 Found 974 hits with Last Name = 'chenard' and Initial = 'l'
Found 974 hits with Last Name = 'chenard' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260806
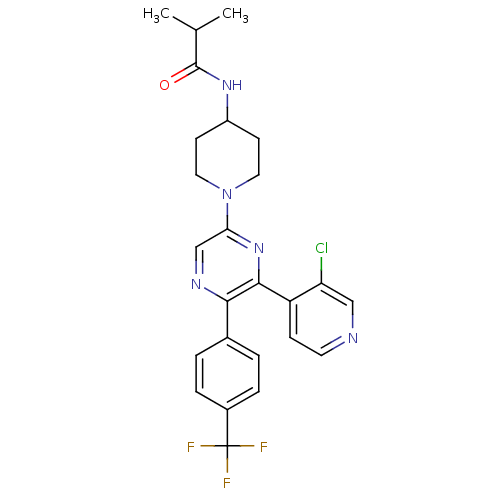
(CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(C)C(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C25H25ClF3N5O/c1-15(2)24(35)32-18-8-11-34(12-9-18)21-14-31-22(16-3-5-17(6-4-16)25(27,28)29)23(33-21)19-7-10-30-13-20(19)26/h3-7,10,13-15,18H,8-9,11-12H2,1-2H3,(H,32,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50260806

(CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(C)C(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C25H25ClF3N5O/c1-15(2)24(35)32-18-8-11-34(12-9-18)21-14-31-22(16-3-5-17(6-4-16)25(27,28)29)23(33-21)19-7-10-30-13-20(19)26/h3-7,10,13-15,18H,8-9,11-12H2,1-2H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50202412
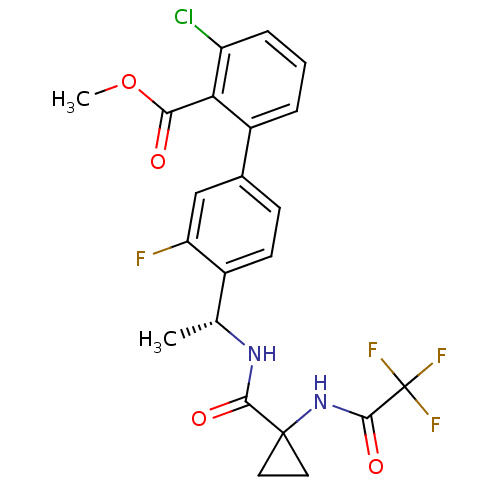
(3-Chloro-3'-fluoro-4'-((R)-1-{[1-(2,2,2-trifluoro-...)Show SMILES COC(=O)c1c(Cl)cccc1-c1ccc([C@@H](C)NC(=O)C2(CC2)NC(=O)C(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C22H19ClF4N2O4/c1-11(28-19(31)21(8-9-21)29-20(32)22(25,26)27)13-7-6-12(10-16(13)24)14-4-3-5-15(23)17(14)18(30)33-2/h3-7,10-11H,8-9H2,1-2H3,(H,28,31)(H,29,32)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
Bioorg Med Chem Lett 18: 5027-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.014
BindingDB Entry DOI: 10.7270/Q2NG4QDX |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50127438
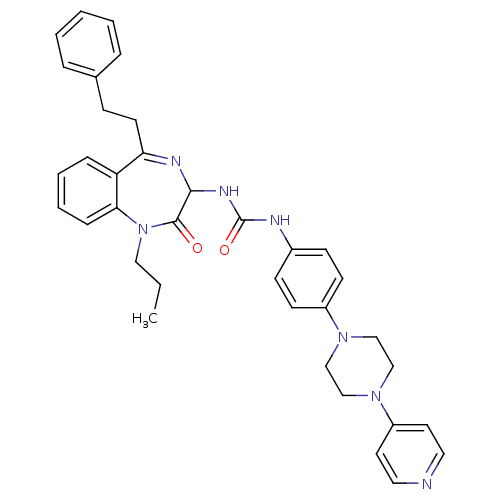
(1-(2-Oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-benzo...)Show SMILES CCCN1c2ccccc2C(CCc2ccccc2)=NC(NC(=O)Nc2ccc(cc2)N2CCN(CC2)c2ccncc2)C1=O |c:20| Show InChI InChI=1S/C36H39N7O2/c1-2-22-43-33-11-7-6-10-31(33)32(17-12-27-8-4-3-5-9-27)39-34(35(43)44)40-36(45)38-28-13-15-29(16-14-28)41-23-25-42(26-24-41)30-18-20-37-21-19-30/h3-11,13-16,18-21,34H,2,12,17,22-26H2,1H3,(H2,38,40,45) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human bradykinin B1 receptor |
Bioorg Med Chem Lett 18: 5027-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.014
BindingDB Entry DOI: 10.7270/Q2NG4QDX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260767
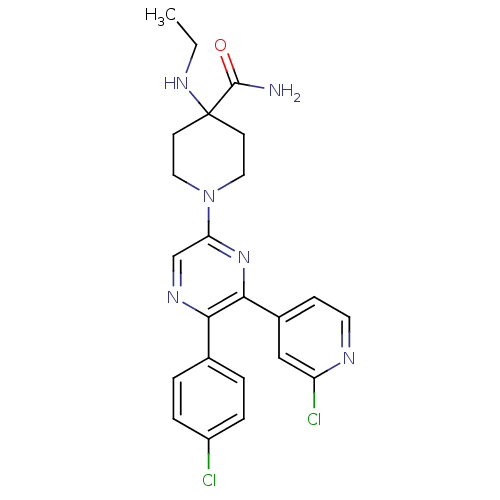
(1-(5-(4-chlorophenyl)-6-(2-chloropyridin-4-yl)pyra...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C23H24Cl2N6O/c1-2-29-23(22(26)32)8-11-31(12-9-23)19-14-28-20(15-3-5-17(24)6-4-15)21(30-19)16-7-10-27-18(25)13-16/h3-7,10,13-14,29H,2,8-9,11-12H2,1H3,(H2,26,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260805
