 Found 4978 hits with Last Name = 'cheng' and Initial = 'ac'
Found 4978 hits with Last Name = 'cheng' and Initial = 'ac' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514220
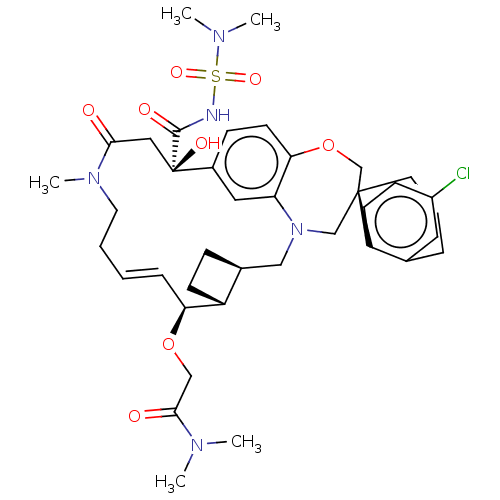
(CHEMBL4535151 | US11274105, Example 188)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCC(=O)N(C)C)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:15| Show InChI InChI=1S/C39H52ClN5O8S/c1-42(2)36(47)23-52-33-10-6-7-18-44(5)35(46)21-39(49,37(48)41-54(50,51)43(3)4)28-12-16-34-32(20-28)45(22-27-11-14-30(27)33)24-38(25-53-34)17-8-9-26-19-29(40)13-15-31(26)38/h6,10,12-13,15-16,19-20,27,30,33,49H,7-9,11,14,17-18,21-25H2,1-5H3,(H,41,48)/b10-6+/t27-,30+,33-,38-,39+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514203
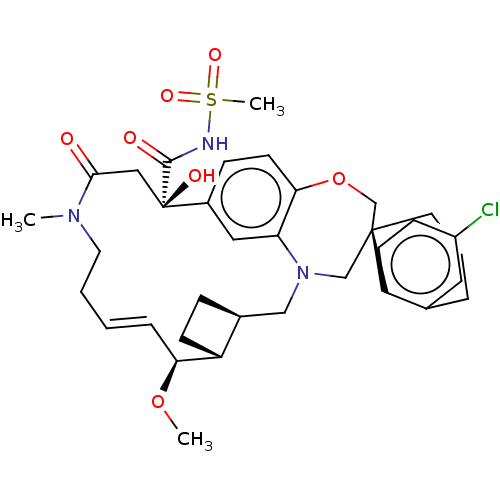
(CHEMBL4593361 | US11274105, Example 6)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(C)(=O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C35H44ClN3O7S/c1-38-16-5-4-8-30(45-2)27-12-9-24(27)20-39-21-34(15-6-7-23-17-26(36)11-13-28(23)34)22-46-31-14-10-25(18-29(31)39)35(42,19-32(38)40)33(41)37-47(3,43)44/h4,8,10-11,13-14,17-18,24,27,30,42H,5-7,9,12,15-16,19-22H2,1-3H3,(H,37,41)/b8-4+/t24-,27+,30-,34-,35+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514222
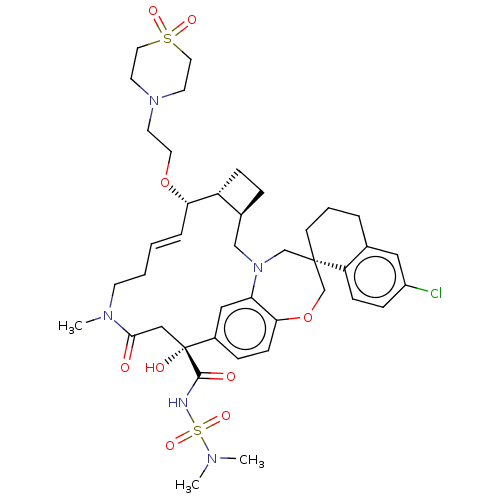
(CHEMBL4580244 | US11274105, Example 193)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCS(=O)(=O)CC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:20| Show InChI InChI=1S/C41H56ClN5O9S2/c1-44(2)58(53,54)43-39(49)41(50)25-38(48)45(3)16-5-4-8-36(55-20-17-46-18-21-57(51,52)22-19-46)33-12-9-30(33)26-47-27-40(28-56-37-14-10-31(41)24-35(37)47)15-6-7-29-23-32(42)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,50H,5-7,9,12,15-22,25-28H2,1-3H3,(H,43,49)/b8-4+/t30-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514196
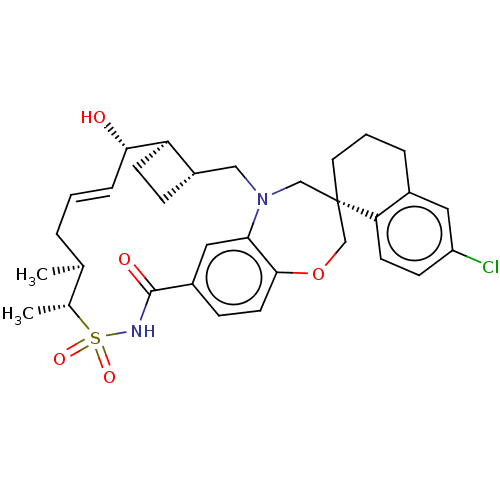
(CHEMBL4476472)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\C[C@H](C)[C@@H](C)S(=O)(=O)NC(=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C32H39ClN2O5S/c1-20-5-3-7-29(36)26-11-8-24(26)17-35-18-32(14-4-6-22-15-25(33)10-12-27(22)32)19-40-30-13-9-23(16-28(30)35)31(37)34-41(38,39)21(20)2/h3,7,9-10,12-13,15-16,20-21,24,26,29,36H,4-6,8,11,14,17-19H2,1-2H3,(H,34,37)/b7-3+/t20-,21+,24-,26+,29-,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514202

