 Found 1045 hits with Last Name = 'chen' and Initial = 'll'
Found 1045 hits with Last Name = 'chen' and Initial = 'll' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308541
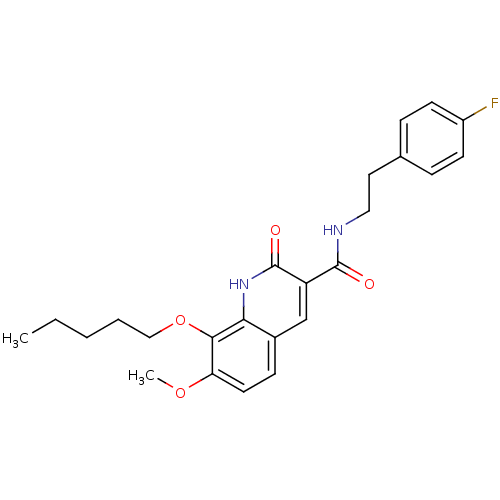
(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c12 Show InChI InChI=1S/C24H27FN2O4/c1-3-4-5-14-31-22-20(30-2)11-8-17-15-19(24(29)27-21(17)22)23(28)26-13-12-16-6-9-18(25)10-7-16/h6-11,15H,3-5,12-14H2,1-2H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308541

(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c12 Show InChI InChI=1S/C24H27FN2O4/c1-3-4-5-14-31-22-20(30-2)11-8-17-15-19(24(29)27-21(17)22)23(28)26-13-12-16-6-9-18(25)10-7-16/h6-11,15H,3-5,12-14H2,1-2H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353091

(CHEMBL1822944)Show SMILES CCOc1c(OC)ccc2cc(C(=O)NCCc3ccncc3)c(=O)[nH]c12 Show InChI InChI=1S/C20H21N3O4/c1-3-27-18-16(26-2)5-4-14-12-15(20(25)23-17(14)18)19(24)22-11-8-13-6-9-21-10-7-13/h4-7,9-10,12H,3,8,11H2,1-2H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353091

(CHEMBL1822944)Show SMILES CCOc1c(OC)ccc2cc(C(=O)NCCc3ccncc3)c(=O)[nH]c12 Show InChI InChI=1S/C20H21N3O4/c1-3-27-18-16(26-2)5-4-14-12-15(20(25)23-17(14)18)19(24)22-11-8-13-6-9-21-10-7-13/h4-7,9-10,12H,3,8,11H2,1-2H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073061
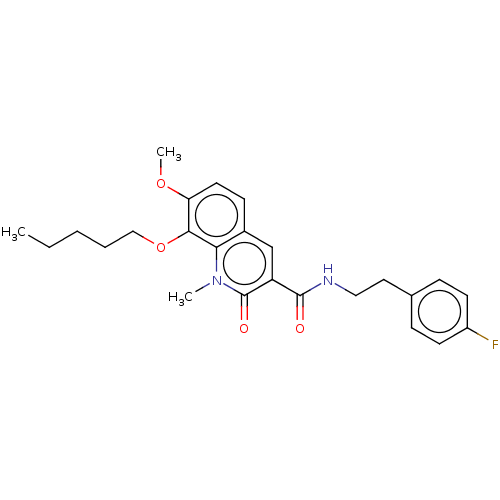
(CHEMBL3410832)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)n(C)c12 Show InChI InChI=1S/C25H29FN2O4/c1-4-5-6-15-32-23-21(31-3)12-9-18-16-20(25(30)28(2)22(18)23)24(29)27-14-13-17-7-10-19(26)11-8-17/h7-12,16H,4-6,13-15H2,1-3H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073061

(CHEMBL3410832)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)n(C)c12 Show InChI InChI=1S/C25H29FN2O4/c1-4-5-6-15-32-23-21(31-3)12-9-18-16-20(25(30)28(2)22(18)23)24(29)27-14-13-17-7-10-19(26)11-8-17/h7-12,16H,4-6,13-15H2,1-3H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073068

(CHEMBL3410813)Show SMILES CCCCCOc1ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c2c1OCCCCC Show InChI InChI=1S/C28H35FN2O4/c1-3-5-7-17-34-24-14-11-21-19-23(27(32)30-16-15-20-9-12-22(29)13-10-20)28(33)31-25(21)26(24)35-18-8-6-4-2/h9-14,19H,3-8,15-18H2,1-2H3,(H,30,32)(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073068