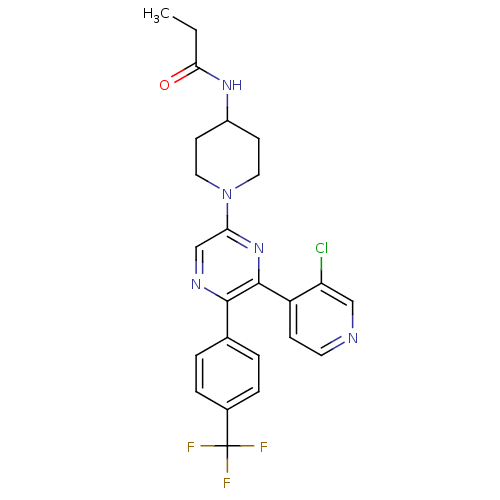
(CHEMBL524804 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CCC(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C24H23ClF3N5O/c1-2-21(34)31-17-8-11-33(12-9-17)20-14-30-22(15-3-5-16(6-4-15)24(26,27)28)23(32-20)18-7-10-29-13-19(18)25/h3-7,10,13-14,17H,2,8-9,11-12H2,1H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50260681
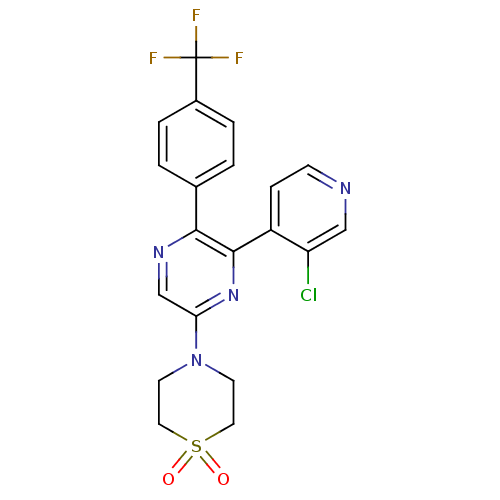
(4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...)Show SMILES FC(F)(F)c1ccc(cc1)-c1ncc(nc1-c1ccncc1Cl)N1CCS(=O)(=O)CC1 Show InChI InChI=1S/C20H16ClF3N4O2S/c21-16-11-25-6-5-15(16)19-18(13-1-3-14(4-2-13)20(22,23)24)26-12-17(27-19)28-7-9-31(29,30)10-8-28/h1-6,11-12H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260681

(4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...)Show SMILES FC(F)(F)c1ccc(cc1)-c1ncc(nc1-c1ccncc1Cl)N1CCS(=O)(=O)CC1 Show InChI InChI=1S/C20H16ClF3N4O2S/c21-16-11-25-6-5-15(16)19-18(13-1-3-14(4-2-13)20(22,23)24)26-12-17(27-19)28-7-9-31(29,30)10-8-28/h1-6,11-12H,7-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260768
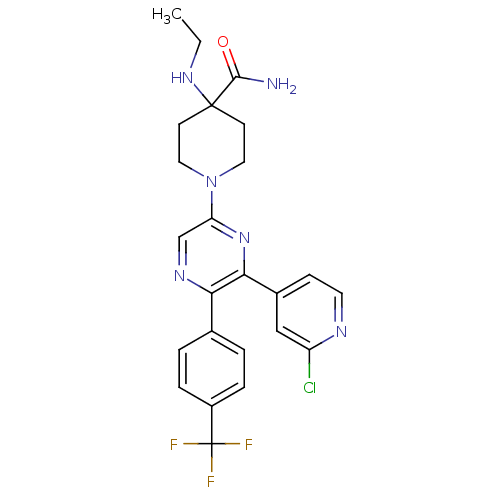
(1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C24H24ClF3N6O/c1-2-32-23(22(29)35)8-11-34(12-9-23)19-14-31-20(15-3-5-17(6-4-15)24(26,27)28)21(33-19)16-7-10-30-18(25)13-16/h3-7,10,13-14,32H,2,8-9,11-12H2,1H3,(H2,29,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448144
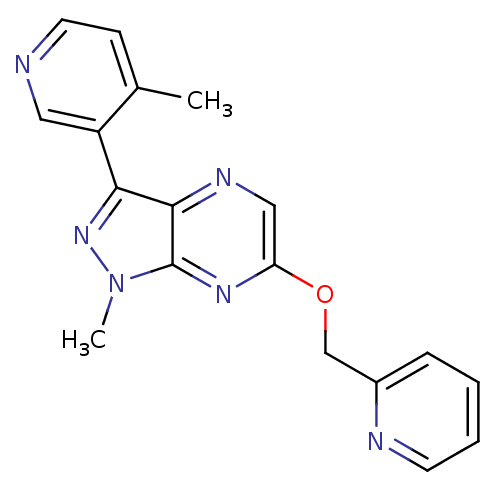
(CHEMBL3122212)Show InChI InChI=1S/C18H16N6O/c1-12-6-8-19-9-14(12)16-17-18(24(2)23-16)22-15(10-21-17)25-11-13-5-3-4-7-20-13/h3-10H,11H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260682
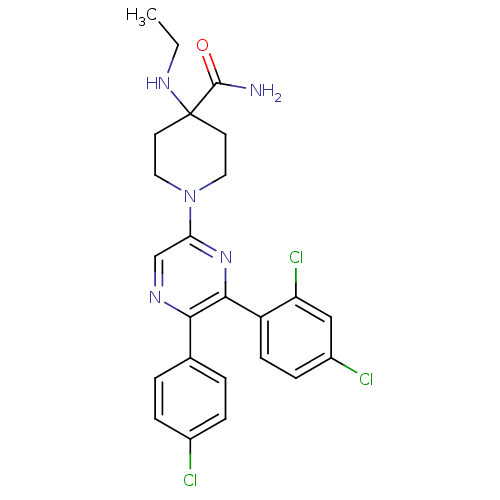
(1-(5-(4-chlorophenyl)-6-(2,4-dichlorophenyl)pyrazi...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccc(Cl)cc1Cl)C(N)=O Show InChI InChI=1S/C24H24Cl3N5O/c1-2-30-24(23(28)33)9-11-32(12-10-24)20-14-29-21(15-3-5-16(25)6-4-15)22(31-20)18-8-7-17(26)13-19(18)27/h3-8,13-14,30H,2,9-12H2,1H3,(H2,28,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(RAT) | BDBM50127438