(CHEMBL4446369 | US11274105, Example 179)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(F)(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C40H52ClF2N5O7S/c1-45(2)56(52,53)44-37(50)40(51)21-36(49)46(3)16-5-4-8-34(54-18-17-47-24-39(42,43)25-47)31-12-9-28(31)22-48-23-38(26-55-35-14-10-29(40)20-33(35)48)15-6-7-27-19-30(41)11-13-32(27)38/h4,8,10-11,13-14,19-20,28,31,34,51H,5-7,9,12,15-18,21-26H2,1-3H3,(H,44,50)/b8-4+/t28-,31+,34-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514199
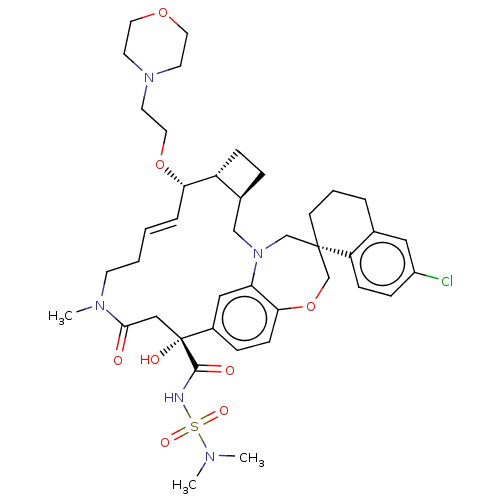
(CHEMBL4553660 | US11274105, Example 182)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCOCC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C41H56ClN5O8S/c1-44(2)56(51,52)43-39(49)41(50)25-38(48)45(3)16-5-4-8-36(54-22-19-46-17-20-53-21-18-46)33-12-9-30(33)26-47-27-40(28-55-37-14-10-31(41)24-35(37)47)15-6-7-29-23-32(42)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,50H,5-7,9,12,15-22,25-28H2,1-3H3,(H,43,49)/b8-4+/t30-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514200
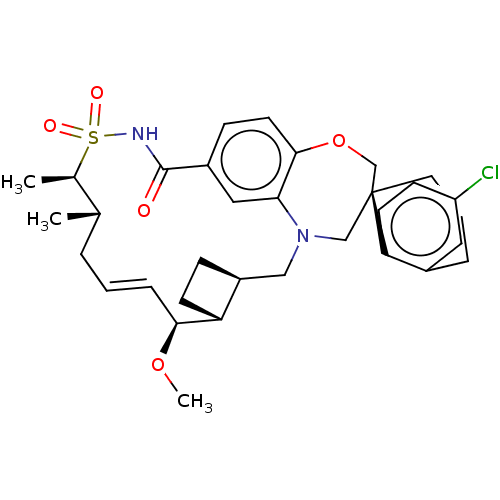
(CHEMBL4446378 | US10703733, Comparative Example 1)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\C[C@H](C)[C@@H](C)S(=O)(=O)NC(=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C33H41ClN2O5S/c1-21-6-4-8-30(40-3)27-12-9-25(27)18-36-19-33(15-5-7-23-16-26(34)11-13-28(23)33)20-41-31-14-10-24(17-29(31)36)32(37)35-42(38,39)22(21)2/h4,8,10-11,13-14,16-17,21-22,25,27,30H,5-7,9,12,15,18-20H2,1-3H3,(H,35,37)/b8-4+/t21-,22+,25-,27+,30-,33-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514215
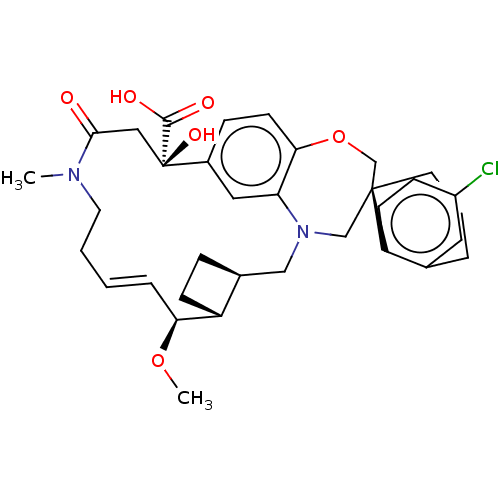
(CHEMBL4577379 | US11274105, Example 4)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-3-7-29(42-2)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(41,32(39)40)18-31(36)38/h3,7,9-10,12-13,16-17,23,26,29,41H,4-6,8,11,14-15,18-21H2,1-2H3,(H,39,40)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514218