(CHEMBL3410813)Show SMILES CCCCCOc1ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c2c1OCCCCC Show InChI InChI=1S/C28H35FN2O4/c1-3-5-7-17-34-24-14-11-21-19-23(27(32)30-16-15-20-9-12-22(29)13-10-20)28(33)31-25(21)26(24)35-18-8-6-4-2/h9-14,19H,3-8,15-18H2,1-2H3,(H,30,32)(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205313
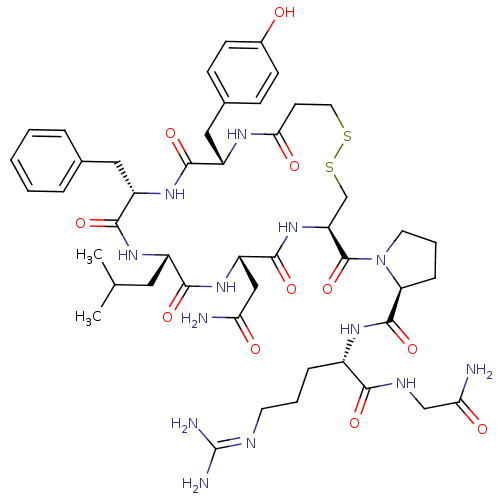
(CHEMBL265859 | d[Leu4]AVP)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C47H67N13O11S2/c1-26(2)20-31-41(66)58-34(23-37(48)62)44(69)59-35(46(71)60-18-7-11-36(60)45(70)55-30(10-6-17-52-47(50)51)40(65)53-24-38(49)63)25-73-72-19-16-39(64)54-32(22-28-12-14-29(61)15-13-28)42(67)57-33(43(68)56-31)21-27-8-4-3-5-9-27/h3-5,8-9,12-15,26,30-36,61H,6-7,10-11,16-25H2,1-2H3,(H2,48,62)(H2,49,63)(H,53,65)(H,54,64)(H,55,70)(H,56,68)(H,57,67)(H,58,66)(H,59,69)(H4,50,51,52)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072968
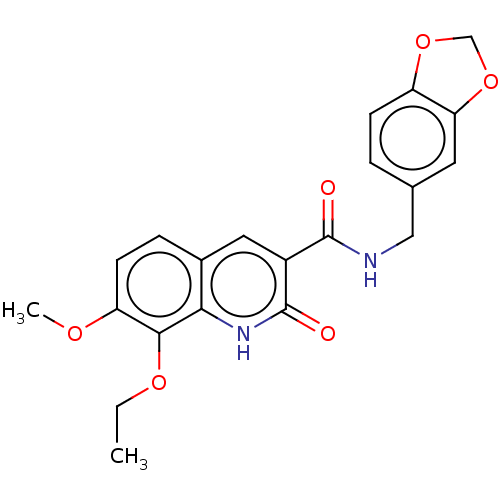
(CHEMBL3410818)Show SMILES CCOc1c(OC)ccc2cc(C(=O)NCc3ccc4OCOc4c3)c(=O)[nH]c12 Show InChI InChI=1S/C21H20N2O6/c1-3-27-19-16(26-2)7-5-13-9-14(21(25)23-18(13)19)20(24)22-10-12-4-6-15-17(8-12)29-11-28-15/h4-9H,3,10-11H2,1-2H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072968

(CHEMBL3410818)Show SMILES CCOc1c(OC)ccc2cc(C(=O)NCc3ccc4OCOc4c3)c(=O)[nH]c12 Show InChI InChI=1S/C21H20N2O6/c1-3-27-19-16(26-2)7-5-13-9-14(21(25)23-18(13)19)20(24)22-10-12-4-6-15-17(8-12)29-11-28-15/h4-9H,3,10-11H2,1-2H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353090
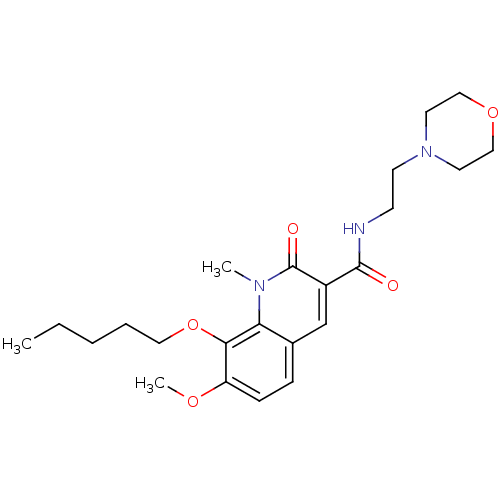
(CHEMBL1822943)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCN3CCOCC3)c(=O)n(C)c12 Show InChI InChI=1S/C23H33N3O5/c1-4-5-6-13-31-21-19(29-3)8-7-17-16-18(23(28)25(2)20(17)21)22(27)24-9-10-26-11-14-30-15-12-26/h7-8,16H,4-6,9-15H2,1-3H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0849 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353090

(CHEMBL1822943)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCN3CCOCC3)c(=O)n(C)c12 Show InChI InChI=1S/C23H33N3O5/c1-4-5-6-13-31-21-19(29-3)8-7-17-16-18(23(28)25(2)20(17)21)22(27)24-9-10-26-11-14-30-15-12-26/h7-8,16H,4-6,9-15H2,1-3H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073005

(CHEMBL3410729)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCc3ccc4OCOc4c3)c(=O)oc12 Show InChI InChI=1S/C24H25NO7/c1-3-4-5-10-29-22-19(28-2)9-7-16-12-17(24(27)32-21(16)22)23(26)25-13-15-6-8-18-20(11-15)31-14-30-18/h6-9,11-12H,3-5,10,13-14H2,1-2H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0869 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073005

(CHEMBL3410729)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCc3ccc4OCOc4c3)c(=O)oc12 Show InChI InChI=1S/C24H25NO7/c1-3-4-5-10-29-22-19(28-2)9-7-16-12-17(24(27)32-21(16)22)23(26)25-13-15-6-8-18-20(11-15)31-14-30-18/h6-9,11-12H,3-5,10,13-14H2,1-2H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073000
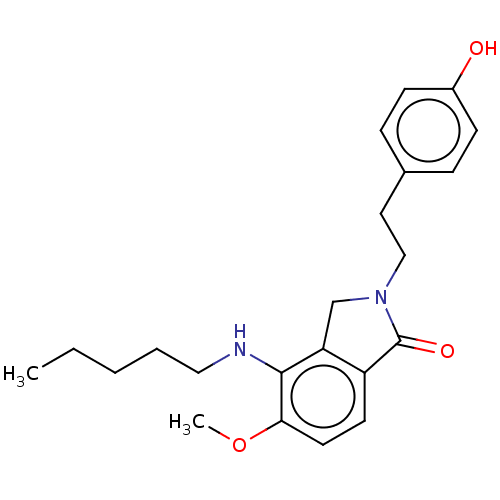
(CHEMBL3410826)Show InChI InChI=1S/C22H28N2O3/c1-3-4-5-13-23-21-19-15-24(14-12-16-6-8-17(25)9-7-16)22(26)18(19)10-11-20(21)27-2/h6-11,23,25H,3-5,12-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0879 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073000

