

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM724 ((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM723 (CHEMBL277908 | Pyrrolinone inhibitor 4 | tert-buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description The inhibition of HIV-1 protease activities were measured with peptide hydrolysis assays. The appearance of products and the corresponding loss of su... | Bioorg Med Chem Lett 16: 859-63 (2006) Article DOI: 10.1016/j.bmcl.2005.11.011 BindingDB Entry DOI: 10.7270/Q29K48FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8530 ((3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-4-[(3aR,8aS)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description The inhibition of HIV-1 protease activities were measured with peptide hydrolysis assays. The appearance of products and the corresponding loss of su... | Bioorg Med Chem Lett 16: 859-63 (2006) Article DOI: 10.1016/j.bmcl.2005.11.011 BindingDB Entry DOI: 10.7270/Q29K48FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM751 (CHEMBL289195 | Hydroxyethylene dipeptide isostere ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM721 ((3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-2-benzyl-4-[(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50467984 (CHEMBL4293287) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... | ACS Med Chem Lett 9: 1039-1044 (2018) Article DOI: 10.1021/acsmedchemlett.8b00344 BindingDB Entry DOI: 10.7270/Q25D8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50467989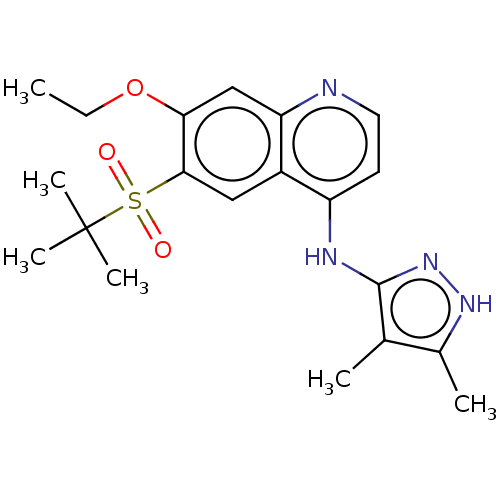 (CHEMBL4282034) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... | ACS Med Chem Lett 9: 1039-1044 (2018) Article DOI: 10.1021/acsmedchemlett.8b00344 BindingDB Entry DOI: 10.7270/Q25D8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM727 ((3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-2-benzyl-4-[(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM726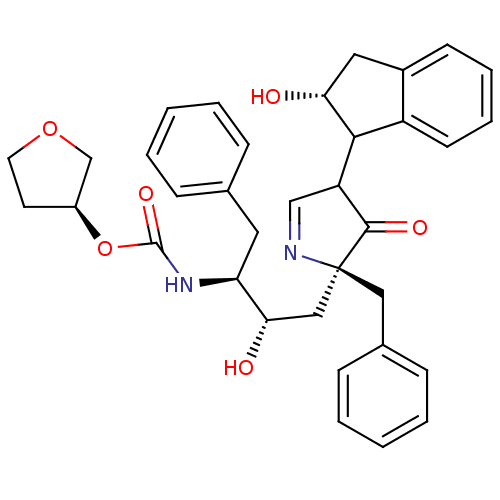 ((3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-2-benzyl-4-[(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Rattus norvegicus) | BDBM50516677 (CHEMBL4514780) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of fluorescent-labelled ligand binding to rat RIP2K preincubated for 10 mins followed by fluorescent-labelled ligand addition and measured... | J Med Chem 62: 6482-6494 (2019) Article DOI: 10.1021/acs.jmedchem.9b00575 BindingDB Entry DOI: 