(1-(2-Oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-benzo...)Show SMILES CCCN1c2ccccc2C(CCc2ccccc2)=NC(NC(=O)Nc2ccc(cc2)N2CCN(CC2)c2ccncc2)C1=O |c:20| Show InChI InChI=1S/C36H39N7O2/c1-2-22-43-33-11-7-6-10-31(33)32(17-12-27-8-4-3-5-9-27)39-34(35(43)44)40-36(45)38-28-13-15-29(16-14-28)41-23-25-42(26-24-41)30-18-20-37-21-19-30/h3-11,13-16,18-21,34H,2,12,17,22-26H2,1H3,(H2,38,40,45) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat bradykinin B1 receptor |
Bioorg Med Chem Lett 18: 5027-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.014
BindingDB Entry DOI: 10.7270/Q2NG4QDX |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50240887
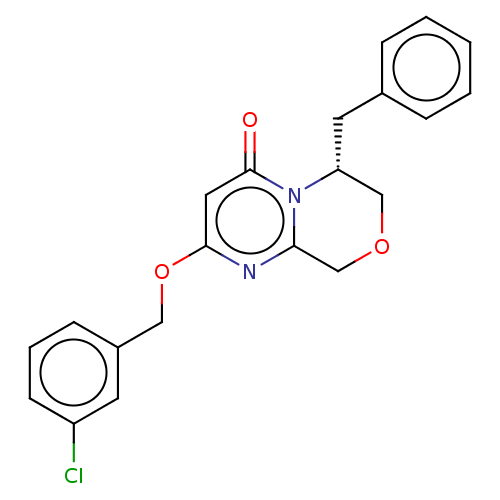
(CHEMBL4066731)Show SMILES Clc1cccc(COc2cc(=O)n3[C@H](Cc4ccccc4)COCc3n2)c1 |r| Show InChI InChI=1S/C21H19ClN2O3/c22-17-8-4-7-16(9-17)12-27-20-11-21(25)24-18(13-26-14-19(24)23-20)10-15-5-2-1-3-6-15/h1-9,11,18H,10,12-14H2/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting |
J Med Chem 60: 7764-7780 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00604
BindingDB Entry DOI: 10.7270/Q2DJ5HS8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260803
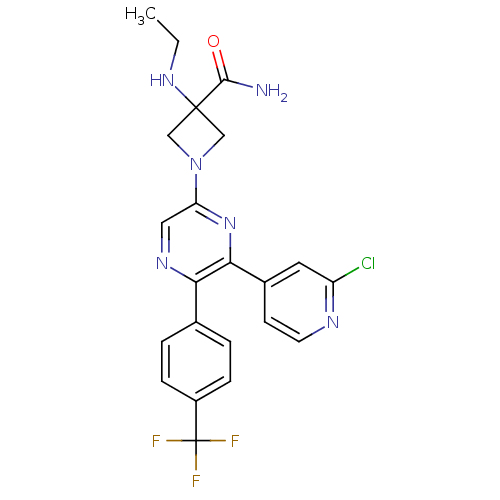
(1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...)Show SMILES CCNC1(CN(C1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C22H20ClF3N6O/c1-2-30-21(20(27)33)11-32(12-21)17-10-29-18(13-3-5-15(6-4-13)22(24,25)26)19(31-17)14-7-8-28-16(23)9-14/h3-10,30H,2,11-12H2,1H3,(H2,27,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448157
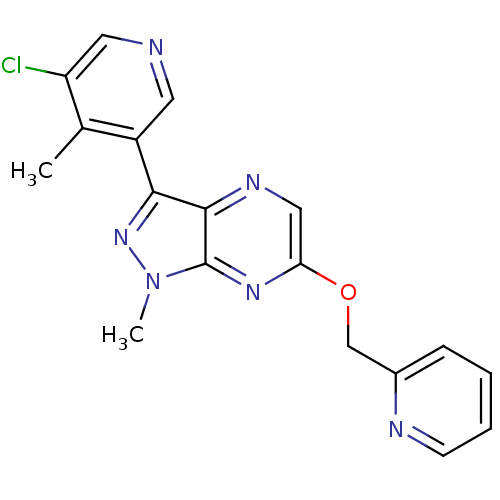
(CHEMBL3122215)Show InChI InChI=1S/C18H15ClN6O/c1-11-13(7-20-8-14(11)19)16-17-18(25(2)24-16)23-15(9-22-17)26-10-12-5-3-4-6-21-12/h3-9H,10H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50240900
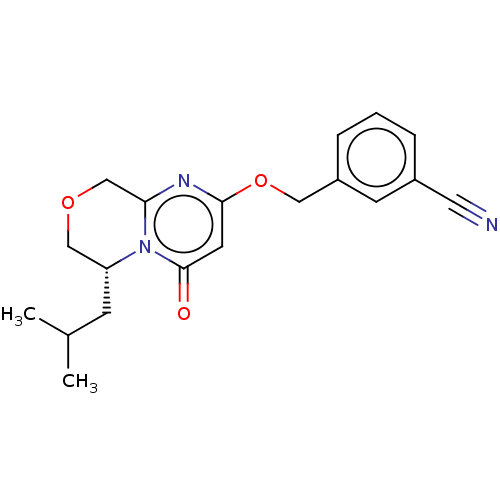
(CHEMBL4091620)Show SMILES CC(C)C[C@@H]1COCc2nc(OCc3cccc(c3)C#N)cc(=O)n12 |r| Show InChI InChI=1S/C19H21N3O3/c1-13(2)6-16-11-24-12-17-21-18(8-19(23)22(16)17)25-10-15-5-3-4-14(7-15)9-20/h3-5,7-8,13,16H,6,10-12H2,1-2H3/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting |
J Med Chem 60: 7764-7780 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00604
BindingDB Entry DOI: 10.7270/Q2DJ5HS8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122928

(5-{6-[5-Chloro-2-(3-methyl-isoxazol-5-ylmethoxy)-b...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cc(Cl)ccc3OCc3cc(C)no3)ncnc12 Show InChI InChI=1S/C23H24ClN7O6/c1-11-5-14(37-30-11)8-35-15-4-3-13(24)6-12(15)7-26-20-16-21(28-9-27-20)31(10-29-16)23-18(33)17(32)19(36-23)22(34)25-2/h3-6,9-10,17-19,23,32-33H,7-8H2,1-2H3,(H,25,34)(H,26,27,28)/t17-,18+,19-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260718