(CHEMBL3410826)Show InChI InChI=1S/C22H28N2O3/c1-3-4-5-13-23-21-19-15-24(14-12-16-6-8-17(25)9-7-16)22(26)18(19)10-11-20(21)27-2/h6-11,23,25H,3-5,12-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072967

(CHEMBL3410817)Show SMILES CCCOc1c(OC)ccc2cc(C(=O)NCCc3ccccc3)c(=O)[nH]c12 Show InChI InChI=1S/C22H24N2O4/c1-3-13-28-20-18(27-2)10-9-16-14-17(22(26)24-19(16)20)21(25)23-12-11-15-7-5-4-6-8-15/h4-10,14H,3,11-13H2,1-2H3,(H,23,25)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370109

(CHEMBL1790723)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H]-1-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6](-[#8])-[#6]-[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](=O)-c1ccc(-[#6]-2=[#6]-3-[#6]=[#6]-[#6](=[#6]-[#6]-3-[#8]-[#6]-3=[#6]\[#6](-[#6]=[#6]-[#6]-2-3)=[#7+](\[#6])-[#6])-[#7](-[#6])-[#6])c(c1)-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#7])-c1ccc(-[#8])cc1 |c:62,64,66,74,t:71| Show InChI InChI=1S/C65H84N14O16S2/c1-7-33(2)54-62(90)72-44(22-23-51(67)82)58(86)73-45(29-52(68)83)59(87)74-46(30-96-97-31-48(81)61(89)76-55(63(91)75-54)34-12-17-38(80)18-13-34)64(92)79-25-9-11-47(79)60(88)71-43(57(85)70-32-66)10-8-24-69-56(84)35-14-19-39(42(26-35)65(93)94)53-40-20-15-36(77(3)4)27-49(40)95-50-28-37(78(5)6)16-21-41(50)53/h12-21,26-28,33,40,43-48,50,54-55,81H,7-11,22-25,29-32,66H2,1-6H3,(H13-,67,68,69,70,71,72,73,74,75,76,80,82,83,84,85,86,87,88,89,90,91,93,94)/p+1/t33-,40?,43+,44-,45-,46-,47-,48?,50?,54-,55+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072967

(CHEMBL3410817)Show SMILES CCCOc1c(OC)ccc2cc(C(=O)NCCc3ccccc3)c(=O)[nH]c12 Show InChI InChI=1S/C22H24N2O4/c1-3-13-28-20-18(27-2)10-9-16-14-17(22(26)24-19(16)20)21(25)23-12-11-15-7-5-4-6-8-15/h4-10,14H,3,11-13H2,1-2H3,(H,23,25)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073001
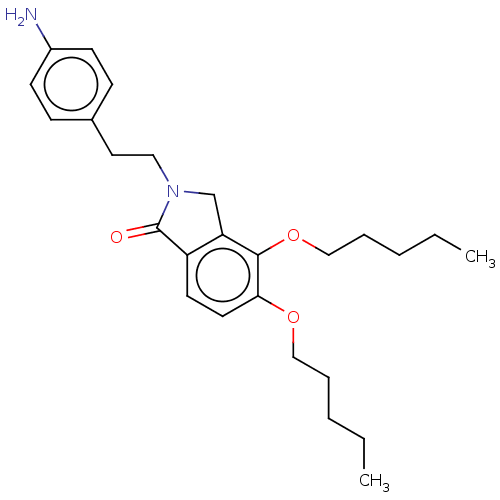
(CHEMBL3410827)Show SMILES CCCCCOc1ccc2C(=O)N(CCc3ccc(N)cc3)Cc2c1OCCCCC Show InChI InChI=1S/C26H36N2O3/c1-3-5-7-17-30-24-14-13-22-23(25(24)31-18-8-6-4-2)19-28(26(22)29)16-15-20-9-11-21(27)12-10-20/h9-14H,3-8,15-19,27H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073001

