

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50005429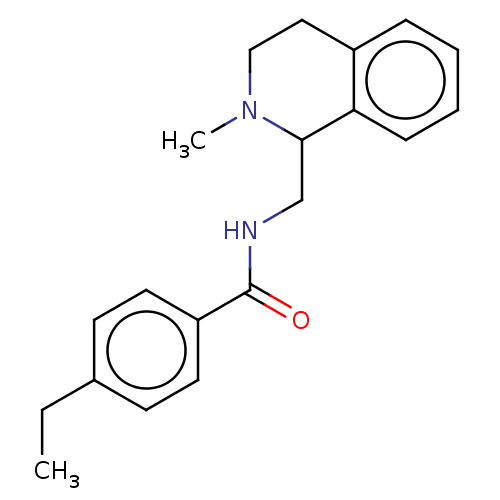 (CHEMBL4070288) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50007985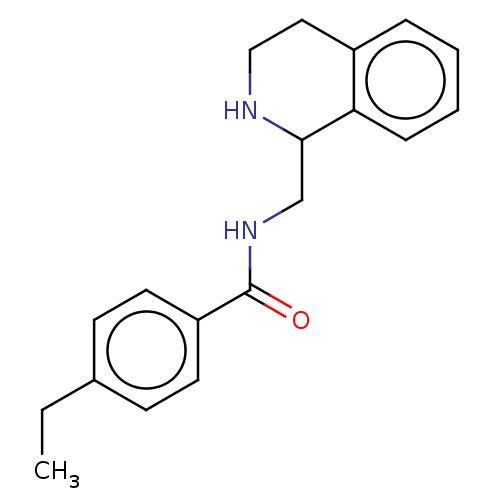 (CHEMBL4097865) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50042064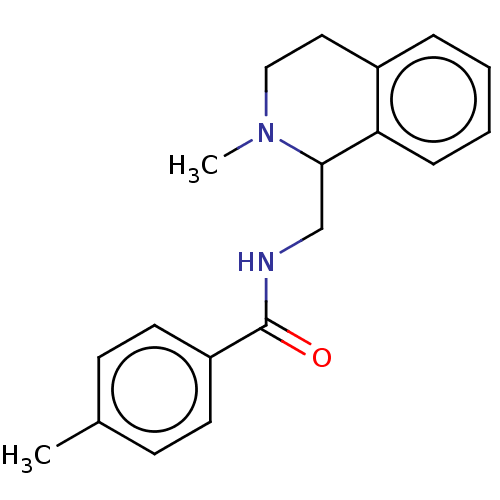 (CHEMBL4097466) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015426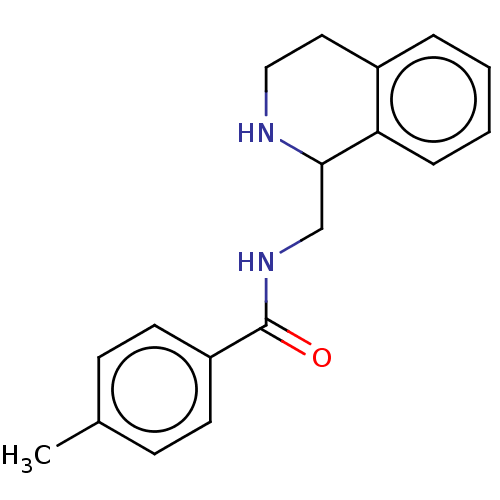 (CHEMBL4070750) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50009239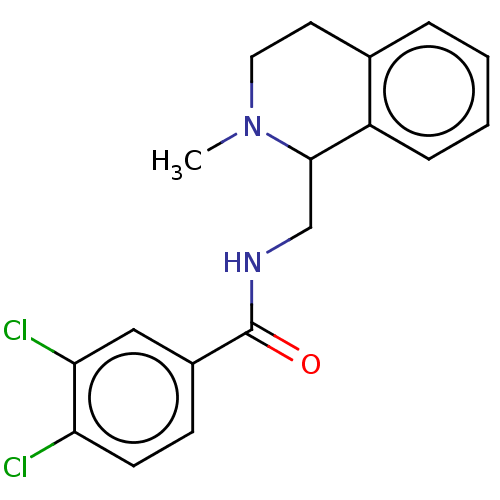 (CHEMBL4092125) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008523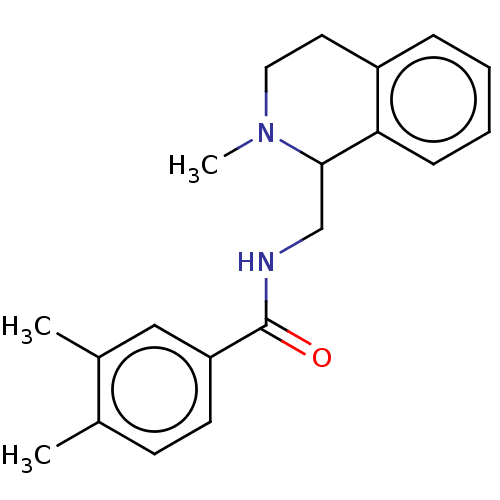 (CHEMBL4071332) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015433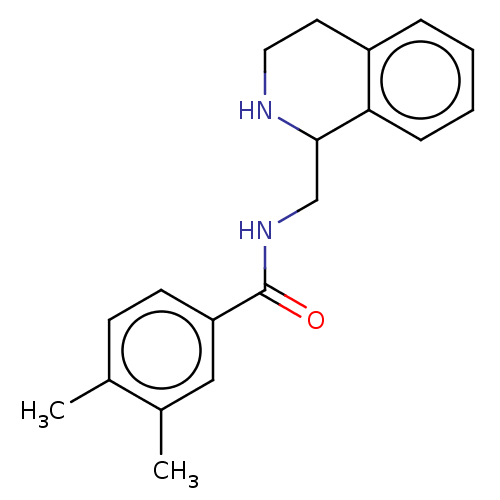 (CHEMBL4105599) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008003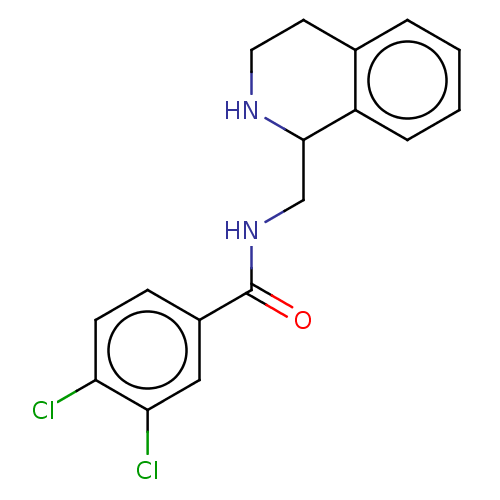 (CHEMBL4065924) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055967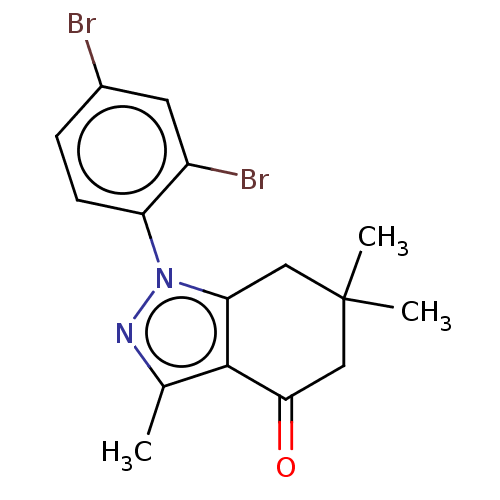 (CHEMBL3325714) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055966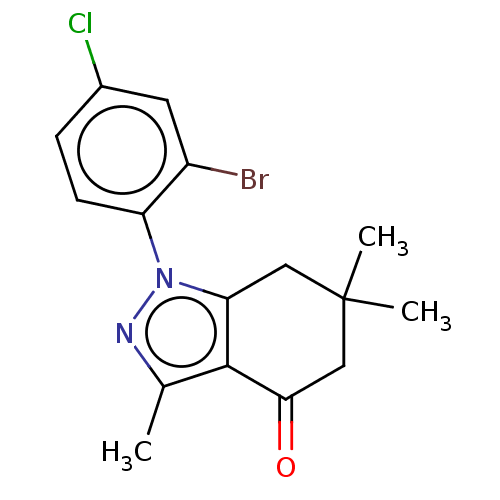 (CHEMBL3325715) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055965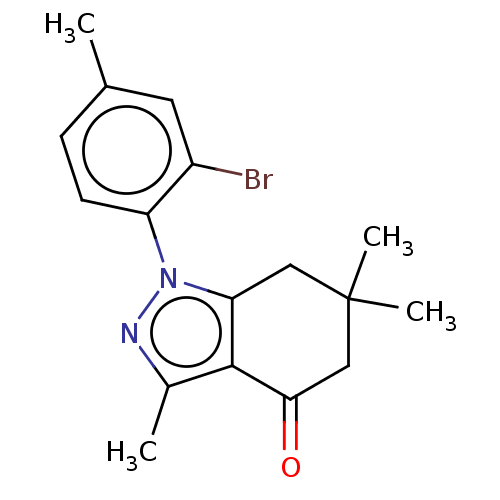 (CHEMBL3325716) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055969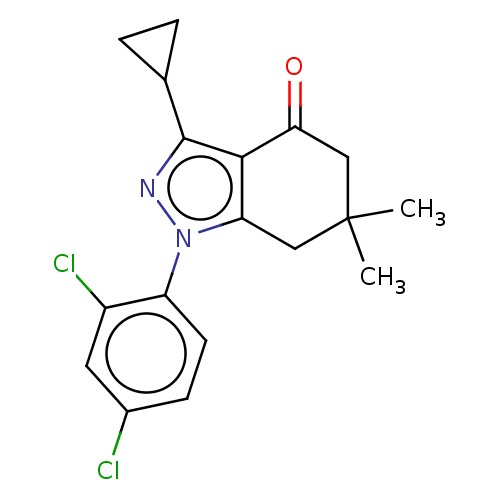 (CHEMBL3325719) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307814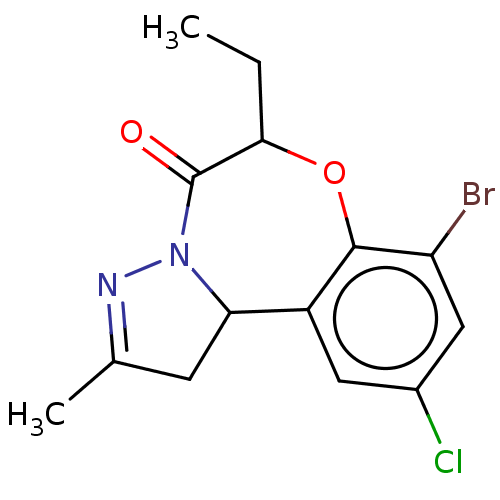 (CHEMBL4176194) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum BuChE using varying levels of butyrylthiocholine iodide as substrate preincubated for 20 mins followed by ... | Eur J Med Chem 147: 194-204 (2018) Article DOI: 10.1016/j.ejmech.2018.02.002 BindingDB Entry DOI: 10.7270/Q2XG9TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055973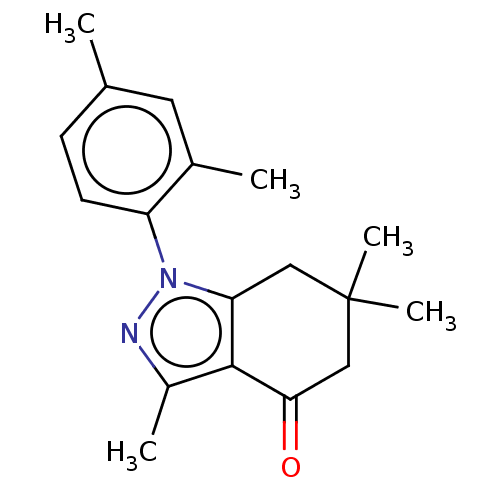 (CHEMBL3325707) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50307397 (CHEMBL4177151) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE using varying levels