(1-(6-(2-chlorophenyl)-5-(4-chlorophenyl)pyrazin-2-...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(Cl)cc2)c(n1)-c1ccccc1Cl)C(N)=O Show InChI InChI=1S/C24H25Cl2N5O/c1-2-29-24(23(27)32)11-13-31(14-12-24)20-15-28-21(16-7-9-17(25)10-8-16)22(30-20)18-5-3-4-6-19(18)26/h3-10,15,29H,2,11-14H2,1H3,(H2,27,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260717
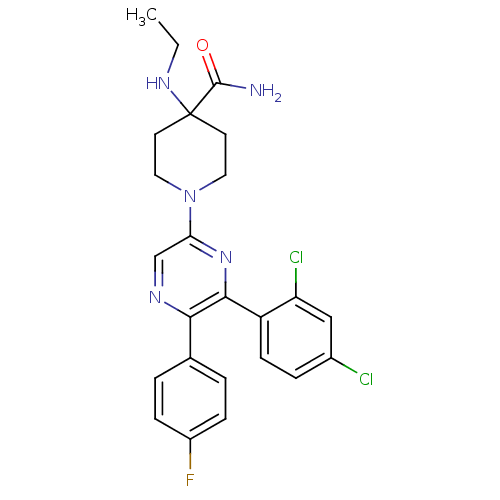
(1-(6-(2,4-dichlorophenyl)-5-(4-fluorophenyl)pyrazi...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(F)cc2)c(n1)-c1ccc(Cl)cc1Cl)C(N)=O Show InChI InChI=1S/C24H24Cl2FN5O/c1-2-30-24(23(28)33)9-11-32(12-10-24)20-14-29-21(15-3-6-17(27)7-4-15)22(31-20)18-8-5-16(25)13-19(18)26/h3-8,13-14,30H,2,9-12H2,1H3,(H2,28,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260804
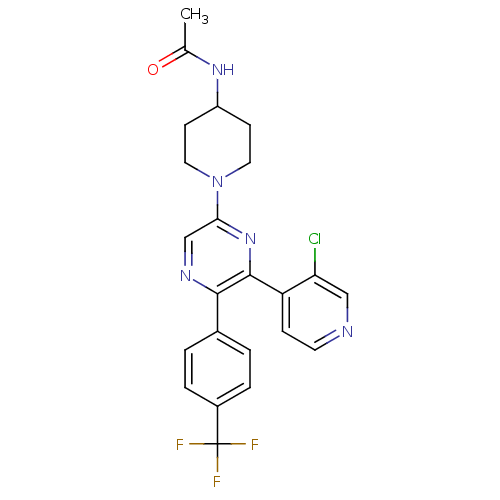
(CHEMBL497556 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...)Show SMILES CC(=O)NC1CCN(CC1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccncc1Cl Show InChI InChI=1S/C23H21ClF3N5O/c1-14(33)30-17-7-10-32(11-8-17)20-13-29-21(15-2-4-16(5-3-15)23(25,26)27)22(31-20)18-6-9-28-12-19(18)24/h2-6,9,12-13,17H,7-8,10-11H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50260803

(1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...)Show SMILES CCNC1(CN(C1)c1cnc(-c2ccc(cc2)C(F)(F)F)c(n1)-c1ccnc(Cl)c1)C(N)=O Show InChI InChI=1S/C22H20ClF3N6O/c1-2-30-21(20(27)33)11-32(12-21)17-10-29-18(13-3-5-15(6-4-13)22(24,25)26)19(31-17)14-7-8-28-16(23)9-14/h3-10,30H,2,11-12H2,1H3,(H2,27,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448161
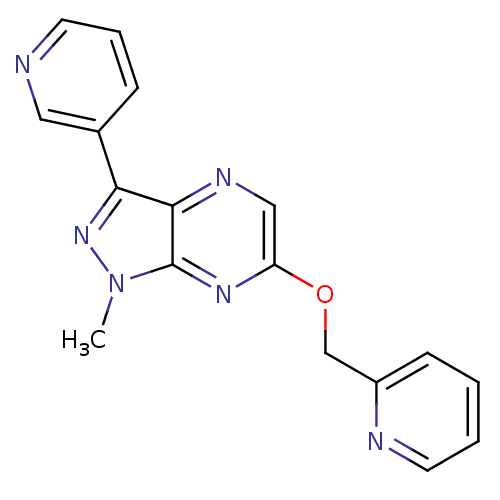
(CHEMBL3122210)Show InChI InChI=1S/C17H14N6O/c1-23-17-16(15(22-23)12-5-4-7-18-9-12)20-10-14(21-17)24-11-13-6-2-3-8-19-13/h2-10H,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182312
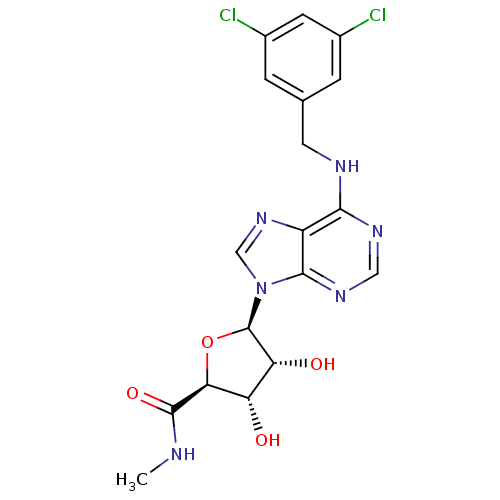
((2S,3S,4R,5R)-5-(6-(3,5-dichlorobenzylamino)-9H-pu...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cc(Cl)cc(Cl)c3)ncnc12 Show InChI InChI=1S/C18H18Cl2N6O4/c1-21-17(29)14-12(27)13(28)18(30-14)26-7-25-11-15(23-6-24-16(11)26)22-5-8-2-9(19)4-10(20)3-8/h2-4,6-7,12-14,18,27-28H,5H2,1H3,(H,21,29)(H,22,23,24)/t12-,13+,14-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448159
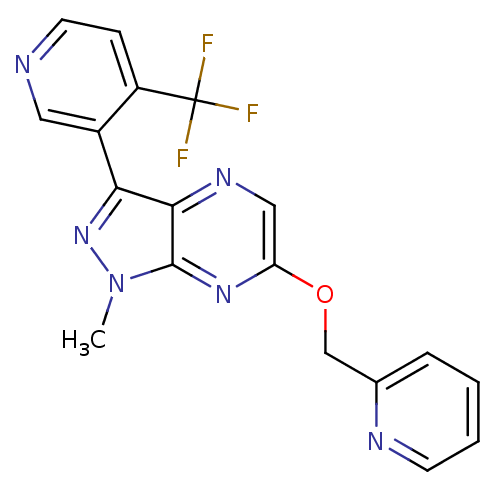