(CHEMBL3410827)Show SMILES CCCCCOc1ccc2C(=O)N(CCc3ccc(N)cc3)Cc2c1OCCCCC Show InChI InChI=1S/C26H36N2O3/c1-3-5-7-17-30-24-14-13-22-23(25(24)31-18-8-6-4-2)19-28(26(22)29)16-15-20-9-11-21(27)12-10-20/h9-14H,3-8,15-19,27H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353092
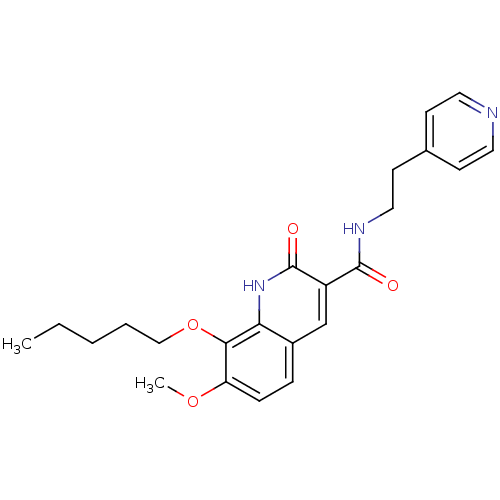
(CHEMBL1822945)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccncc3)c(=O)[nH]c12 Show InChI InChI=1S/C23H27N3O4/c1-3-4-5-14-30-21-19(29-2)7-6-17-15-18(23(28)26-20(17)21)22(27)25-13-10-16-8-11-24-12-9-16/h6-9,11-12,15H,3-5,10,13-14H2,1-2H3,(H,25,27)(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205305
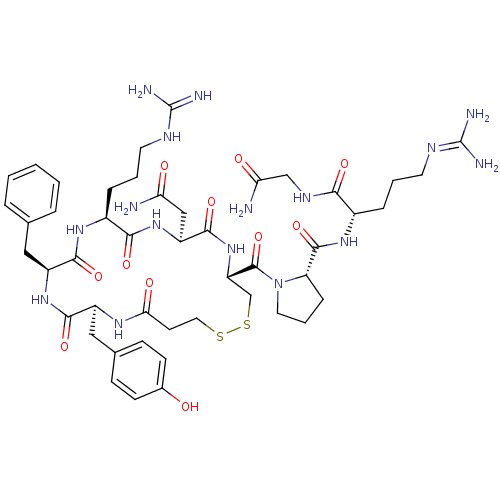
(CHEMBL375324 | d[Arg4]AVP)Show SMILES [#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H]-1-[#6]-[#16]-[#16]-[#6]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C47H68N16O11S2/c48-36(65)23-33-43(72)62-34(45(74)63-19-6-11-35(63)44(73)59-29(9-4-17-54-46(50)51)39(68)56-24-37(49)66)25-76-75-20-16-38(67)57-31(22-27-12-14-28(64)15-13-27)41(70)60-32(21-26-7-2-1-3-8-26)42(71)58-30(40(69)61-33)10-5-18-55-47(52)53/h1-3,7-8,12-15,29-35,64H,4-6,9-11,16-25H2,(H2,48,65)(H2,49,66)(H,56,68)(H,57,67)(H,58,71)(H,59,73)(H,60,70)(H,61,69)(H,62,72)(H4,50,51,54)(H4,52,53,55)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50353092

(CHEMBL1822945)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccncc3)c(=O)[nH]c12 Show InChI InChI=1S/C23H27N3O4/c1-3-4-5-14-30-21-19(29-2)7-6-17-15-18(23(28)26-20(17)21)22(27)25-13-10-16-8-11-24-12-9-16/h6-9,11-12,15H,3-5,10,13-14H2,1-2H3,(H,25,27)(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205309
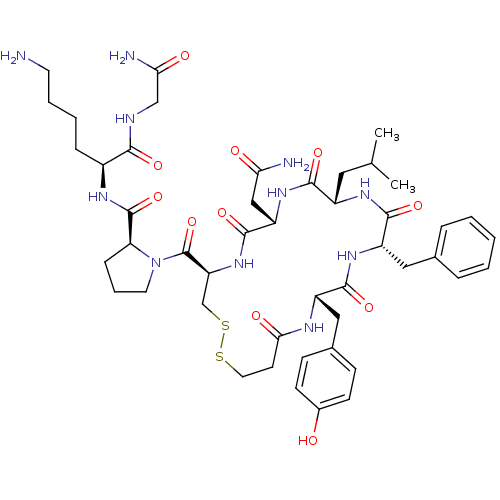
(CHEMBL412972 | d[Leu4,Lys8]VP)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)NCC(N)=O Show InChI InChI=1S/C47H67N11O11S2/c1-27(2)21-32-42(64)56-35(24-38(49)60)45(67)57-36(47(69)58-19-8-12-37(58)46(68)53-31(11-6-7-18-48)41(63)51-25-39(50)61)26-71-70-20-17-40(62)52-33(23-29-13-15-30(59)16-14-29)43(65)55-34(44(66)54-32)22-28-9-4-3-5-10-28/h3-5,9-10,13-16,27,31-37,59H,6-8,11-12,17-26,48H2,1-2H3,(H2,49,60)(H2,50,61)(H,51,63)(H,52,62)(H,53,68)(H,54,66)(H,55,65)(H,56,64)(H,57,67)/t31-,32-,33-,34-,35-,36-,37-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370113

(CHEMBL1790719)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)C(O)CSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](NC1=O)C(C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C60H71N11O18S2/c1-4-28(2)47-55(83)69-48(29(3)72)56(84)66-40(24-46(62)77)52(80)67-41(25-90-91-26-43(76)54(82)70-49(57(85)68-47)30-9-12-32(73)13-10-30)58(86)71-20-6-8-42(71)53(81)65-39(51(79)64-27-61)7-5-19-63-50(78)31-11-16-35-38(21-31)60(89-59(35)87)36-17-14-33(74)22-44(36)88-45-23-34(75)15-18-37(45)60/h9-18,21-23,28-29,39-43,47-49,72-76H,4-8,19-20,24-27,61H2,1-3H3,(H2,62,77)(H,63,78)(H,64,79)(H,65,81)(H,66,84)(H,67,80)(H,68,85)(H,69,83)(H,70,82)/t28-,29?,39+,40-,41-,42-,43?,47-,48-,49+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073059

(CHEMBL3410831)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccccc3F)c(=O)[nH]c12 Show InChI InChI=1S/C24H27FN2O4/c1-3-4-7-14-31-22-20(30-2)11-10-17-15-18(24(29)27-21(17)22)23(28)26-13-12-16-8-5-6-9-19(16)25/h5-6,8-11,15H,3-4,7,12-14H2,1-2H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50073059

(CHEMBL3410831)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccccc3F)c(=O)[nH]c12 Show InChI InChI=1S/C24H27FN2O4/c1-3-4-7-14-31-22-20(30-2)11-10-17-15-18(24(29)27-21(17)22)23(28)26-13-12-16-8-5-6-9-19(16)25/h5-6,8-11,15H,3-4,7,12-14H2,1-2H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370117