10.7270/Q2WS8XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50516677 (CHEMBL4514780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of recombinant human full-length FLAG/His-tagged RIP2 measured after 2 hrs by ADP-Glo luminescence assay | J Med Chem 62: 6482-6494 (2019) Article DOI: 10.1021/acs.jmedchem.9b00575 BindingDB Entry DOI: 10.7270/Q2WS8XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Rattus norvegicus) | BDBM50184765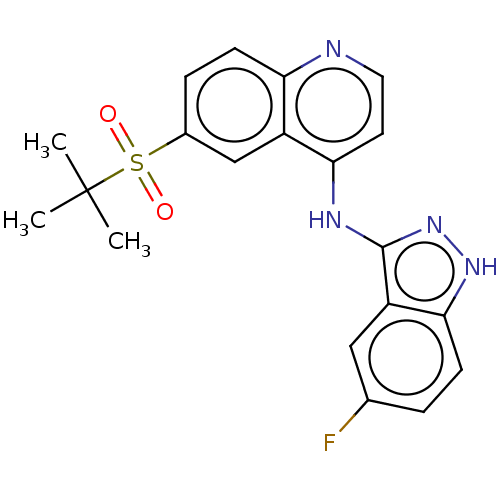 (CHEMBL3823499) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of rat RIPK2 preincubated for 10 mins followed by addition of fluorescent-labeled 2-Methyl-5-(2-propen-l-yloxy)aniline measured after 10 m... | J Med Chem 59: 4867-80 (2016) Article DOI: 10.1021/acs.jmedchem.6b00211 BindingDB Entry DOI: 10.7270/Q2G162SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM727 ((3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-2-benzyl-4-[(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description The inhibition of HIV-1 protease activities were measured with peptide hydrolysis assays. The appearance of products and the corresponding loss of su... | Bioorg Med Chem Lett 16: 859-63 (2006) Article DOI: 10.1016/j.bmcl.2005.11.011 BindingDB Entry DOI: 10.7270/Q29K48FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM726 ((3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-2-benzyl-4-[(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description The inhibition of HIV-1 protease activities were measured with peptide hydrolysis assays. The appearance of products and the corresponding loss of su... | Bioorg Med Chem Lett 16: 859-63 (2006) Article DOI: 10.1016/j.bmcl.2005.11.011 BindingDB Entry DOI: 10.7270/Q29K48FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8529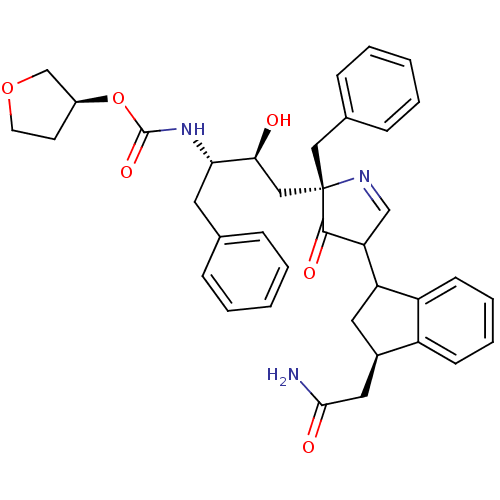 ((3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-2-benzyl-4-[(3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description The inhibition of HIV-1 protease activities were measured with peptide hydrolysis assays. The appearance of products and the corresponding loss of su... | Bioorg Med Chem Lett 16: 859-63 (2006) Article DOI: 10.1016/j.bmcl.2005.11.011 BindingDB Entry DOI: 10.7270/Q29K48FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM8533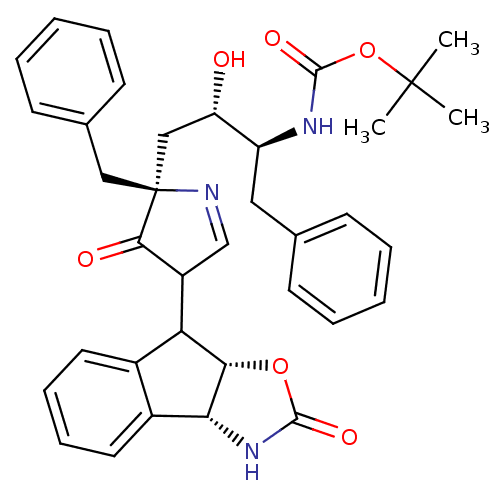 (monopyrrolinone-based inhibitor (-)-20 | monopyrro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description