(CHEMBL3122213)Show SMILES Cn1nc(-c2cnccc2C(F)(F)F)c2ncc(OCc3ccccn3)nc12 Show InChI InChI=1S/C18H13F3N6O/c1-27-17-16(15(26-27)12-8-22-7-5-13(12)18(19,20)21)24-9-14(25-17)28-10-11-4-2-3-6-23-11/h2-9H,10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50260720
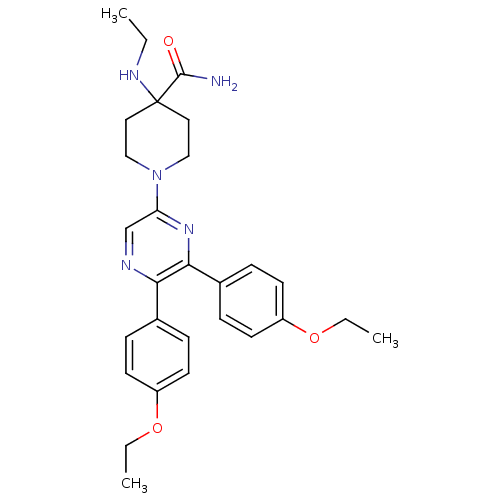
(1-(5,6-bis(4-ethoxyphenyl)pyrazin-2-yl)-4-(ethylam...)Show SMILES CCNC1(CCN(CC1)c1cnc(-c2ccc(OCC)cc2)c(n1)-c1ccc(OCC)cc1)C(N)=O Show InChI InChI=1S/C28H35N5O3/c1-4-31-28(27(29)34)15-17-33(18-16-28)24-19-30-25(20-7-11-22(12-8-20)35-5-2)26(32-24)21-9-13-23(14-10-21)36-6-3/h7-14,19,31H,4-6,15-18H2,1-3H3,(H2,29,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 18: 3376-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.022
BindingDB Entry DOI: 10.7270/Q2QR4Z0T |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50096326
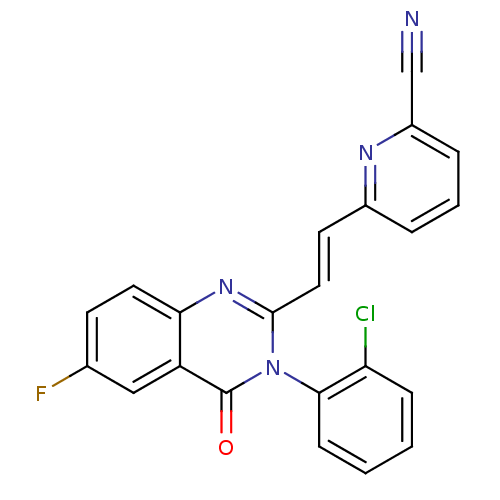
(6-{(E)-2-[3-(2-Chloro-phenyl)-6-fluoro-4-oxo-3,4-d...)Show SMILES Fc1ccc2nc(\C=C\c3cccc(n3)C#N)n(-c3ccccc3Cl)c(=O)c2c1 |(1.2,-4.46,;2.55,-5.21,;2.55,-6.75,;3.88,-7.52,;5.21,-6.75,;6.56,-7.52,;7.91,-6.73,;9.25,-7.5,;9.25,-9.04,;10.6,-9.81,;10.6,-11.35,;11.93,-12.12,;13.26,-11.35,;13.26,-9.78,;11.92,-9.04,;14.58,-8.99,;15.91,-8.22,;7.91,-5.18,;9.25,-4.41,;9.22,-2.88,;10.53,-2.1,;11.88,-2.85,;11.91,-4.39,;10.57,-5.18,;10.57,-6.72,;6.56,-4.41,;6.54,-2.87,;5.21,-5.19,;3.88,-4.42,)| Show InChI InChI=1S/C22H12ClFN4O/c23-18-6-1-2-7-20(18)28-21(11-9-15-4-3-5-16(13-25)26-15)27-19-10-8-14(24)12-17(19)22(28)29/h1-12H/b11-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by PDSP Ki Database
| |
Mol Pharmacol 58: 1310-7 (2000)
Article DOI: 10.1124/mol.58.6.1310
BindingDB Entry DOI: 10.7270/Q24X56B1 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50240888
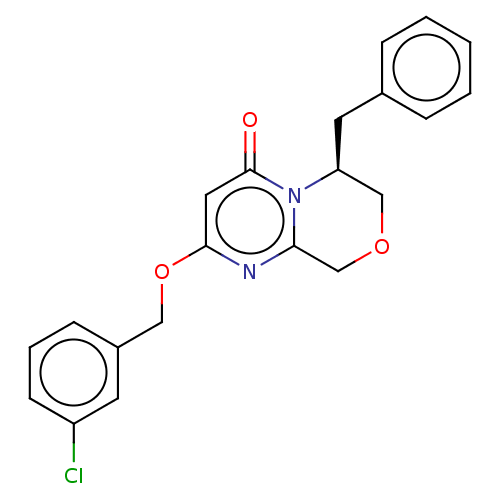
(CHEMBL4094256)Show SMILES Clc1cccc(COc2cc(=O)n3[C@@H](Cc4ccccc4)COCc3n2)c1 |r| Show InChI InChI=1S/C21H19ClN2O3/c22-17-8-4-7-16(9-17)12-27-20-11-21(25)24-18(13-26-14-19(24)23-20)10-15-5-2-1-3-6-15/h1-9,11,18H,10,12-14H2/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting |
J Med Chem 60: 7764-7780 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00604
BindingDB Entry DOI: 10.7270/Q2DJ5HS8 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50240881
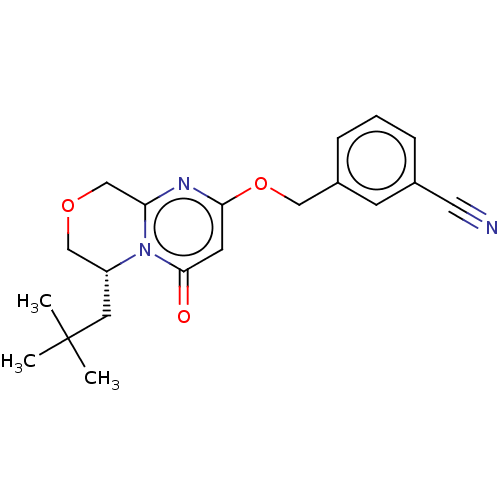
(CHEMBL4090712)Show SMILES CC(C)(C)C[C@@H]1COCc2nc(OCc3cccc(c3)C#N)cc(=O)n12 |r| Show InChI InChI=1S/C20H23N3O3/c1-20(2,3)9-16-12-25-13-17-22-18(8-19(24)23(16)17)26-11-15-6-4-5-14(7-15)10-21/h4-8,16H,9,11-13H2,1-3H3/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting |
J Med Chem 60: 7764-7780 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00604
BindingDB Entry DOI: 10.7270/Q2DJ5HS8 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50240885
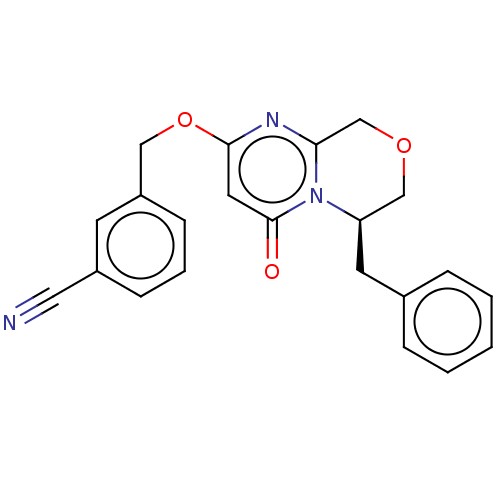
(CHEMBL4064010)Show SMILES O=c1cc(OCc2cccc(c2)C#N)nc2COC[C@@H](Cc3ccccc3)n12 |r| Show InChI InChI=1S/C22H19N3O3/c23-12-17-7-4-8-18(9-17)13-28-21-11-22(26)25-19(14-27-15-20(25)24-21)10-16-5-2-1-3-6-16/h1-9,11,19H,10,13-15H2/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting |
J Med Chem 60: 7764-7780 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00604
BindingDB Entry DOI: 10.7270/Q2DJ5HS8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
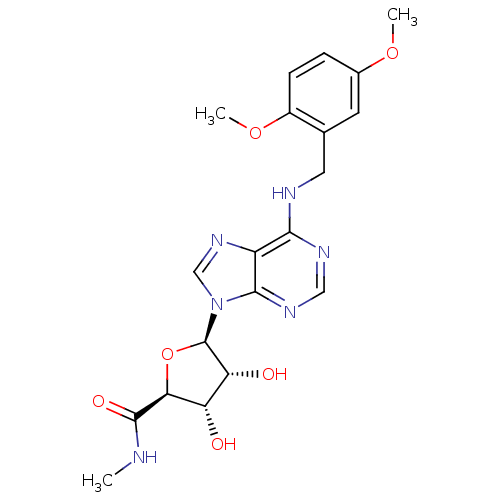
(Homo sapiens (Human)) | BDBM50182322
((2S,3S,4R,5R)-5-(6-(2,5-dimethoxybenzylamino)-9H-p...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cc(OC)ccc3OC)ncnc12 Show InChI InChI=1S/C20H24N6O6/c1-21-19(29)16-14(27)15(28)20(32-16)26-9-25-13-17(23-8-24-18(13)26)22-7-10-6-11(30-2)4-5-12(10)31-3/h4-6,8-9,14-16,20,27-28H,7H2,1-3H3,(H,21,29)(H,22,23,24)/t14-,15+,16-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50240890
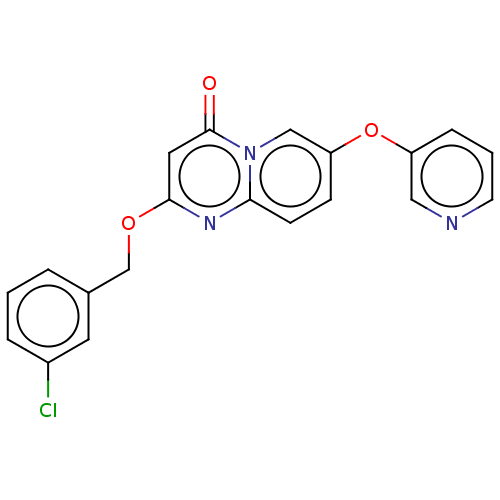
(CHEMBL4078647)Show SMILES Clc1cccc(COc2cc(=O)n3cc(Oc4cccnc4)ccc3n2)c1 Show InChI InChI=1S/C20H14ClN3O3/c21-15-4-1-3-14(9-15)13-26-19-10-20(25)24-12-17(6-7-18(24)23-19)27-16-5-2-8-22-11-16/h1-12H,13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting |
J Med Chem 60: 7764-7780 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00604
BindingDB Entry DOI: 10.7270/Q2DJ5HS8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50118812
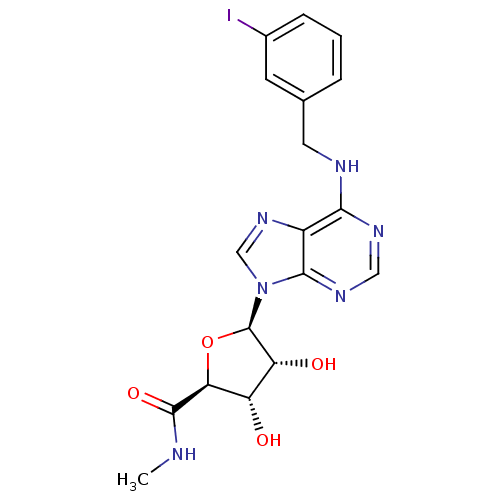
((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122927
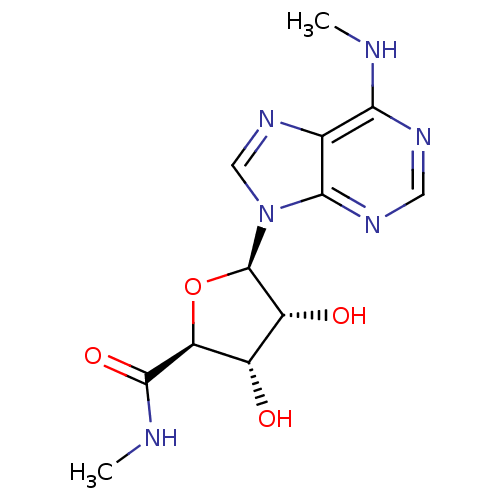
((2S,3S,4R,5R)-3,4-dihydroxy-N-methyl-5-(6-(methyla...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-13-9-5-10(16-3-15-9)18(4-17-5)12-7(20)6(19)8(22-12)11(21)14-2/h3-4,6-8,12,19-20H,1-2H3,(H,14,21)(H,13,15,16)/t6-,7+,8-,12+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122927

((2S,3S,4R,5R)-3,4-dihydroxy-N-methyl-5-(6-(methyla...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-13-9-5-10(16-3-15-9)18(4-17-5)12-7(20)6(19)8(22-12)11(21)14-2/h3-4,6-8,12,19-20H,1-2H3,(H,14,21)(H,13,15,16)/t6-,7+,8-,12+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448149