(CHEMBL1790720)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)C(O)CSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C61H72N12O18S2/c1-3-29(2)49-57(86)68-40(18-19-47(63)78)53(82)69-41(25-48(64)79)54(83)70-42(26-92-93-27-44(77)56(85)72-50(58(87)71-49)30-8-11-32(74)12-9-30)59(88)73-21-5-7-43(73)55(84)67-39(52(81)66-28-62)6-4-20-65-51(80)31-10-15-35-38(22-31)61(91-60(35)89)36-16-13-33(75)23-45(36)90-46-24-34(76)14-17-37(46)61/h8-17,22-24,29,39-44,49-50,74-77H,3-7,18-21,25-28,62H2,1-2H3,(H2,63,78)(H2,64,79)(H,65,80)(H,66,81)(H,67,84)(H,68,86)(H,69,82)(H,70,83)(H,71,87)(H,72,85)/t29-,39+,40-,41-,42-,43-,44?,49-,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205296

(CHEMBL385739 | d[Arg4,Dab8]VP)Show SMILES NCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSCCC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O Show InChI InChI=1S/C45H64N14O11S2/c46-16-14-29(38(64)52-23-36(48)62)55-43(69)34-9-5-18-59(34)44(70)33-24-72-71-19-15-37(63)53-30(21-26-10-12-27(60)13-11-26)40(66)56-31(20-25-6-2-1-3-7-25)41(67)54-28(8-4-17-51-45(49)50)39(65)57-32(22-35(47)61)42(68)58-33/h1-3,6-7,10-13,28-34,60H,4-5,8-9,14-24,46H2,(H2,47,61)(H2,48,62)(H,52,64)(H,53,63)(H,54,67)(H,55,69)(H,56,66)(H,57,65)(H,58,68)(H4,49,50,51)/t28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205291
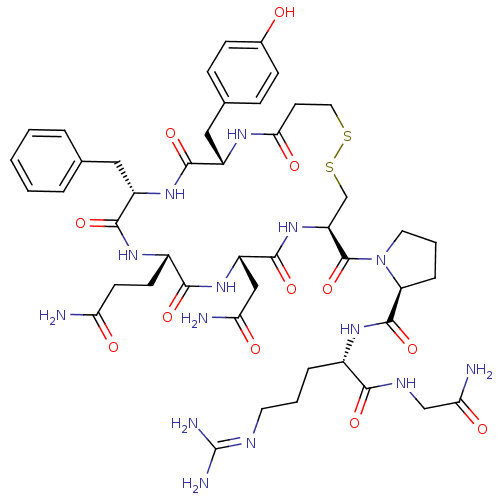
((S)-1-((4R,7S,10S,13S,16S)-7-(2-amino-2-oxoethyl)-...)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52)/t28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205305

(CHEMBL375324 | d[Arg4]AVP)Show SMILES [#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H]-1-[#6]-[#16]-[#16]-[#6]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccc(-[#8])cc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C47H68N16O11S2/c48-36(65)23-33-43(72)62-34(45(74)63-19-6-11-35(63)44(73)59-29(9-4-17-54-46(50)51)39(68)56-24-37(49)66)25-76-75-20-16-38(67)57-31(22-27-12-14-28(64)15-13-27)41(70)60-32(21-26-7-2-1-3-8-26)42(71)58-30(40(69)61-33)10-5-18-55-47(52)53/h1-3,7-8,12-15,29-35,64H,4-6,9-11,16-25H2,(H2,48,65)(H2,49,66)(H,56,68)(H,57,67)(H,58,71)(H,59,73)(H,60,70)(H,61,69)(H,62,72)(H4,50,51,54)(H4,52,53,55)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370106

(CHEMBL1790712)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@](C)(NC(=O)CC2(CCCCC2)SSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](NC1=O)[C@@H](C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCN)C(=O)N[C@H](C(N)=O)c1ccc(O)cc1)c1ccc(O)cc1 Show InChI InChI=1S/C52H75N11O13S2/c1-5-28(2)40-47(73)59-41(29(3)64)48(74)57-35(25-38(54)67)45(71)58-36(27-77-78-52(21-7-6-8-22-52)26-39(68)62-51(4,50(76)61-40)31-15-19-33(66)20-16-31)49(75)63-24-10-12-37(63)46(72)56-34(11-9-23-53)44(70)60-42(43(55)69)30-13-17-32(65)18-14-30/h13-20,28-29,34-37,40-42,64-66H,5-12,21-27,53H2,1-4H3,(H2,54,67)(H2,55,69)(H,56,72)(H,57,74)(H,58,71)(H,59,73)(H,60,70)(H,61,76)(H,62,68)/t28-,29-,34+,35-,36-,37-,40-,41-,42+,51-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072992

(CHEMBL3410819)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCc3ccc4OCOc4c3)c(=O)n(C)c12 Show InChI InChI=1S/C25H28N2O6/c1-4-5-6-11-31-23-20(30-3)10-8-17-13-18(25(29)27(2)22(17)23)24(28)26-14-16-7-9-19-21(12-16)33-15-32-19/h7-10,12-13H,4-6,11,14-15H2,1-3H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072992

(CHEMBL3410819)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCc3ccc4OCOc4c3)c(=O)n(C)c12 Show InChI InChI=1S/C25H28N2O6/c1-4-5-6-11-31-23-20(30-3)10-8-17-13-18(25(29)27(2)22(17)23)24(28)26-14-16-7-9-19-21(12-16)33-15-32-19/h7-10,12-13H,4-6,11,14-15H2,1-3H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50145126

