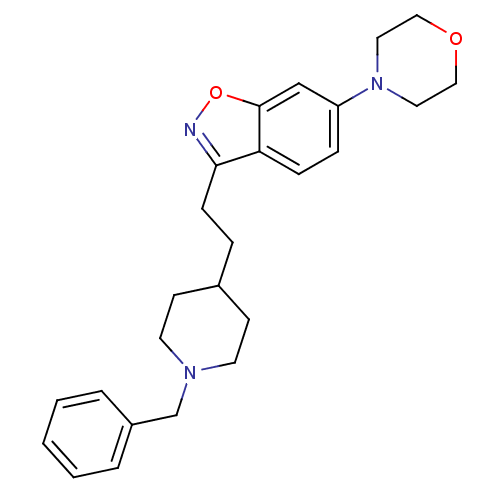
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-morpholinobe...)Show SMILES C(Cc1noc2cc(ccc12)N1CCOCC1)C1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H31N3O2/c1-2-4-21(5-3-1)19-27-12-10-20(11-13-27)6-9-24-23-8-7-22(18-25(23)30-26-24)28-14-16-29-17-15-28/h1-5,7-8,18,20H,6,9-17,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31594
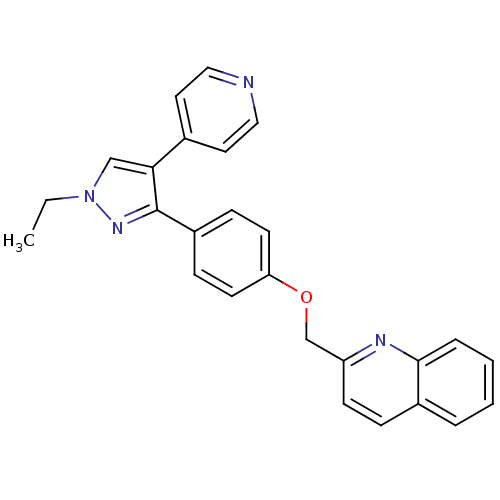
(substituted pyrazole, 11)Show SMILES CCn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C26H22N4O/c1-2-30-17-24(19-13-15-27-16-14-19)26(29-30)21-8-11-23(12-9-21)31-18-22-10-7-20-5-3-4-6-25(20)28-22/h3-17H,2,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032163
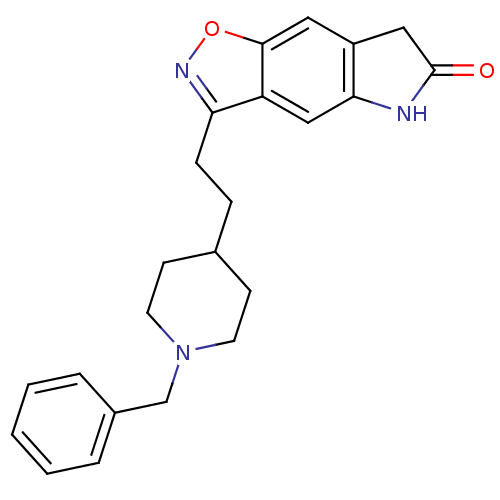
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5H-isoxazolo[5...)Show SMILES O=C1Cc2cc3onc(CCC4CCN(Cc5ccccc5)CC4)c3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-22-19(14-21(18)24-23)20(25-28-22)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31605
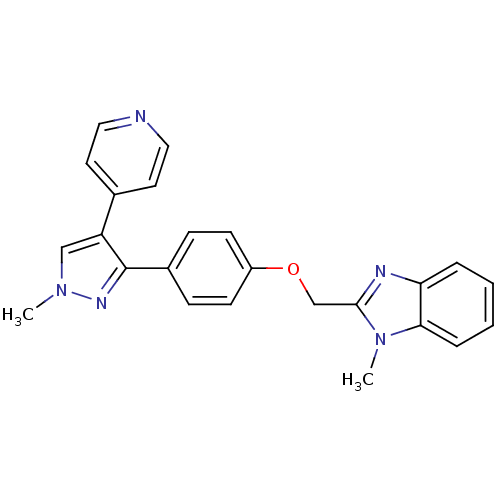
(methyl substituted pyrazole, 26)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2nc3ccccc3n2C)cc1)-c1ccncc1 Show InChI InChI=1S/C24H21N5O/c1-28-15-20(17-11-13-25-14-12-17)24(27-28)18-7-9-19(10-8-18)30-16-23-26-21-5-3-4-6-22(21)29(23)2/h3-15H,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50229040
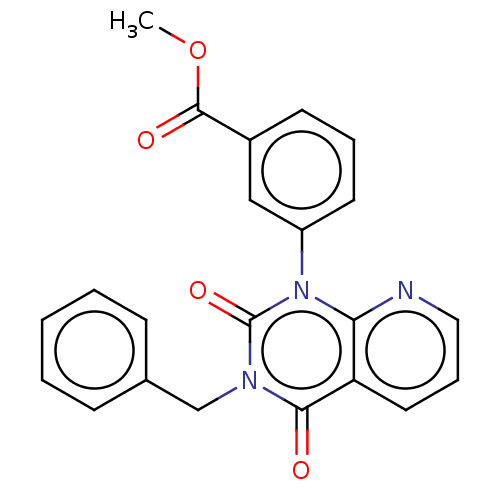
(CHEMBL156140)Show SMILES COC(=O)c1cccc(c1)-n1c2ncccc2c(=O)n(Cc2ccccc2)c1=O Show InChI InChI=1S/C22H17N3O4/c1-29-21(27)16-9-5-10-17(13-16)25-19-18(11-6-12-23-19)20(26)24(22(25)28)14-15-7-3-2-4-8-15/h2-13H,14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]rolipram binding to Phosphodiesterase 4 in rat brain |
J Med Chem 34: 624-8 (1991)
BindingDB Entry DOI: 10.7270/Q2WD42SG |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31596
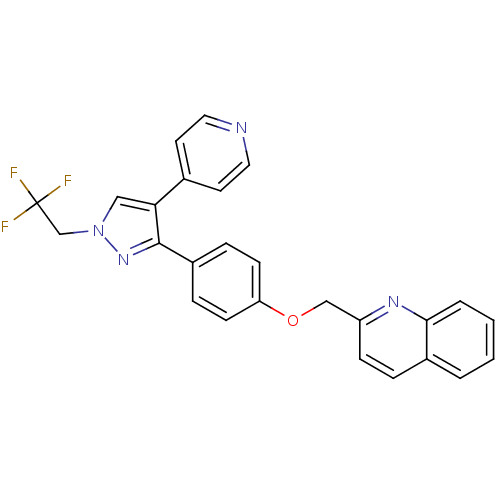