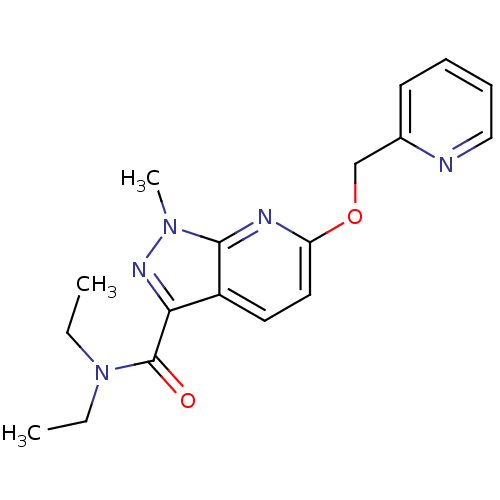
(CHEMBL3122224)Show InChI InChI=1S/C18H21N5O2/c1-4-23(5-2)18(24)16-14-9-10-15(20-17(14)22(3)21-16)25-12-13-8-6-7-11-19-13/h6-11H,4-5,12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448163
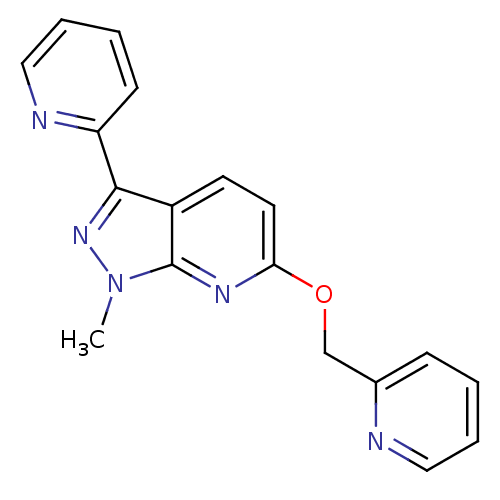
(CHEMBL3122208)Show InChI InChI=1S/C18H15N5O/c1-23-18-14(17(22-23)15-7-3-5-11-20-15)8-9-16(21-18)24-12-13-6-2-4-10-19-13/h2-11H,12H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50240884
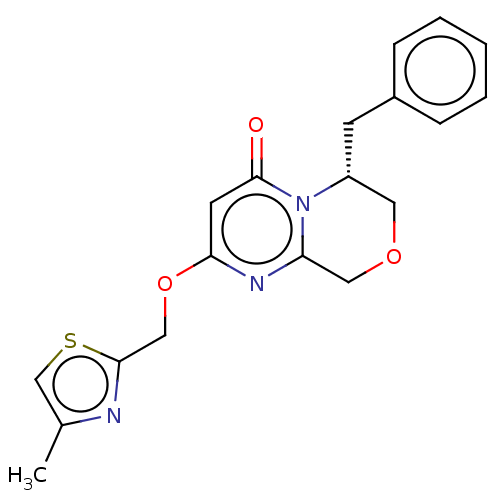
(CHEMBL4085572)Show SMILES Cc1csc(COc2cc(=O)n3[C@H](Cc4ccccc4)COCc3n2)n1 |r| Show InChI InChI=1S/C19H19N3O3S/c1-13-12-26-18(20-13)11-25-17-8-19(23)22-15(9-24-10-16(22)21-17)7-14-5-3-2-4-6-14/h2-6,8,12,15H,7,9-11H2,1H3/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting |
J Med Chem 60: 7764-7780 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00604
BindingDB Entry DOI: 10.7270/Q2DJ5HS8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50122926
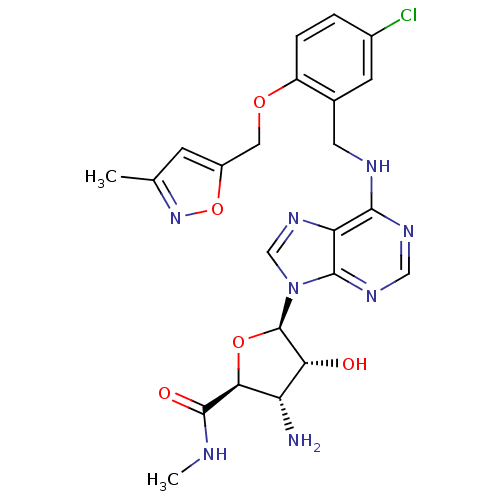
(3-Amino-5-{6-[5-chloro-2-(3-methyl-isoxazol-5-ylme...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OCc3cc(C)no3)ncnc12 Show InChI InChI=1S/C23H25ClN8O5/c1-11-5-14(37-31-11)8-35-15-4-3-13(24)6-12(15)7-27-20-17-21(29-9-28-20)32(10-30-17)23-18(33)16(25)19(36-23)22(34)26-2/h3-6,9-10,16,18-19,23,33H,7-8,25H2,1-2H3,(H,26,34)(H,27,28,29)/t16-,18+,19-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity using [125I]-ABA against human Adenosine A3 receptor |
J Med Chem 46: 353-5 (2003)
Article DOI: 10.1021/jm0255724
BindingDB Entry DOI: 10.7270/Q2416WDM |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50240882
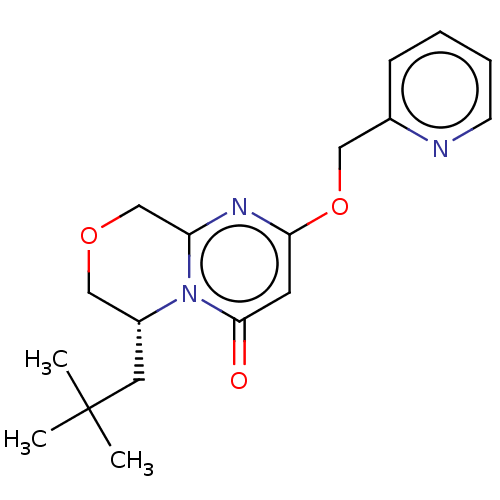
(CHEMBL4063837)Show SMILES CC(C)(C)C[C@@H]1COCc2nc(OCc3ccccn3)cc(=O)n12 |r| Show InChI InChI=1S/C18H23N3O3/c1-18(2,3)9-14-11-23-12-15-20-16(8-17(22)21(14)15)24-10-13-6-4-5-7-19-13/h4-8,14H,9-12H2,1-3H3/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting |
J Med Chem 60: 7764-7780 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00604
BindingDB Entry DOI: 10.7270/Q2DJ5HS8 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448162

(CHEMBL3122209)Show InChI InChI=1S/C18H15N5O/c1-23-18-15(17(22-23)13-5-4-9-19-11-13)7-8-16(21-18)24-12-14-6-2-3-10-20-14/h2-11H,12H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448158
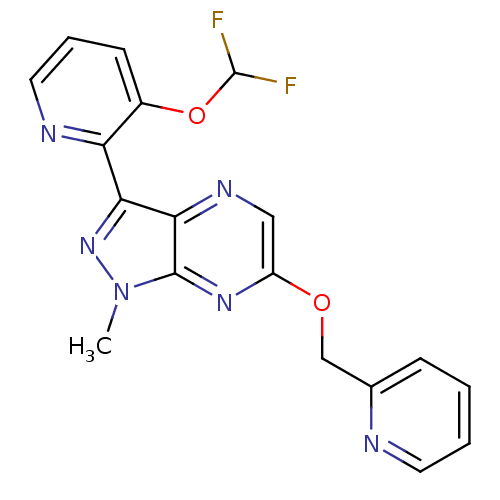