((2S)-2-{[(2R)-1-{[(4S,7R,10S,13R,16S)-13-benzyl-7-...)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C47H67N13O11S2/c1-26(2)20-31-41(66)58-34(23-37(48)62)44(69)59-35(46(71)60-18-7-11-36(60)45(70)55-30(10-6-17-52-47(50)51)40(65)53-24-38(49)63)25-73-72-19-16-39(64)54-32(22-28-12-14-29(61)15-13-28)42(67)57-33(43(68)56-31)21-27-8-4-3-5-9-27/h3-5,8-9,12-15,26,30-36,61H,6-7,10-11,16-25H2,1-2H3,(H2,48,62)(H2,49,63)(H,53,65)(H,54,64)(H,55,70)(H,56,68)(H,57,67)(H,58,66)(H,59,69)(H4,50,51,52)/t30-,31-,32-,33+,34+,35+,36+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Binding affinity against human vasopressin V1b receptor was determined by using plasma membranes from CHO cells stably transfected with VP/OT recepto... |
J Med Chem 47: 2375-88 (2004)
Article DOI: 10.1021/jm030611c
BindingDB Entry DOI: 10.7270/Q2GF0V8C |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205297
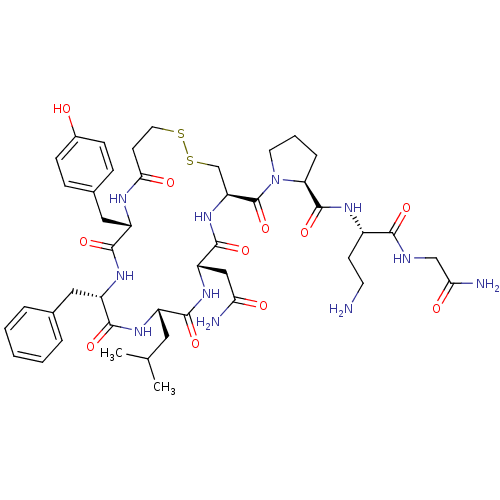
(CHEMBL412973 | d[Leu4,Dab8]VP)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCN)C(=O)NCC(N)=O Show InChI InChI=1S/C45H63N11O11S2/c1-25(2)19-30-40(62)54-33(22-36(47)58)43(65)55-34(45(67)56-17-6-9-35(56)44(66)51-29(14-16-46)39(61)49-23-37(48)59)24-69-68-18-15-38(60)50-31(21-27-10-12-28(57)13-11-27)41(63)53-32(42(64)52-30)20-26-7-4-3-5-8-26/h3-5,7-8,10-13,25,29-35,57H,6,9,14-24,46H2,1-2H3,(H2,47,58)(H2,48,59)(H,49,61)(H,50,60)(H,51,66)(H,52,64)(H,53,63)(H,54,62)(H,55,65)/t29-,30-,31-,32-,33-,34-,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205300
(CHEMBL221436 | d[Val4]AVP)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N13O11S2/c1-25(2)38-44(69)56-32(22-35(47)61)41(66)57-33(45(70)59-18-7-11-34(59)43(68)54-29(10-6-17-51-46(49)50)39(64)52-23-36(48)62)24-72-71-19-16-37(63)53-30(21-27-12-14-28(60)15-13-27)40(65)55-31(42(67)58-38)20-26-8-4-3-5-9-26/h3-5,8-9,12-15,25,29-34,38,60H,6-7,10-11,16-24H2,1-2H3,(H2,47,61)(H2,48,62)(H,52,64)(H,53,63)(H,54,68)(H,55,65)(H,56,69)(H,57,66)(H,58,67)(H4,49,50,51)/t29-,30-,31-,32-,33-,34-,38-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370110