(substituted pyrazole, 13)Show SMILES FC(F)(F)Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C26H19F3N4O/c27-26(28,29)17-33-15-23(18-11-13-30-14-12-18)25(32-33)20-6-9-22(10-7-20)34-16-21-8-5-19-3-1-2-4-24(19)31-21/h1-15H,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31593
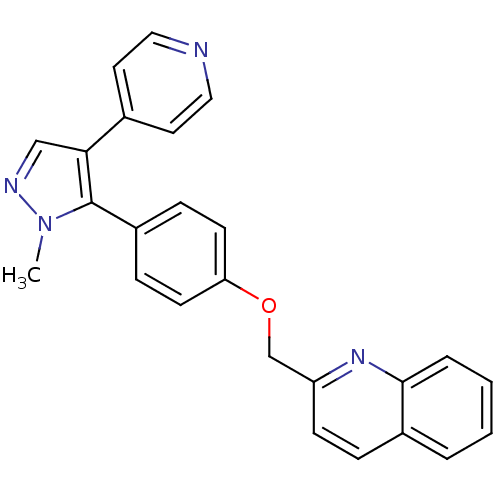
(substituted pyrazole, 10)Show SMILES Cn1ncc(c1-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C25H20N4O/c1-29-25(23(16-27-29)18-12-14-26-15-13-18)20-7-10-22(11-8-20)30-17-21-9-6-19-4-2-3-5-24(19)28-21/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.54 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50229041
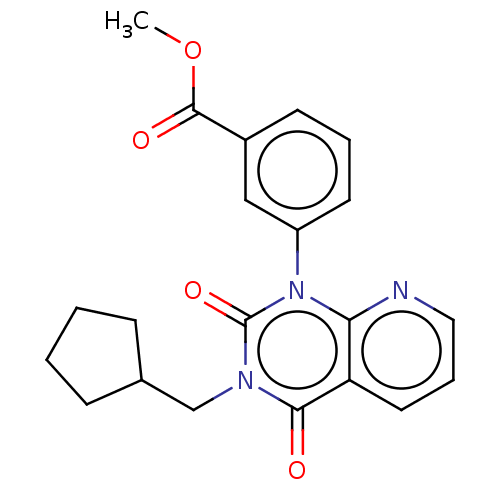
(CHEMBL351567)Show SMILES COC(=O)c1cccc(c1)-n1c2ncccc2c(=O)n(CC2CCCC2)c1=O Show InChI InChI=1S/C21H21N3O4/c1-28-20(26)15-8-4-9-16(12-15)24-18-17(10-5-11-22-18)19(25)23(21(24)27)13-14-6-2-3-7-14/h4-5,8-12,14H,2-3,6-7,13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]rolipram binding to Phosphodiesterase 4 in rat brain |
J Med Chem 34: 624-8 (1991)
BindingDB Entry DOI: 10.7270/Q2WD42SG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]rolipram binding to Phosphodiesterase 4 in rat brain |
J Med Chem 34: 624-8 (1991)
BindingDB Entry DOI: 10.7270/Q2WD42SG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- rolipram binding to phosphodiesterase 4 of rat cerebral cortex membranes. |
J Med Chem 34: 291-8 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9MG8 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50228824
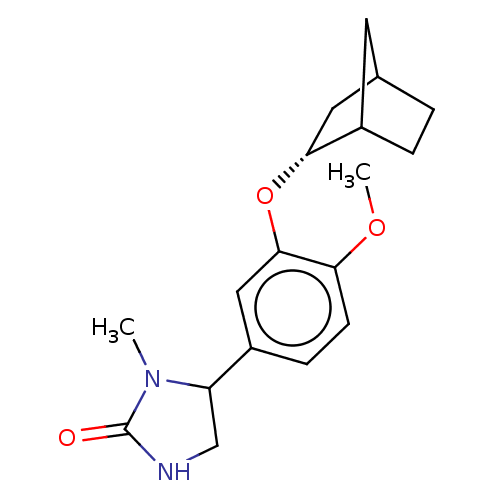
(CHEMBL2112408)Show SMILES COc1ccc(cc1O[C@@H]1CC2CCC1C2)C1CNC(=O)N1C |r,THB:8:9:15:13.12| Show InChI InChI=1S/C18H24N2O3/c1-20-14(10-19-18(20)21)12-5-6-15(22-2)17(9-12)23-16-8-11-3-4-13(16)7-11/h5-6,9,11,13-14,16H,3-4,7-8,10H2,1-2H3,(H,19,21)/t11?,13?,14?,16-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- rolipram binding to rat cerebral cortex membranes |
J Med Chem 34: 291-8 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9MG8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032165
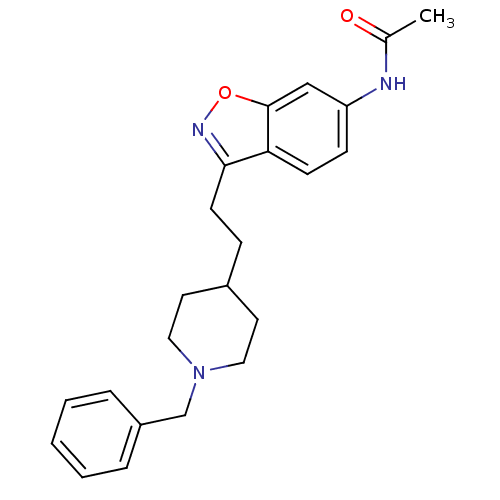
(CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...)Show SMILES CC(=O)Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1 Show InChI InChI=1S/C23H27N3O2/c1-17(27)24-20-8-9-21-22(25-28-23(21)15-20)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032165

(CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...)Show SMILES CC(=O)Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1 Show InChI InChI=1S/C23H27N3O2/c1-17(27)24-20-8-9-21-22(25-28-23(21)15-20)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50228833
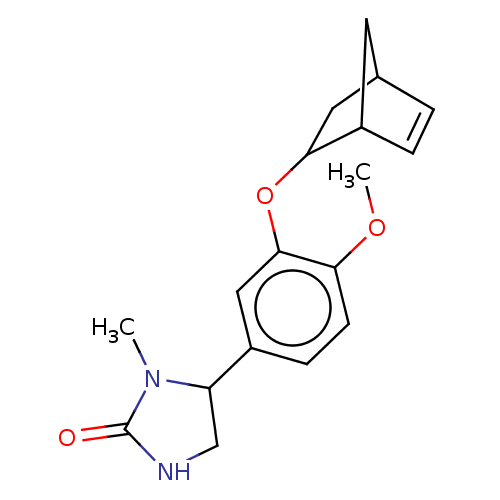
(CHEMBL318836)Show SMILES COc1ccc(cc1OC1CC2CC1C=C2)C1CNC(=O)N1C |c:16,TLB:8:9:14.15:12| Show InChI InChI=1S/C18H22N2O3/c1-20-14(10-19-18(20)21)12-5-6-15(22-2)17(9-12)23-16-8-11-3-4-13(16)7-11/h3-6,9,11,13-14,16H,7-8,10H2,1-2H3,(H,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- rolipram binding to rat cerebral cortex membranes |
J Med Chem 34: 291-8 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9MG8 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50229038
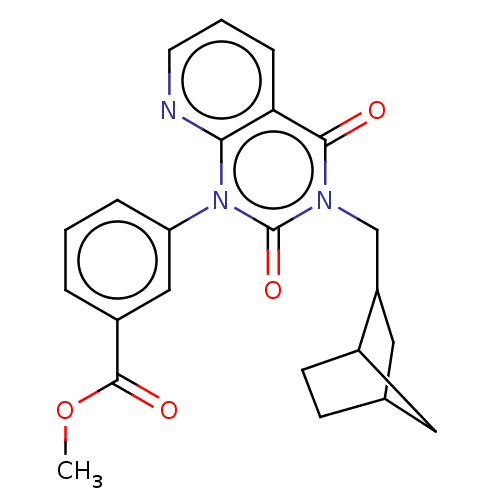
(CHEMBL345917)Show SMILES COC(=O)c1cccc(c1)-n1c2ncccc2c(=O)n(CC2CC3CCC2C3)c1=O |THB:20:21:27:25.24| Show InChI InChI=1S/C23H23N3O4/c1-30-22(28)16-4-2-5-18(12-16)26-20-19(6-3-9-24-20)21(27)25(23(26)29)13-17-11-14-7-8-15(17)10-14/h2-6,9,12,14-15,17H,7-8,10-11,13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]rolipram binding to Phosphodiesterase 4 in rat brain |
J Med Chem 34: 624-8 (1991)
BindingDB Entry DOI: 10.7270/Q2WD42SG |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31607

(methyl substituted pyrazole, 28)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2nc3ccccn3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C22H18N6O/c1-27-14-19(16-9-11-23-12-10-16)22(26-27)17-5-7-18(8-6-17)29-15-20-24-21-4-2-3-13-28(21)25-20/h2-14H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032160
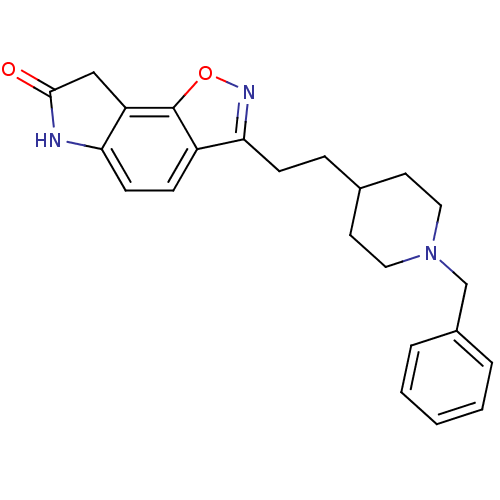