The inhibition of HIV-1 protease activities were measured with peptide hydrolysis assays. The appearance of products and the corresponding loss of su... | Bioorg Med Chem Lett 16: 859-63 (2006) Article DOI: 10.1016/j.bmcl.2005.11.011 BindingDB Entry DOI: 10.7270/Q29K48FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50467980 (CHEMBL4292228) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... | ACS Med Chem Lett 9: 1039-1044 (2018) Article DOI: 10.1021/acsmedchemlett.8b00344 BindingDB Entry DOI: 10.7270/Q25D8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50467979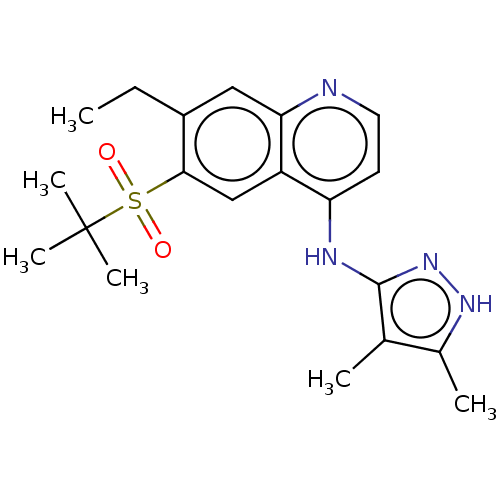 (CHEMBL4285837) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... | ACS Med Chem Lett 9: 1039-1044 (2018) Article DOI: 10.1021/acsmedchemlett.8b00344 BindingDB Entry DOI: 10.7270/Q25D8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50467986 (CHEMBL4289904) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... | ACS Med Chem Lett 9: 1039-1044 (2018) Article DOI: 10.1021/acsmedchemlett.8b00344 BindingDB Entry DOI: 10.7270/Q25D8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM25198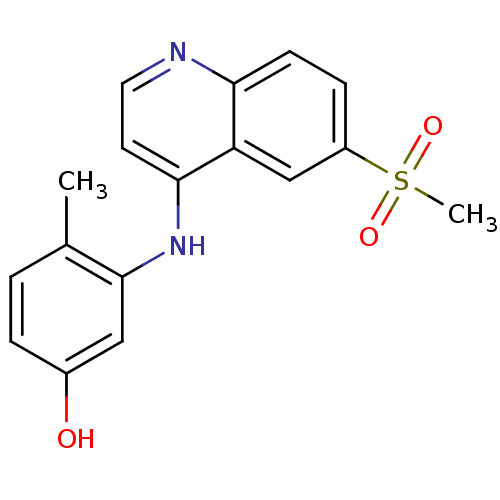 (3-[(6-methanesulfonylquinolin-4-yl)amino]-4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of full length FLAG-His-tagged RIP2K (unknown origin) expressed in baculovirus expression system preincubated for 10 mins foll... | J Med Chem 59: 4867-80 (2016) Article DOI: 10.1021/acs.jmedchem.6b00211 BindingDB Entry DOI: 10.7270/Q2G162SQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM25198 (3-[(6-methanesulfonylquinolin-4-yl)amino]-4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of full length FLAG-His-tagged RIP2K (unknown origin) expressed in baculovirus expression system preincubated for 10 mins foll... | J Med Chem 59: 4867-80 (2016) Article DOI: 10.1021/acs.jmedchem.6b00211 BindingDB Entry DOI: 10.7270/Q2G162SQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nucleotide-binding oligomerization domain-containing protein 2 (Homo sapiens (Human)) | BDBM50516677 (CHEMBL4514780) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human NOD2 expressed in HEK293 cells assessed as reduction in MDP-induced IL8 production measured after 22 hrs by HTRF fluorescence ass... | J Med Chem 62: 6482-6494 (2019) Article DOI: 10.1021/acs.jmedchem.9b00575 BindingDB Entry DOI: 