(CHEMBL1790721)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)CCSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C61H72N12O17S2/c1-3-30(2)50-57(85)68-41(18-19-47(63)77)54(82)69-42(27-48(64)78)55(83)70-43(28-92-91-23-20-49(79)71-51(58(86)72-50)31-8-11-33(74)12-9-31)59(87)73-22-5-7-44(73)56(84)67-40(53(81)66-29-62)6-4-21-65-52(80)32-10-15-36-39(24-32)61(90-60(36)88)37-16-13-34(75)25-45(37)89-46-26-35(76)14-17-38(46)61/h8-17,24-26,30,40-44,50-51,74-76H,3-7,18-23,27-29,62H2,1-2H3,(H2,63,77)(H2,64,78)(H,65,80)(H,66,81)(H,67,84)(H,68,85)(H,69,82)(H,70,83)(H,71,79)(H,72,86)/t30-,40+,41-,42-,43-,44-,50-,51+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205304
(CHEMBL435323 | dVDAVP)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N13O11S2/c1-25(2)38-44(69)56-32(22-35(47)61)41(66)57-33(45(70)59-18-7-11-34(59)43(68)54-29(10-6-17-51-46(49)50)39(64)52-23-36(48)62)24-72-71-19-16-37(63)53-30(21-27-12-14-28(60)15-13-27)40(65)55-31(42(67)58-38)20-26-8-4-3-5-9-26/h3-5,8-9,12-15,25,29-34,38,60H,6-7,10-11,16-24H2,1-2H3,(H2,47,61)(H2,48,62)(H,52,64)(H,53,63)(H,54,68)(H,55,65)(H,56,69)(H,57,66)(H,58,67)(H4,49,50,51)/t29-,30+,31+,32+,33+,34+,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50205301
(CHEMBL375188 | d[Leu4,Orn8]VP)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN)C(=O)NCC(N)=O Show InChI InChI=1S/C46H65N11O11S2/c1-26(2)20-31-41(63)55-34(23-37(48)59)44(66)56-35(46(68)57-18-7-11-36(57)45(67)52-30(10-6-17-47)40(62)50-24-38(49)60)25-70-69-19-16-39(61)51-32(22-28-12-14-29(58)15-13-28)42(64)54-33(43(65)53-31)21-27-8-4-3-5-9-27/h3-5,8-9,12-15,26,30-36,58H,6-7,10-11,16-25,47H2,1-2H3,(H2,48,59)(H2,49,60)(H,50,62)(H,51,61)(H,52,67)(H,53,65)(H,54,64)(H,55,63)(H,56,66)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50145118
(CHEMBL412353 | d[Val4]AVP)Show SMILES CC(C)[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C46H65N13O11S2/c1-25(2)38-44(69)56-32(22-35(47)61)41(66)57-33(45(70)59-18-7-11-34(59)43(68)54-29(10-6-17-51-46(49)50)39(64)52-23-36(48)62)24-72-71-19-16-37(63)53-30(21-27-12-14-28(60)15-13-27)40(65)55-31(42(67)58-38)20-26-8-4-3-5-9-26/h3-5,8-9,12-15,25,29-34,38,60H,6-7,10-11,16-24H2,1-2H3,(H2,47,61)(H2,48,62)(H,52,64)(H,53,63)(H,54,68)(H,55,65)(H,56,69)(H,57,66)(H,58,67)(H4,49,50,51)/t29-,30-,31+,32+,33+,34+,38-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Ohio
Curated by ChEMBL
| Assay Description
Binding affinity against human vasopressin V1b receptor was determined by using plasma membranes from CHO cells stably transfected with VP/OT recepto... |
J Med Chem 47: 2375-88 (2004)
Article DOI: 10.1021/jm030611c
BindingDB Entry DOI: 10.7270/Q2GF0V8C |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370114
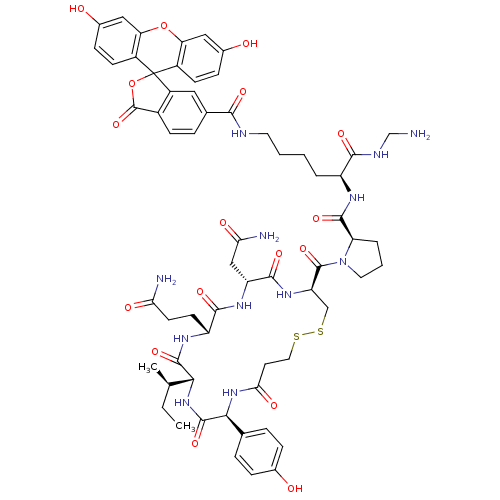
(CHEMBL1790718)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)CCSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C62H74N12O17S2/c1-3-31(2)51-58(86)69-42(19-20-48(64)78)55(83)70-43(28-49(65)79)56(84)71-44(29-93-92-24-21-50(80)72-52(59(87)73-51)32-9-12-34(75)13-10-32)60(88)74-23-6-8-45(74)57(85)68-41(54(82)67-30-63)7-4-5-22-66-53(81)33-11-16-37-40(25-33)62(91-61(37)89)38-17-14-35(76)26-46(38)90-47-27-36(77)15-18-39(47)62/h9-18,25-27,31,41-45,51-52,75-77H,3-8,19-24,28-30,63H2,1-2H3,(H2,64,78)(H2,65,79)(H,66,81)(H,67,82)(H,68,85)(H,69,86)(H,70,83)(H,71,84)(H,72,80)(H,73,87)/t31-,41+,42-,43-,44-,45-,51-,52+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205308
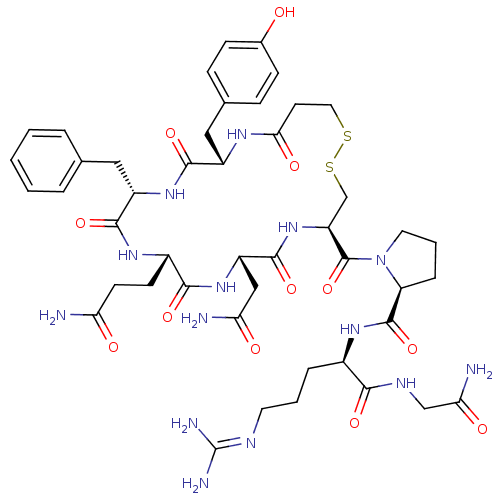
((S)-1-((4R,7S,10S,13S,16S)-7-(2-amino-2-oxoethyl)-...)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H64N14O12S2/c47-35(62)15-14-29-40(67)58-32(22-36(48)63)43(70)59-33(45(72)60-18-5-9-34(60)44(71)56-28(8-4-17-52-46(50)51)39(66)53-23-37(49)64)24-74-73-19-16-38(65)54-30(21-26-10-12-27(61)13-11-26)41(68)57-31(42(69)55-29)20-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,61H,4-5,8-9,14-24H2,(H2,47,62)(H2,48,63)(H2,49,64)(H,53,66)(H,54,65)(H,55,69)(H,56,71)(H,57,68)(H,58,67)(H,59,70)(H4,50,51,52)/t28-,29+,30+,31+,32+,33+,34+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50205300