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6H-isoxazolo[5...)Show SMILES O=C1Cc2c(N1)ccc1c(CCC3CCN(Cc4ccccc4)CC3)noc21 Show InChI InChI=1S/C23H25N3O2/c27-22-14-19-20(24-22)9-7-18-21(25-28-23(18)19)8-6-16-10-12-26(13-11-16)15-17-4-2-1-3-5-17/h1-5,7,9,16H,6,8,10-15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Acetylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034001
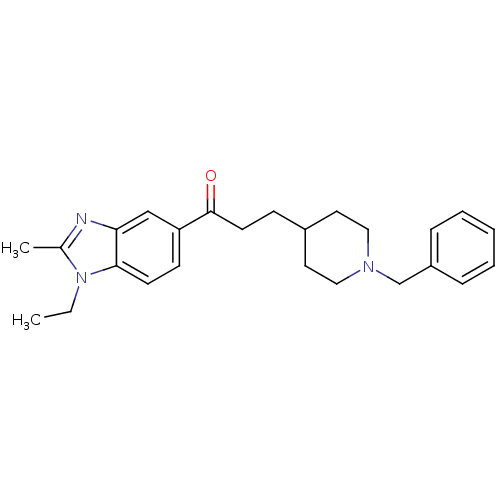
(3-(1-Benzyl-piperidin-4-yl)-1-(1-ethyl-2-methyl-1H...)Show SMILES CCn1c(C)nc2cc(ccc12)C(=O)CCC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H31N3O/c1-3-28-19(2)26-23-17-22(10-11-24(23)28)25(29)12-9-20-13-15-27(16-14-20)18-21-7-5-4-6-8-21/h4-8,10-11,17,20H,3,9,12-16,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31595

(substituted pyrazole, 12)Show SMILES CCn1ncc(c1-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C26H22N4O/c1-2-30-26(24(17-28-30)19-13-15-27-16-14-19)21-8-11-23(12-9-21)31-18-22-10-7-20-5-3-4-6-25(20)29-22/h3-17H,2,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.76 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50350800
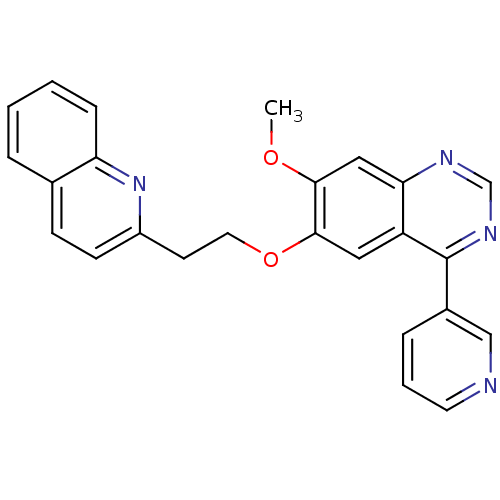
(CHEMBL1819135)Show SMILES COc1cc2ncnc(-c3cccnc3)c2cc1OCCc1ccc2ccccc2n1 Show InChI InChI=1S/C25H20N4O2/c1-30-23-14-22-20(25(28-16-27-22)18-6-4-11-26-15-18)13-24(23)31-12-10-19-9-8-17-5-2-3-7-21(17)29-19/h2-9,11,13-16H,10,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat PDE10A expressed in Sf9 cells using [3H]cAMP after 30 mins by scintillation proximity assay |
J Med Chem 54: 4536-47 (2011)
Article DOI: 10.1021/jm2001508
BindingDB Entry DOI: 10.7270/Q2PN961Q |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31604
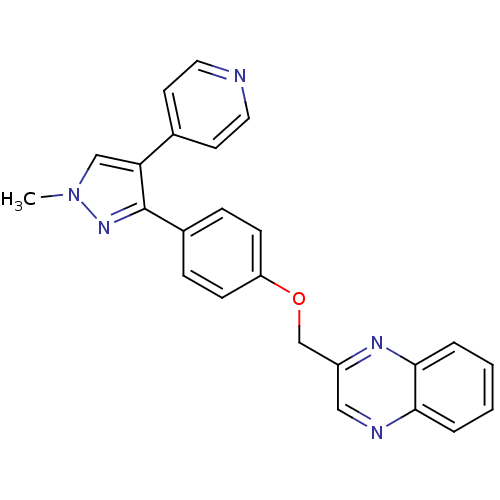
(methyl substituted pyrazole, 25)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2cnc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C24H19N5O/c1-29-15-21(17-10-12-25-13-11-17)24(28-29)18-6-8-20(9-7-18)30-16-19-14-26-22-4-2-3-5-23(22)27-19/h2-15H,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.09 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039729