of butyrylthiocholine iodide as substrate preincubated for 20 mins followed by subst... | Eur J Med Chem 147: 194-204 (2018) Article DOI: 10.1016/j.ejmech.2018.02.002 BindingDB Entry DOI: 10.7270/Q2XG9TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055972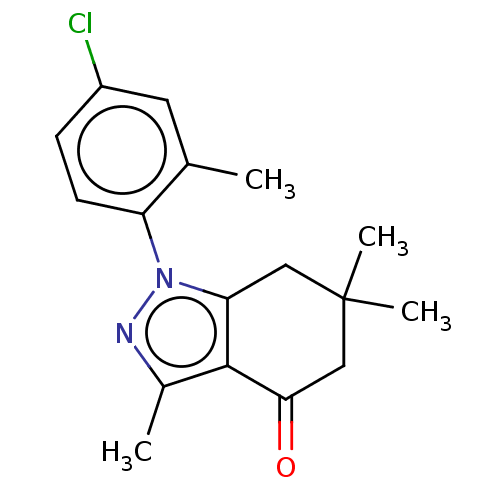 (CHEMBL3325710) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50055964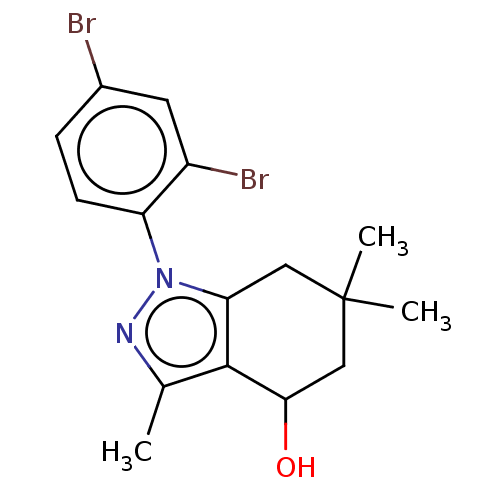 (CHEMBL3325841) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from rat mu opioid receptor expressed in HEK cells after 60 mins | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b... | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21008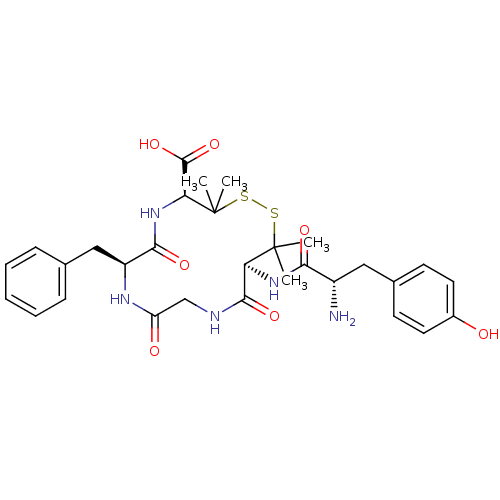 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human delta opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b... | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins by H... | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344933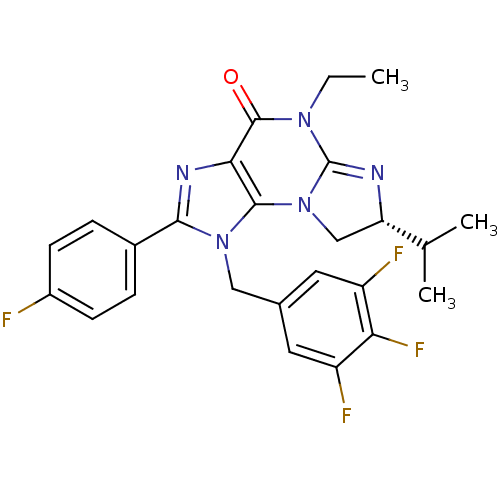 ((R)-5-ethyl-2-(4-fluorophenyl)-7-isopropyl-1-(3,4,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50359218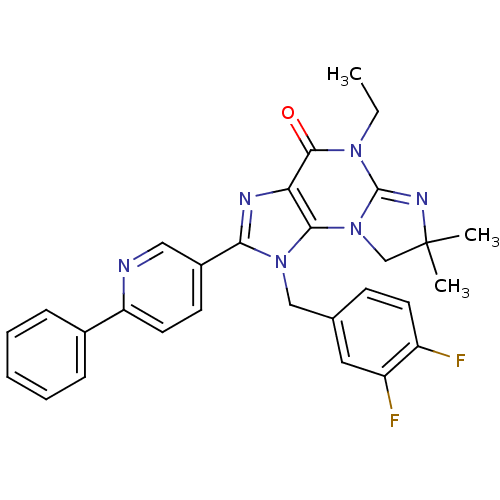 (CHEMBL1923313) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in LPS-stimulated human whole blood assessed as inhibition of ATP-induced IL1-beta production treated 30 mins be... | Bioorg Med Chem Lett 21: 7287-90 (2011) Article DOI: 10.1016/j.bmcl.2011.10.037 BindingDB Entry DOI: 10.7270/Q2DV1K8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50359221 (CHEMBL1923316) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in LPS-stimulated human whole blood assessed as inhibition of ATP-induced IL1-beta production treated 30 mins be... | Bioorg Med Chem Lett 21: 7287-90 (2011) Article DOI: 10.1016/j.bmcl.2011.10.037 BindingDB Entry DOI: 10.7270/Q2DV1K8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344920 ((R)-1-(3,4-difluorobenzyl)-5-ethyl-7-isopropyl-2-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition and measured afte... | Eur J Med Chem 147: 194-204 (2018) Article DOI: 10.1016/j.ejmech.2018.02.002 BindingDB Entry DOI: 10.7270/Q2XG9TP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50359223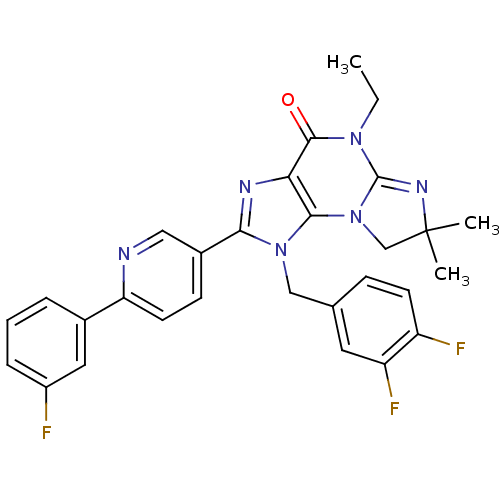 (CHEMBL1923318) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in LPS-stimulated human whole blood assessed as inhibition of ATP-induced IL1-beta production treated 30 mins be... | Bioorg Med Chem Lett 21: 7287-90 (2011) Article DOI: 10.1016/j.bmcl.2011.10.037 BindingDB Entry DOI: 10.7270/Q2DV1K8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344903 ((R)-1-(3,4-difluorobenzyl)-2-(6-(3,3-difluoropiper...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344937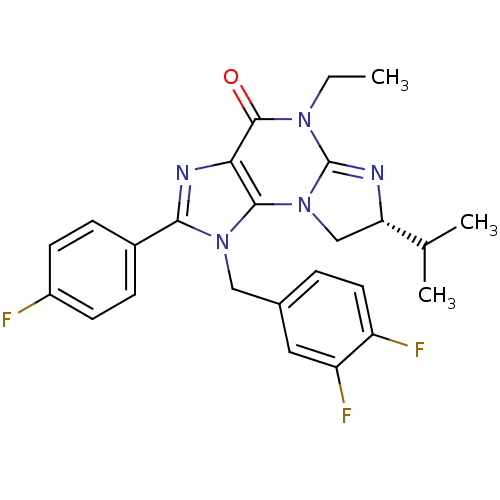 ((R)-1-(3,4-difluorobenzyl)-5-ethyl-2-(4-fluorophen...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344936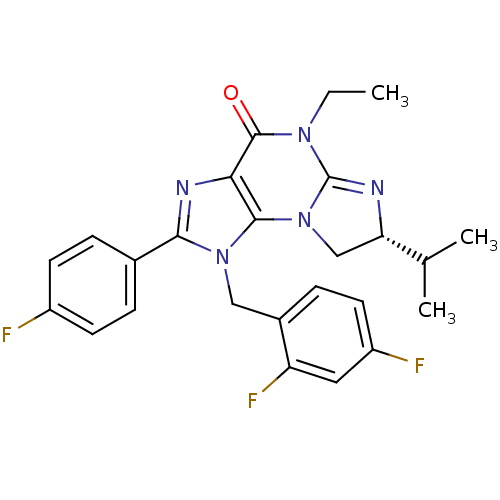 ((R)-1-(2,4-difluorobenzyl)-5-ethyl-2-(4-fluorophen...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344914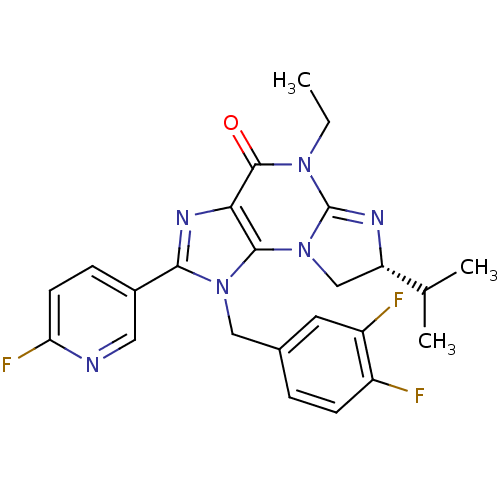 ((R)-1-(3,4-difluorobenzyl)-5-ethyl-2-(6-fluoropyri...