(CHEMBL221436 | d[Val4]AVP)Show SMILES [#6]-[#6](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6]-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N13O11S2/c1-25(2)38-44(69)56-32(22-35(47)61)41(66)57-33(45(70)59-18-7-11-34(59)43(68)54-29(10-6-17-51-46(49)50)39(64)52-23-36(48)62)24-72-71-19-16-37(63)53-30(21-27-12-14-28(60)15-13-27)40(65)55-31(42(67)58-38)20-26-8-4-3-5-9-26/h3-5,8-9,12-15,25,29-34,38,60H,6-7,10-11,16-24H2,1-2H3,(H2,47,61)(H2,48,62)(H,52,64)(H,53,63)(H,54,68)(H,55,65)(H,56,69)(H,57,66)(H,58,67)(H4,49,50,51)/t29-,30-,31-,32-,33-,34-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from vasopressin V2 receptor in rat kidney membranes |
J Med Chem 50: 835-47 (2007)
Article DOI: 10.1021/jm060928n
BindingDB Entry DOI: 10.7270/Q2G161NH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370112

(CHEMBL1790729)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)C(O)CSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](NC1=O)C(C)O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C61H73N11O18S2/c1-4-29(2)48-56(84)70-49(30(3)73)57(85)67-41(25-47(63)78)53(81)68-42(26-91-92-27-44(77)55(83)71-50(58(86)69-48)31-10-13-33(74)14-11-31)59(87)72-21-7-9-43(72)54(82)66-40(52(80)65-28-62)8-5-6-20-64-51(79)32-12-17-36-39(22-32)61(90-60(36)88)37-18-15-34(75)23-45(37)89-46-24-35(76)16-19-38(46)61/h10-19,22-24,29-30,40-44,48-50,73-77H,4-9,20-21,25-28,62H2,1-3H3,(H2,63,78)(H,64,79)(H,65,80)(H,66,82)(H,67,85)(H,68,81)(H,69,86)(H,70,84)(H,71,83)/t29-,30?,40+,41-,42-,43-,44?,48-,49-,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072965
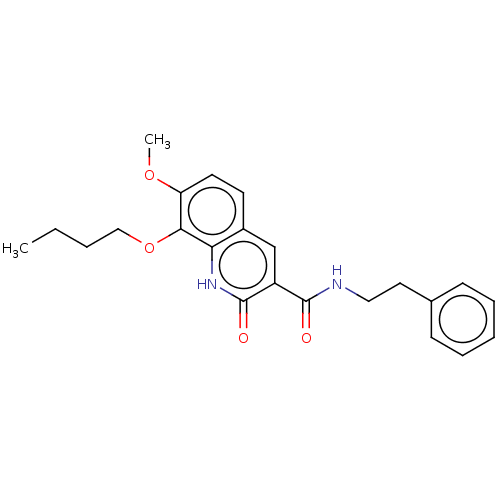
(CHEMBL3410815)Show SMILES CCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccccc3)c(=O)[nH]c12 Show InChI InChI=1S/C23H26N2O4/c1-3-4-14-29-21-19(28-2)11-10-17-15-18(23(27)25-20(17)21)22(26)24-13-12-16-8-6-5-7-9-16/h5-11,15H,3-4,12-14H2,1-2H3,(H,24,26)(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072965

(CHEMBL3410815)Show SMILES CCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccccc3)c(=O)[nH]c12 Show InChI InChI=1S/C23H26N2O4/c1-3-4-14-29-21-19(28-2)11-10-17-15-18(23(27)25-20(17)21)22(26)24-13-12-16-8-6-5-7-9-16/h5-11,15H,3-4,12-14H2,1-2H3,(H,24,26)(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Binding affinity to CB2 receptor (unknown origin) |
Eur J Med Chem 93: 16-32 (2015)
Article DOI: 10.1016/j.ejmech.2015.01.054
BindingDB Entry DOI: 10.7270/Q28P626S |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50370115
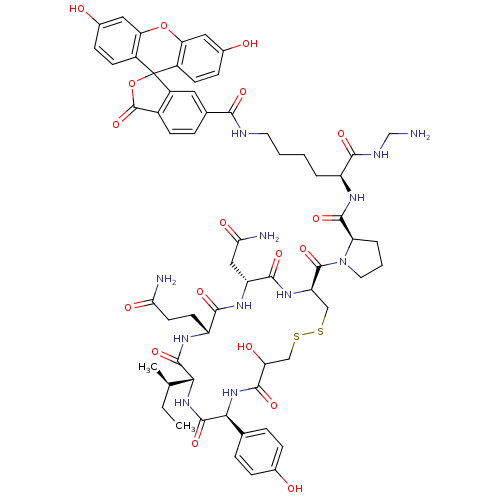
(CHEMBL1790717)Show SMILES CC[C@@H](C)[C@H]1NC(=O)[C@@H](NC(=O)C(O)CSSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCCNC(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)NCN)c1ccc(O)cc1 Show InChI InChI=1S/C62H74N12O18S2/c1-3-30(2)50-58(87)69-41(19-20-48(64)79)54(83)70-42(26-49(65)80)55(84)71-43(27-93-94-28-45(78)57(86)73-51(59(88)72-50)31-9-12-33(75)13-10-31)60(89)74-22-6-8-44(74)56(85)68-40(53(82)67-29-63)7-4-5-21-66-52(81)32-11-16-36-39(23-32)62(92-61(36)90)37-17-14-34(76)24-46(37)91-47-25-35(77)15-18-38(47)62/h9-18,23-25,30,40-45,50-51,75-78H,3-8,19-22,26-29,63H2,1-2H3,(H2,64,79)(H2,65,80)(H,66,81)(H,67,82)(H,68,85)(H,69,87)(H,70,83)(H,71,84)(H,72,88)(H,73,86)/t30-,40+,41-,42-,43-,44-,45?,50-,51+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity for human oxytocin receptor |
J Med Chem 45: 2579-88 (2002)
BindingDB Entry DOI: 10.7270/Q2W37X10 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data