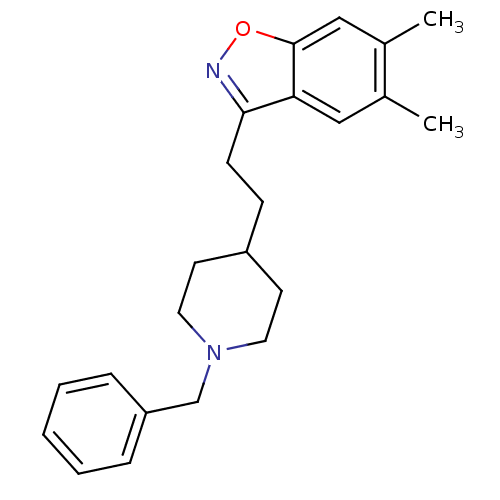
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dimethylbe...)Show InChI InChI=1S/C23H28N2O/c1-17-14-21-22(24-26-23(21)15-18(17)2)9-8-19-10-12-25(13-11-19)16-20-6-4-3-5-7-20/h3-7,14-15,19H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for the in vitro inhibition of the Butyrylcholinesterase from horse serum |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
In vitro inhibition of Butyrylcholinesterase from human erythrocytes |
J Med Chem 38: 2802-8 (1995)
BindingDB Entry DOI: 10.7270/Q2Q52NN0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Butyrylcholinesterase obtained from human serum was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034002

(3-(1-Benzyl-piperidin-4-yl)-1-(2-methyl-benzothiaz...)Show InChI InChI=1S/C23H26N2OS/c1-17-24-21-9-8-20(15-23(21)27-17)22(26)10-7-18-11-13-25(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039713
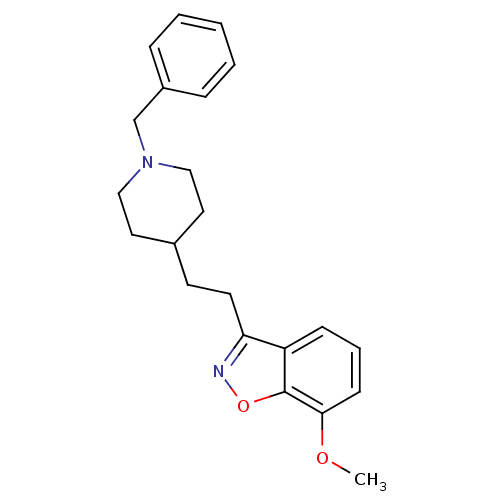
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-7-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-21-9-5-8-19-20(23-26-22(19)21)11-10-17-12-14-24(15-13-17)16-18-6-3-2-4-7-18/h2-9,17H,10-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039712
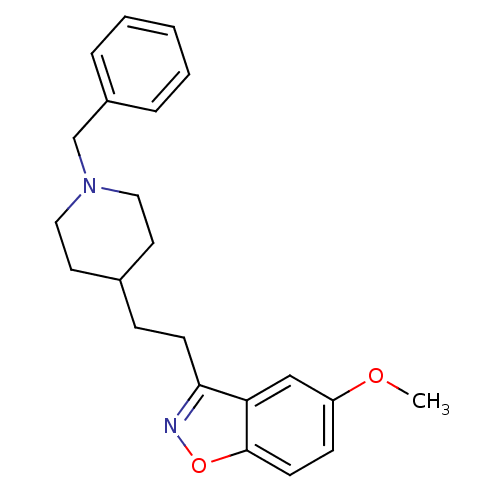
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-19-8-10-22-20(15-19)21(23-26-22)9-7-17-11-13-24(14-12-17)16-18-5-3-2-4-6-18/h2-6,8,10,15,17H,7,9,11-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50228829
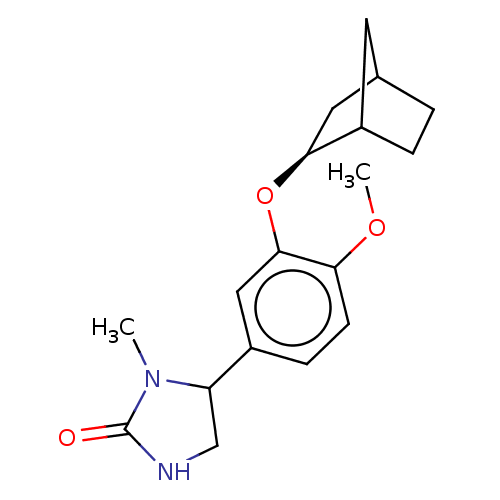
(CHEMBL2111611)Show SMILES COc1ccc(cc1O[C@H]1CC2CCC1C2)C1CNC(=O)N1C |r,THB:8:9:15:13.12| Show InChI InChI=1S/C18H24N2O3/c1-20-14(10-19-18(20)21)12-5-6-15(22-2)17(9-12)23-16-8-11-3-4-13(16)7-11/h5-6,9,11,13-14,16H,3-4,7-8,10H2,1-2H3,(H,19,21)/t11?,13?,14?,16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- rolipram binding to phosphodiesterase 4 of rat cerebral cortex membranes. |
J Med Chem 34: 291-8 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9MG8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039728