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344934 ((R)-1-(3,5-difluorobenzyl)-5-ethyl-2-(4-fluorophen...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50359225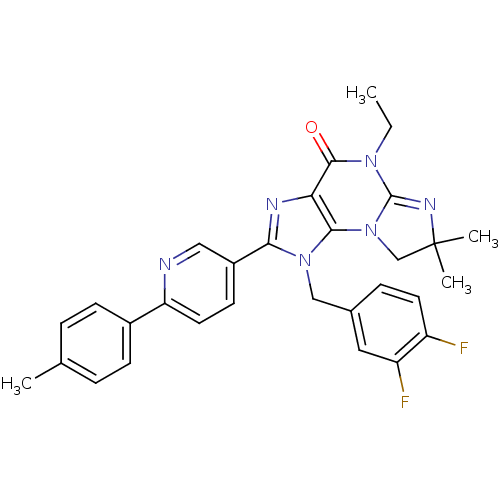 (CHEMBL1923320) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in LPS-stimulated human whole blood assessed as inhibition of ATP-induced IL1-beta production treated 30 mins be... | Bioorg Med Chem Lett 21: 7287-90 (2011) Article DOI: 10.1016/j.bmcl.2011.10.037 BindingDB Entry DOI: 10.7270/Q2DV1K8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50013629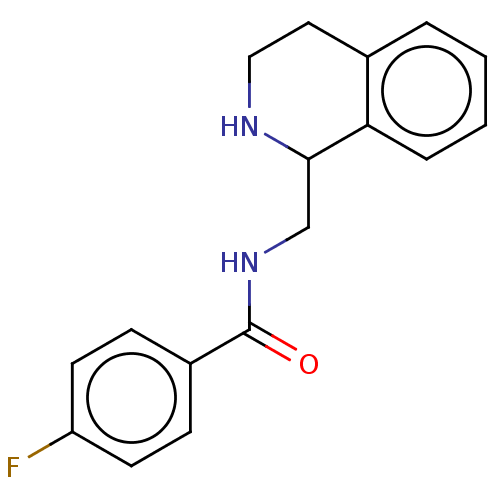 (CHEMBL4062124) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50003238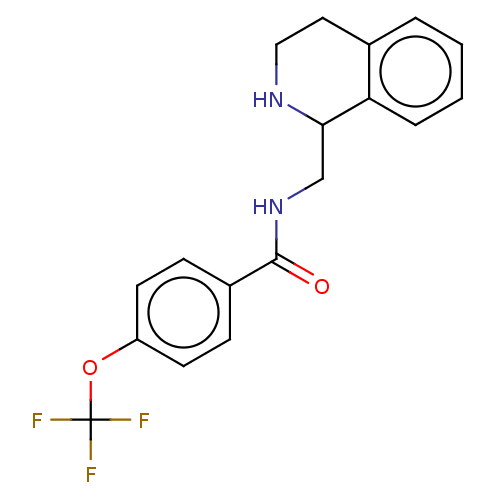 (CHEMBL4082299) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344918 ((R)-4-(1-(3,4-difluorobenzyl)-5-ethyl-7-isopropyl-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344929 ((R)-7-tert-butyl-1-(3,4-difluorobenzyl)-5-ethyl-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344913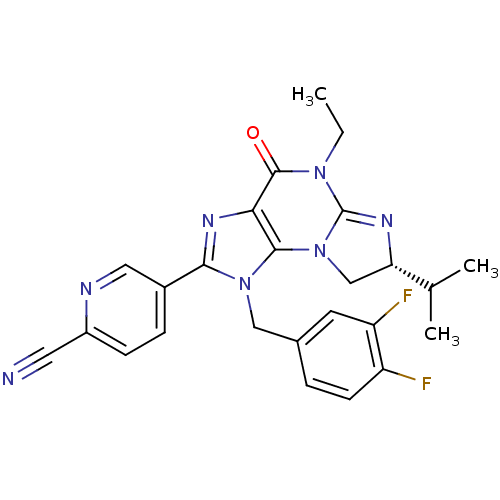 ((R)-5-(1-(3,4-difluorobenzyl)-5-ethyl-7-isopropyl-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344907 ((R)-1-(3,4-difluorobenzyl)-5-ethyl-7-isopropyl-2-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344906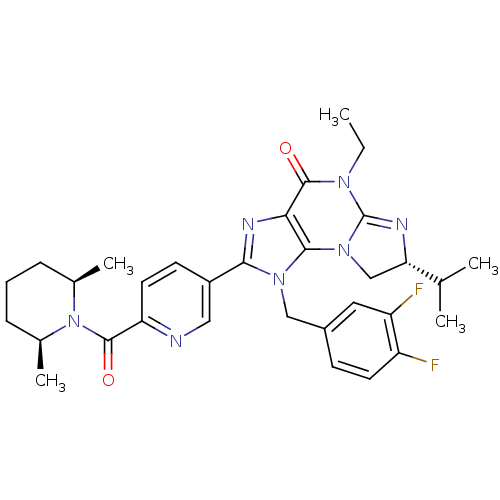 ((R)-1-(3,4-difluorobenzyl)-2-(6-((2R,6S)-2,6-dimet...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344926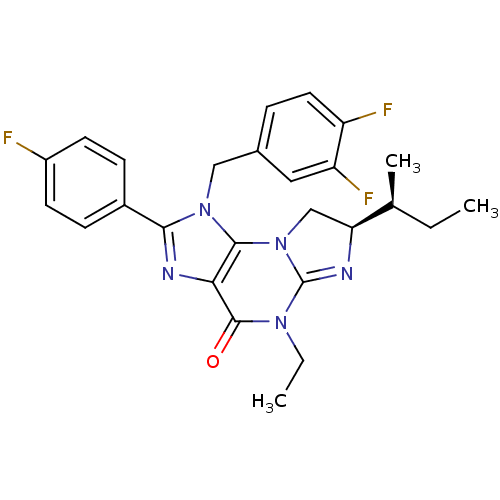 ((R)-7-sec-butyl-1-(3,4-difluorobenzyl)-5-ethyl-2-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50359227 (CHEMBL1923322) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in LPS-stimulated human whole blood assessed as inhibition of ATP-induced IL1-beta production treated 30 mins be... | Bioorg Med Chem Lett 21: 7287-90 (2011) Article DOI: 10.1016/j.bmcl.2011.10.037 BindingDB Entry DOI: 10.7270/Q2DV1K8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344912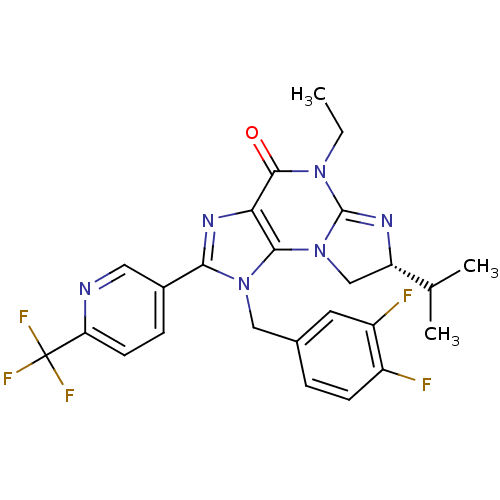 ((R)-1-(3,4-difluorobenzyl)-5-ethyl-7-isopropyl-2-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50009239 (CHEMBL4092125) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50007981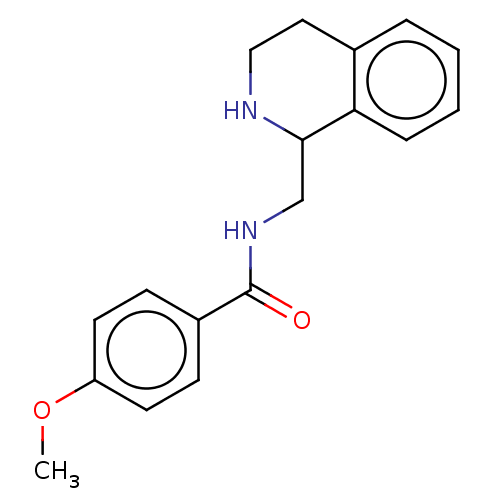 (CHEMBL4100317) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50359231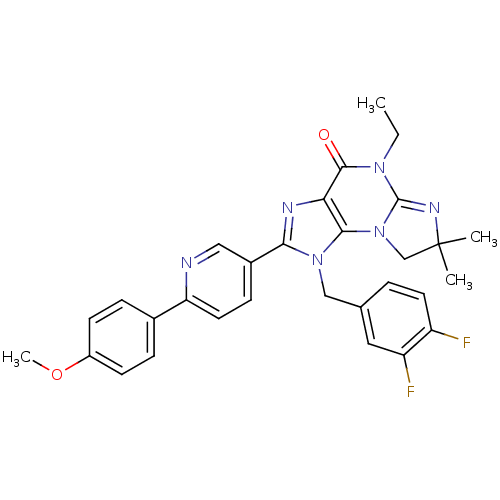 (CHEMBL1923323) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in LPS-stimulated human whole blood assessed as inhibition of ATP-induced IL1-beta production treated 30 mins be... | Bioorg Med Chem Lett 21: 7287-90 (2011) Article DOI: 10.1016/j.bmcl.2011.10.037 BindingDB Entry DOI: 10.7270/Q2DV1K8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50344904 ((R)-1-(3,4-difluorobenzyl)-2-(6-(4,4-difluoropiper...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor by calcium flux assay | Bioorg Med Chem Lett 21: 3805-8 (2011) Article DOI: 10.1016/j.bmcl.2011.04.034 BindingDB Entry DOI: 10.7270/Q25B02SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 234 total ) | Next | Last >> |