10.7270/Q2WS8XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50516676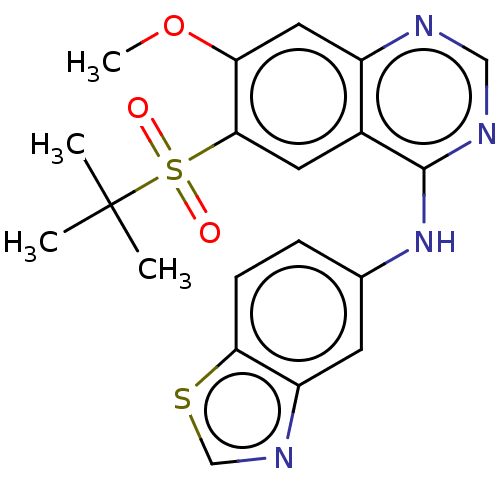 (CHEMBL4473105) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of fluorescent-labelled ligand binding to human RIP2K preincubated for 10 mins followed by fluorescent-labelled ligand addition and measur... | J Med Chem 62: 6482-6494 (2019) Article DOI: 10.1021/acs.jmedchem.9b00575 BindingDB Entry DOI: 10.7270/Q2WS8XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50184765 (CHEMBL3823499) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... | ACS Med Chem Lett 9: 1039-1044 (2018) Article DOI: 10.1021/acsmedchemlett.8b00344 BindingDB Entry DOI: 10.7270/Q25D8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50467980 (CHEMBL4292228) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIPK2 in human whole blood assessed as reduction in MDP-stimulated TNFalpha levels incubated for 30 mins followed by MDP addition measu... | ACS Med Chem Lett 9: 1039-1044 (2018) Article DOI: 10.1021/acsmedchemlett.8b00344 BindingDB Entry DOI: 10.7270/Q25D8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50516677 (CHEMBL4514780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of fluorescent-labelled ligand binding to human RIP2K preincubated for 10 mins followed by fluorescent-labelled ligand addition and measur... | J Med Chem 62: 6482-6494 (2019) Article DOI: 10.1021/acs.jmedchem.9b00575 BindingDB Entry DOI: 10.7270/Q2WS8XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50184765 (CHEMBL3823499) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of full length FLAG-His-tagged RIP2K (unknown origin) expressed in baculovirus expression system preincubated for 10 mins foll... | J Med Chem 59: 4867-80 (2016) Article DOI: 10.1021/acs.jmedchem.6b00211 BindingDB Entry DOI: 10.7270/Q2G162SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM728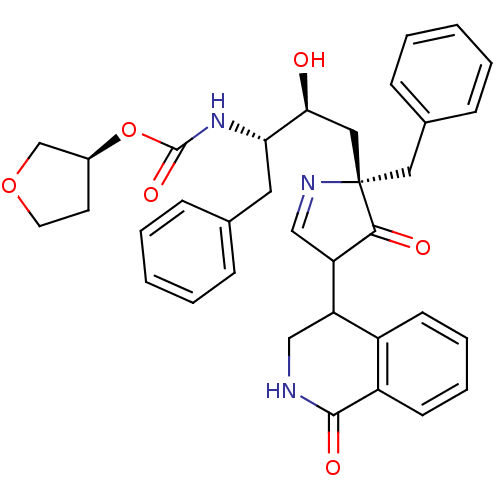 ((3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-2-benzyl-3-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM728 ((3S)-oxolan-3-yl N-[(2S,3S)-4-[(2S)-2-benzyl-3-oxo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description The inhibition of HIV-1 protease activities were measured with peptide hydrolysis assays. The appearance of products and the corresponding loss of su... | Bioorg Med Chem Lett 16: 859-63 (2006) Article DOI: 10.1016/j.bmcl.2005.11.011 BindingDB Entry DOI: 10.7270/Q29K48FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50467988 (CHEMBL4285462) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... | ACS Med Chem Lett 9: 1039-1044 (2018) Article DOI: 10.1021/acsmedchemlett.8b00344 BindingDB Entry DOI: 10.7270/Q25D8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50184479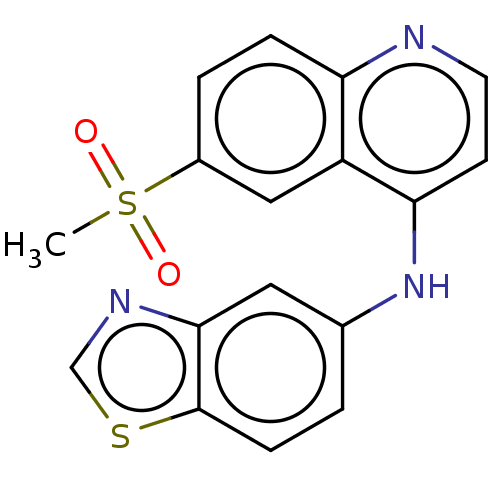 (CHEMBL3822791) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of full length FLAG-His-tagged RIP2K (unknown origin) expressed in baculovirus expression system preincubated for 10 mins foll... | J Med Chem 59: 4867-80 (2016) Article DOI: 10.1021/acs.jmedchem.6b00211 BindingDB Entry DOI: 10.7270/Q2G162SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50128684 (CHEMBL3628627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length FLAG-6His-TEV tagged human RIP2 expressed in Sf9 cells assessed as reduction in autophosphorylation pre-incubated for 30 mi... | Bioorg Med Chem 23: 7000-6 (2015) Article DOI: 10.1016/j.bmc.2015.09.038 BindingDB Entry DOI: 10.7270/Q26D5VTM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50467982 (CHEMBL4281602) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full length His/flag-tagged RIPK2 (unknown origin) expressed in baculovirus expression system using fluorescently labeled substrate inc... | ACS Med Chem Lett 9: 1039-1044 (2018) Article DOI: 10.1021/acsmedchemlett.8b00344 BindingDB Entry DOI: 10.7270/Q25D8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50128684 (CHEMBL3628627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length FLAG-6His-TEV tagged human RIP2 expressed in Sf9 cells by fluorescent polarization assay | Bioorg Med Chem 23: 7000-6 (2015) Article DOI: 10.1016/j.bmc.2015.09.038 BindingDB Entry DOI: 10.7270/Q26D5VTM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50184727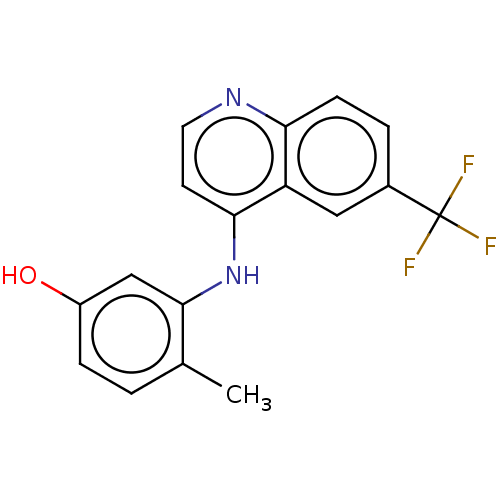 (CHEMBL3822938) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of full length FLAG-His-tagged RIP2K (unknown origin) expressed in baculovirus expression system preincubated for 10 mins foll... | J Med Chem 59: 4867-80 (2016) Article DOI: 10.1021/acs.jmedchem.6b00211 BindingDB Entry DOI: 10.7270/Q2G162SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50045333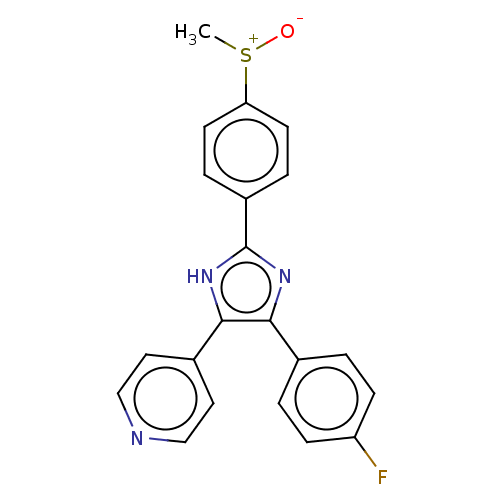 (CHEBI:90705 | SB-203580) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length FLAG-6His-TEV tagged human RIP2 expressed in Sf9 cells assessed as reduction in autophosphorylation pre-incubated for 30 mi... | Bioorg