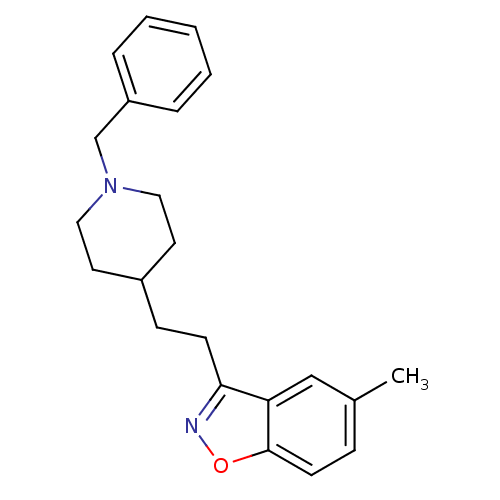
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methylbenzo[...)Show InChI InChI=1S/C22H26N2O/c1-17-7-10-22-20(15-17)21(23-25-22)9-8-18-11-13-24(14-12-18)16-19-5-3-2-4-6-19/h2-7,10,15,18H,8-9,11-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039717
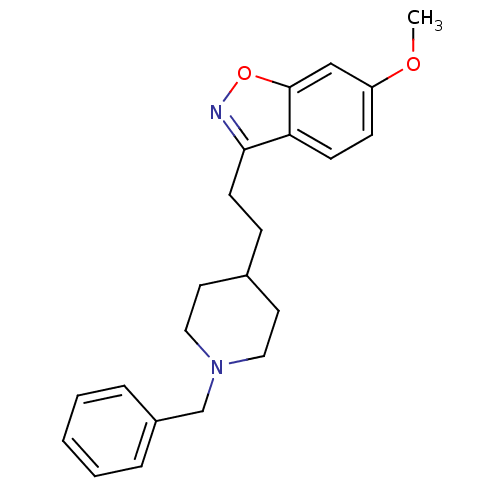
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-19-8-9-20-21(23-26-22(20)15-19)10-7-17-11-13-24(14-12-17)16-18-5-3-2-4-6-18/h2-6,8-9,15,17H,7,10-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM50350802
(CHEMBL1819121)Show InChI InChI=1S/C21H23N3O2/c1-25-19-11-17-18(12-20(19)26-2)22-14-23-21(17)24-10-6-9-16(13-24)15-7-4-3-5-8-15/h3-5,7-8,11-12,14,16H,6,9-10,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE3A |
J Med Chem 54: 4536-47 (2011)
Article DOI: 10.1021/jm2001508
BindingDB Entry DOI: 10.7270/Q2PN961Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039723
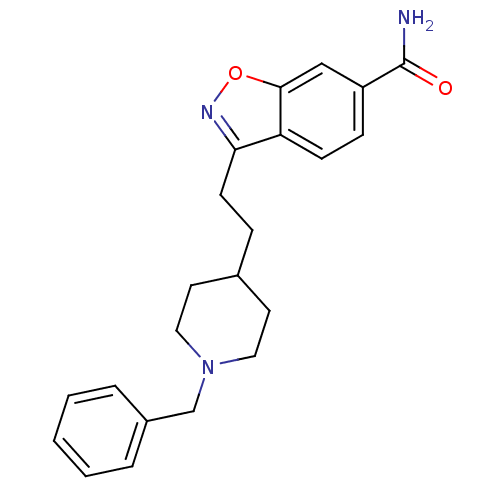
(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C22H25N3O2/c23-22(26)18-7-8-19-20(24-27-21(19)14-18)9-6-16-10-12-25(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16H,6,9-13,15H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039727
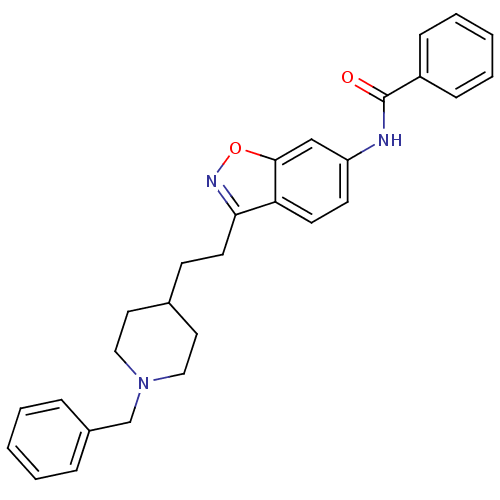
(CHEMBL93123 | N-(3-(2-(1-benzylpiperidin-4-yl)ethy...)Show SMILES O=C(Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1)c1ccccc1 Show InChI InChI=1S/C28H29N3O2/c32-28(23-9-5-2-6-10-23)29-24-12-13-25-26(30-33-27(25)19-24)14-11-21-15-17-31(18-16-21)20-22-7-3-1-4-8-22/h1-10,12-13,19,21H,11,14-18,20H2,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated for the in vitro inhibition of the Acetylcholinesterase (AChE) from human erythrocytes |
J Med Chem 37: 2721-34 (1994)
BindingDB Entry DOI: 10.7270/Q2VT1R4X |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50350797
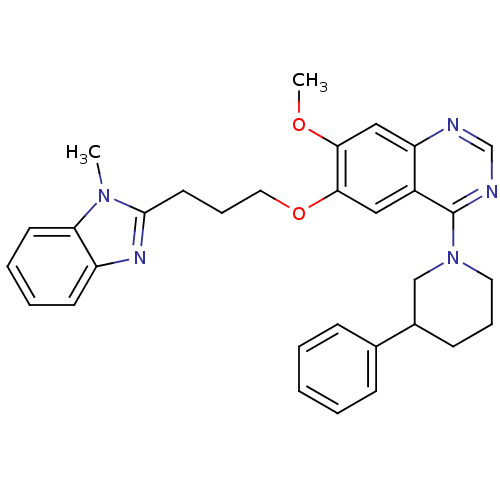