(CHEMBL3122214)Show SMILES Cn1nc(-c2ncccc2OC(F)F)c2ncc(OCc3ccccn3)nc12 Show InChI InChI=1S/C18H14F2N6O2/c1-26-17-16(15(25-26)14-12(28-18(19)20)6-4-8-22-14)23-9-13(24-17)27-10-11-5-2-3-7-21-11/h2-9,18H,10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448150
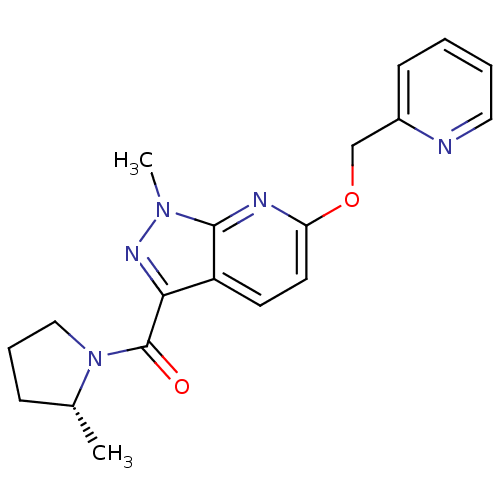
(CHEMBL3122225)Show SMILES C[C@@H]1CCCN1C(=O)c1nn(C)c2nc(OCc3ccccn3)ccc12 |r| Show InChI InChI=1S/C19H21N5O2/c1-13-6-5-11-24(13)19(25)17-15-8-9-16(21-18(15)23(2)22-17)26-12-14-7-3-4-10-20-14/h3-4,7-10,13H,5-6,11-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50182326

((2S,3S,4R,5R)-5-(6-(2-(2-amino-2-oxoethoxy)-5-chlo...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1N)n1cnc2c(NCc3cc(Cl)ccc3OCC(N)=O)ncnc12 Show InChI InChI=1S/C20H23ClN8O5/c1-24-19(32)16-13(23)15(31)20(34-16)29-8-28-14-17(26-7-27-18(14)29)25-5-9-4-10(21)2-3-11(9)33-6-12(22)30/h2-4,7-8,13,15-16,20,31H,5-6,23H2,1H3,(H2,22,30)(H,24,32)(H,25,26,27)/t13-,15+,16-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem Lett 16: 2525-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.088
BindingDB Entry DOI: 10.7270/Q2J38S4Z |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50240879
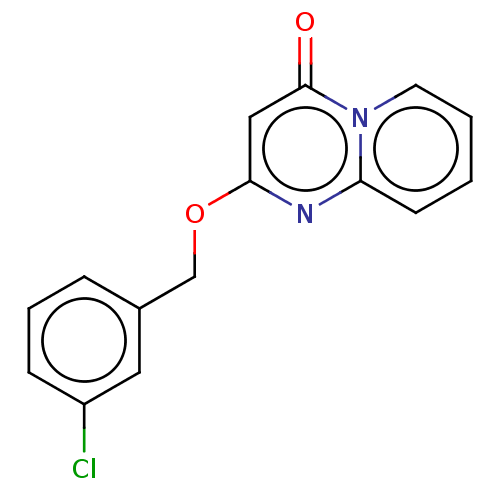
(CHEMBL4089073)Show InChI InChI=1S/C15H11ClN2O2/c16-12-5-3-4-11(8-12)10-20-14-9-15(19)18-7-2-1-6-13(18)17-14/h1-9H,10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting |
J Med Chem 60: 7764-7780 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00604
BindingDB Entry DOI: 10.7270/Q2DJ5HS8 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448154
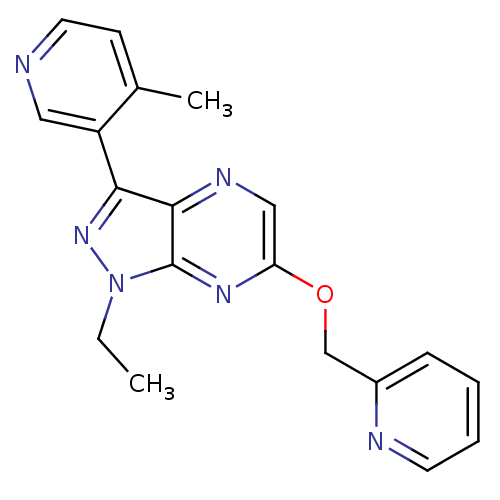
(CHEMBL3122218)Show InChI InChI=1S/C19H18N6O/c1-3-25-19-18(17(24-25)15-10-20-9-7-13(15)2)22-11-16(23-19)26-12-14-6-4-5-8-21-14/h4-11H,3,12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448148
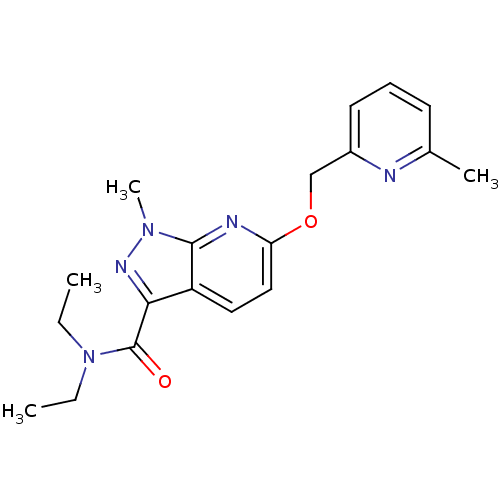
(CHEMBL3122223)Show SMILES CCN(CC)C(=O)c1nn(C)c2nc(OCc3cccc(C)n3)ccc12 Show InChI InChI=1S/C19H23N5O2/c1-5-24(6-2)19(25)17-15-10-11-16(21-18(15)23(4)22-17)26-12-14-9-7-8-13(3)20-14/h7-11H,5-6,12H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50448155
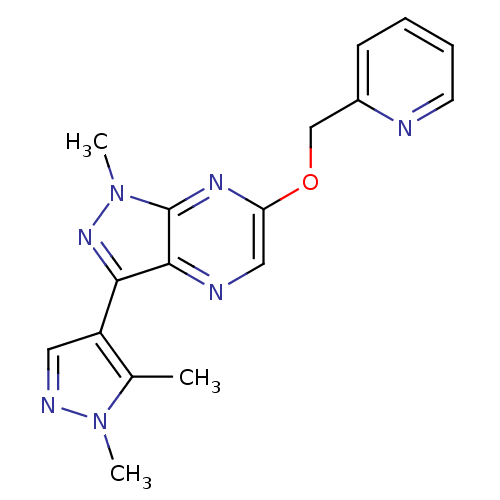
(CHEMBL3122217)Show InChI InChI=1S/C17H17N7O/c1-11-13(8-20-23(11)2)15-16-17(24(3)22-15)21-14(9-19-16)25-10-12-6-4-5-7-18-12/h4-9H,10H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis |
J Med Chem 57: 861-77 (2014)
Article DOI: 10.1021/jm401622k
BindingDB Entry DOI: 10.7270/Q27P90WR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data