Med Chem 23: 7000-6 (2015) Article DOI: 10.1016/j.bmc.2015.09.038 BindingDB Entry DOI: 10.7270/Q26D5VTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50184764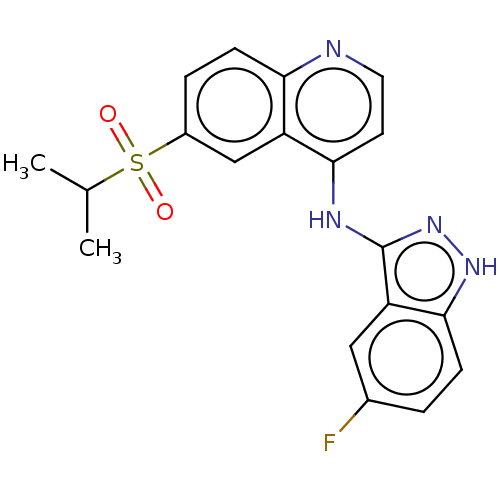 (CHEMBL3822577) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of full length FLAG-His-tagged RIP2K (unknown origin) expressed in baculovirus expression system preincubated for 10 mins foll... | J Med Chem 59: 4867-80 (2016) Article DOI: 10.1021/acs.jmedchem.6b00211 BindingDB Entry DOI: 10.7270/Q2G162SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM720 (Pyrrolinone deriv. 1 | Pyrrolinone inhibitor 1 | t...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM25195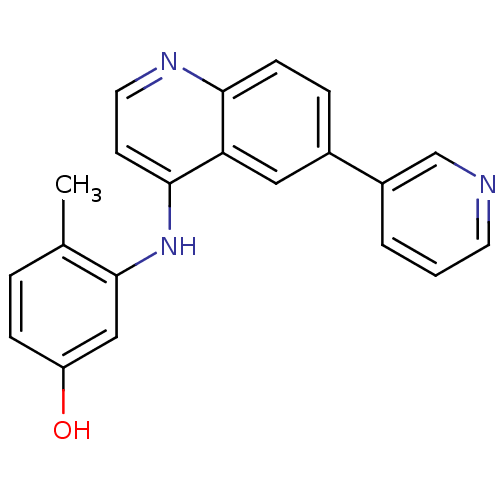 (4-methyl-3-{[6-(pyridin-3-yl)quinolin-4-yl]amino}p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of full length FLAG-His-tagged RIP2K (unknown origin) expressed in baculovirus expression system preincubated for 10 mins foll... | J Med Chem 59: 4867-80 (2016) Article DOI: 10.1021/acs.jmedchem.6b00211 BindingDB Entry DOI: 10.7270/Q2G162SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50467984 (CHEMBL4293287) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIPK2 in human whole blood assessed as reduction in MDP-stimulated TNFalpha levels incubated for 30 mins followed by MDP addition measu... | ACS Med Chem Lett 9: 1039-1044 (2018) Article DOI: 10.1021/acsmedchemlett.8b00344 BindingDB Entry DOI: 10.7270/Q25D8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM725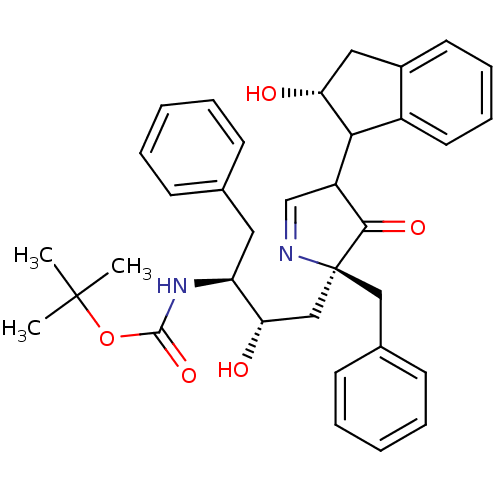 (Boc-Monopyrrolinone Inhibitor | Pyrrolinone deriv....) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.9 | n/a | n/a | n/a | n/a | 5.5 | 30 |
University of Pennsylvania | Assay Description All enzyme assays were performed under initial velocity and steady-state conditions. The conditions for the enzyme catalyzed hydrolysis of the cleava... | J Med Chem 46: 1831-44 (2003) Article DOI: 10.1021/jm0204587 BindingDB Entry DOI: 10.7270/Q2GQ6VZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50128687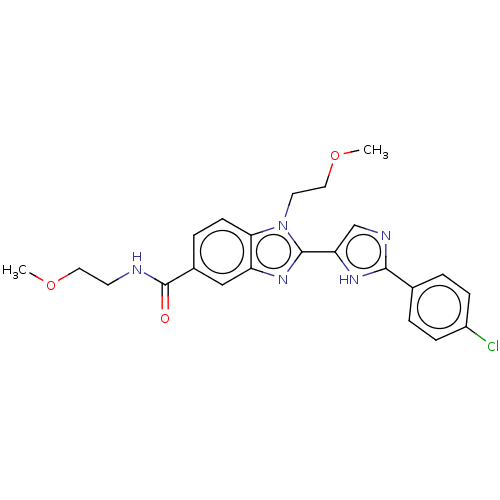 (CHEMBL3628628) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of full-length FLAG-6His-TEV tagged human RIP2 expressed in Sf9 cells assessed as reduction in autophosphorylation pre-incubated for 30 mi... | Bioorg Med Chem 23: 7000-6 (2015) Article DOI: 10.1016/j.bmc.2015.09.038 BindingDB Entry DOI: 