(CHEMBL1819132)Show SMILES COc1cc2ncnc(N3CCCC(C3)c3ccccc3)c2cc1OCCCc1nc2ccccc2n1C Show InChI InChI=1S/C31H33N5O2/c1-35-27-14-7-6-13-25(27)34-30(35)15-9-17-38-29-18-24-26(19-28(29)37-2)32-21-33-31(24)36-16-8-12-23(20-36)22-10-4-3-5-11-22/h3-7,10-11,13-14,18-19,21,23H,8-9,12,15-17,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat PDE10A expressed in Sf9 cells using [3H]cAMP after 30 mins by scintillation proximity assay |
J Med Chem 54: 4536-47 (2011)
Article DOI: 10.1021/jm2001508
BindingDB Entry DOI: 10.7270/Q2PN961Q |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50228825
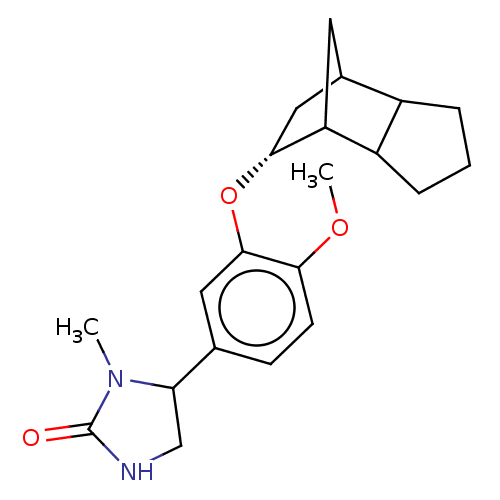
(CHEMBL2112409)Show SMILES COc1ccc(cc1O[C@@H]1CC2CC1C1CCCC21)C1CNC(=O)N1C |r,TLB:15:14:12:9.10,8:9:12:14.18,THB:17:18:12:9.10| Show InChI InChI=1S/C21H28N2O3/c1-23-17(11-22-21(23)24)12-6-7-18(25-2)20(9-12)26-19-10-13-8-16(19)15-5-3-4-14(13)15/h6-7,9,13-17,19H,3-5,8,10-11H2,1-2H3,(H,22,24)/t13?,14?,15?,16?,17?,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- rolipram binding to rat cerebral cortex membranes |
J Med Chem 34: 291-8 (1991)
BindingDB Entry DOI: 10.7270/Q2NV9MG8 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31590

(pyrazole, 2)Show SMILES C(Oc1ccc(cc1)-c1n[nH]cc1Cc1ccncc1)c1ccc2ccccc2n1 Show InChI InChI=1S/C25H20N4O/c1-2-4-24-19(3-1)5-8-22(28-24)17-30-23-9-6-20(7-10-23)25-21(16-27-29-25)15-18-11-13-26-14-12-18/h1-14,16H,15,17H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31597
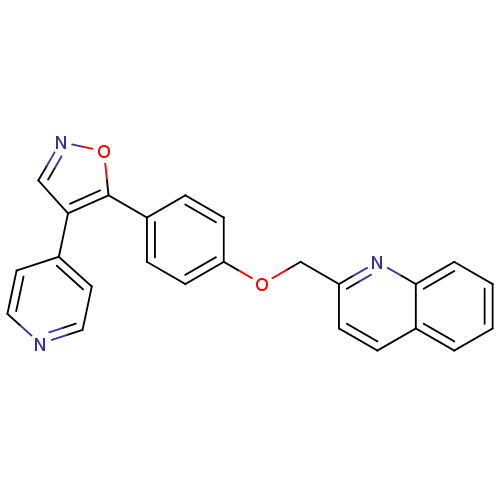
(isoxazole, 14)Show SMILES C(Oc1ccc(cc1)-c1oncc1-c1ccncc1)c1ccc2ccccc2n1 Show InChI InChI=1S/C24H17N3O2/c1-2-4-23-18(3-1)5-8-20(27-23)16-28-21-9-6-19(7-10-21)24-22(15-26-29-24)17-11-13-25-14-12-17/h1-15H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11.7 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM31600
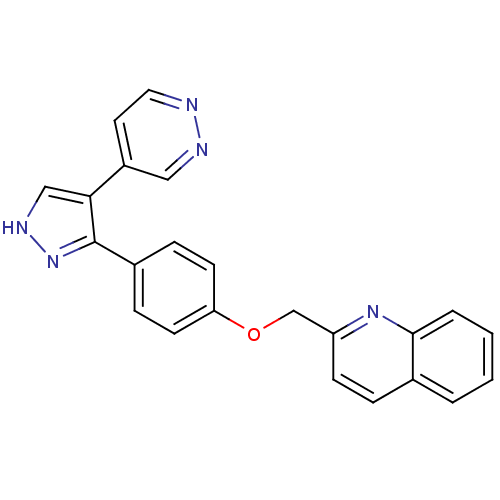
(pyrazole, 17)Show SMILES C(Oc1ccc(cc1)-c1n[nH]cc1-c1ccnnc1)c1ccc2ccccc2n1 Show InChI InChI=1S/C23H17N5O/c1-2-4-22-16(3-1)5-8-19(27-22)15-29-20-9-6-17(7-10-20)23-21(14-26-28-23)18-11-12-24-25-13-18/h1-14H,15H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.9 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Pfizer