10.7270/Q26D5VTM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nucleotide-binding oligomerization domain-containing protein 2 (Homo sapiens (Human)) | BDBM50516677 (CHEMBL4514780) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of NOD2 in human monocytes assessed as reduction in MDP-induced TNFalpha production preincubated for 30 mins followed by MDP-stimulation a... | J Med Chem 62: 6482-6494 (2019) Article DOI: 10.1021/acs.jmedchem.9b00575 BindingDB Entry DOI: 10.7270/Q2WS8XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Rattus norvegicus) | BDBM50467984 (CHEMBL4293287) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIPK2 in rat whole blood assessed as reduction in MDP-stimulated TNFalpha levels incubated for 30 mins followed by MDP addition measure... | ACS Med Chem Lett 9: 1039-1044 (2018) Article DOI: 10.1021/acsmedchemlett.8b00344 BindingDB Entry DOI: 10.7270/Q25D8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50467986 (CHEMBL4289904) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIPK2 in human whole blood assessed as reduction in MDP-stimulated TNFalpha levels incubated for 30 mins followed by MDP addition measu... | ACS Med Chem Lett 9: 1039-1044 (2018) Article DOI: 10.1021/acsmedchemlett.8b00344 BindingDB Entry DOI: 10.7270/Q25D8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 3 (Homo sapiens (Human)) | BDBM50184765 (CHEMBL3823499) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIPK3 (unknown origin) preincubated for 10 mins followed by addition of fluorescent-labeled 2-Methyl-5-(2-propen-l-yloxy)aniline measur... | J Med Chem 59: 4867-80 (2016) Article DOI: 10.1021/acs.jmedchem.6b00211 BindingDB Entry DOI: 10.7270/Q2G162SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50184765 (CHEMBL3823499) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP2K in MDP-stimulated HEK-293 cells over-expressing NOD2 assessed as IL8 secretion | J Med Chem 59: 4867-80 (2016) Article DOI: 10.1021/acs.jmedchem.6b00211 BindingDB Entry DOI: 10.7270/Q2G162SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM25198 (3-[(6-methanesulfonylquinolin-4-yl)amino]-4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP2K in MDP-stimulated HEK-293 cells over-expressing NOD2 assessed as IL8 secretion | J Med Chem 59: 4867-80 (2016) Article DOI: 10.1021/acs.jmedchem.6b00211 BindingDB Entry DOI: 10.7270/Q2G162SQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 204 total ) | Next | Last >> |