| Assay Description
PDE activity was measured using a plate-based Scintillation Proximity Assay (SPA). For competitive enzyme inhibition assays, the substrate [3H]cAMP c... |
J Med Chem 52: 5188-96 (2009)
Article DOI: 10.1021/jm900521k
BindingDB Entry DOI: 10.7270/Q290223B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50350803
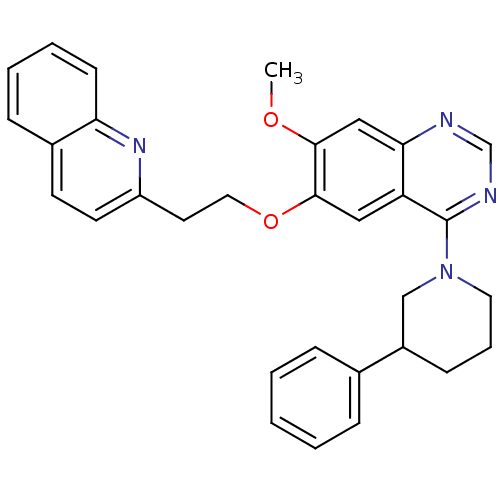
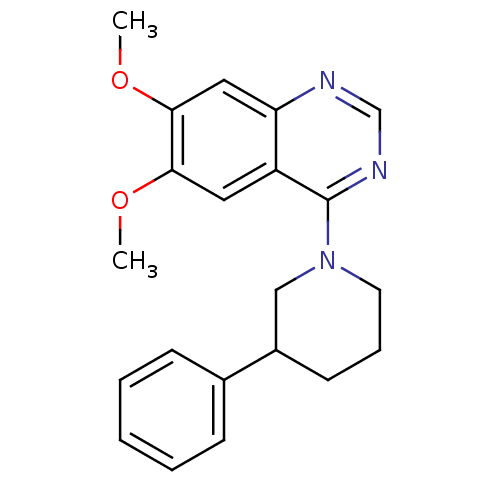
(CHEMBL1819131)Show SMILES COc1cc2ncnc(N3CCCC(C3)c3ccccc3)c2cc1OCCc1ccc2ccccc2n1 Show InChI InChI=1S/C31H30N4O2/c1-36-29-19-28-26(18-30(29)37-17-15-25-14-13-23-10-5-6-12-27(23)34-25)31(33-21-32-28)35-16-7-11-24(20-35)22-8-3-2-4-9-22/h2-6,8-10,12-14,18-19,21,24H,7,11,15-17,20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat PDE10A expressed in Sf9 cells using [3H]cAMP after 30 mins by scintillation proximity assay |
J Med Chem 54: 4536-47 (2011)
Article DOI: 10.1021/jm2001508
BindingDB Entry DOI: 10.7270/Q2PN961Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034003
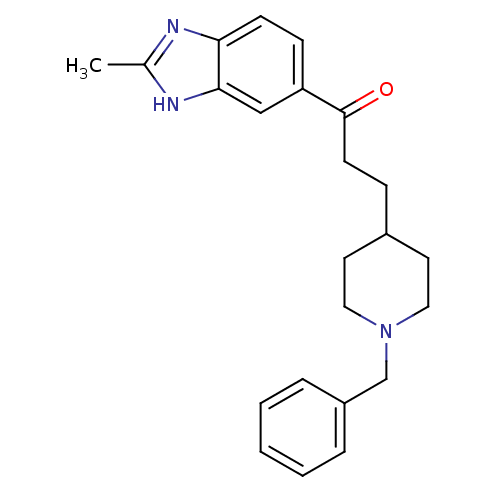
(3-(1-Benzyl-piperidin-4-yl)-1-(2-methyl-1H-benzoim...)Show SMILES Cc1nc2ccc(cc2[nH]1)C(=O)CCC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C23H27N3O/c1-17-24-21-9-8-20(15-22(21)25-17)23(27)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of Acetylcholinesterase obtained from human erythrocytes was determined in vitro |
J Med Chem 38: 1084-9 (1995)
BindingDB Entry DOI: 10.7270/Q21N81S7 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM50350801

(CHEMBL1819136)Show InChI InChI=1S/C21H19N3O3/c1-24-13-22-18-12-19(26-2)20(11-16(18)21(24)25)27-10-9-15-8-7-14-5-3-4-6-17(14)23-15/h3-8,11-13H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat PDE10A expressed in Sf9 cells using [3H]cAMP after 30 mins by scintillation proximity assay |
J Med Chem 54: 4536-47 (2011)
Article DOI: 10.1021/jm2001508
BindingDB Entry DOI: 10.7270/Q2PN961Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data