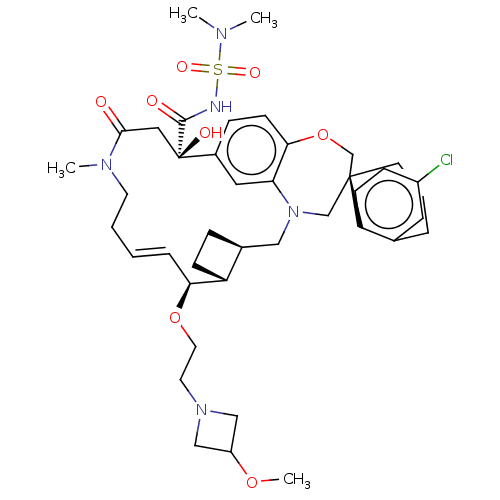
(CHEMBL4539543 | US11274105, Example 197)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(C1)OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C41H56ClN5O8S/c1-44(2)56(51,52)43-39(49)41(50)22-38(48)45(3)17-6-5-9-36(54-19-18-46-24-32(25-46)53-4)33-13-10-29(33)23-47-26-40(27-55-37-15-11-30(41)21-35(37)47)16-7-8-28-20-31(42)12-14-34(28)40/h5,9,11-12,14-15,20-21,29,32-33,36,50H,6-8,10,13,16-19,22-27H2,1-4H3,(H,43,49)/b9-5+/t29-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514201

(CHEMBL4547370 | US11274105, Example 191)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCOCC(F)F)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:16| Show InChI InChI=1S/C39H51ClF2N4O8S/c1-44(2)55(50,51)43-37(48)39(49)21-36(47)45(3)16-5-4-8-33(53-18-17-52-23-35(41)42)30-12-9-27(30)22-46-24-38(25-54-34-14-10-28(39)20-32(34)46)15-6-7-26-19-29(40)11-13-31(26)38/h4,8,10-11,13-14,19-20,27,30,33,35,49H,5-7,9,12,15-18,21-25H2,1-3H3,(H,43,48)/b8-4+/t27-,30+,33-,38-,39+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514219

(CHEMBL4438074 | US11274105, Example 181)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:17| Show InChI InChI=1S/C40H53ClFN5O7S/c1-44(2)55(51,52)43-38(49)40(50)21-37(48)45(3)16-5-4-8-35(53-18-17-46-23-31(42)24-46)32-12-9-28(32)22-47-25-39(26-54-36-14-10-29(40)20-34(36)47)15-6-7-27-19-30(41)11-13-33(27)39/h4,8,10-11,13-14,19-20,28,31-32,35,50H,5-7,9,12,15-18,21-26H2,1-3H3,(H,43,49)/b8-4+/t28-,32+,35-,39-,40+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514214

(CHEMBL4542646 | US11274105, Example 41)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C33H39ClN2O6/c1-35-14-3-2-6-28(37)25-10-7-22(25)18-36-19-32(13-4-5-21-15-24(34)9-11-26(21)32)20-42-29-12-8-23(16-27(29)36)33(41,31(39)40)17-30(35)38/h2,6,8-9,11-12,15-16,22,25,28,37,41H,3-5,7,10,13-14,17-20H2,1H3,(H,39,40)/b6-2+/t22-,25+,28-,32-,33+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514216
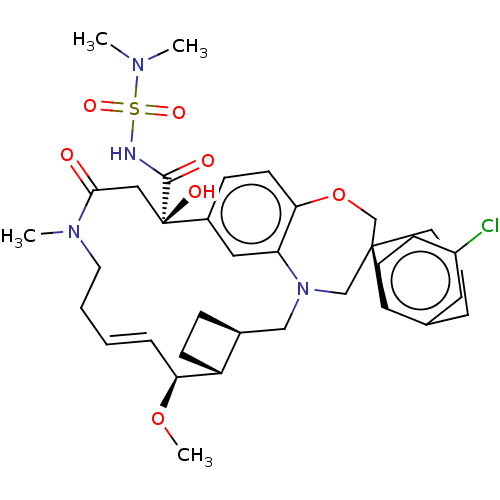
(CHEMBL4528051 | US11274105, Example 5)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C36H47ClN4O7S/c1-39(2)49(45,46)38-34(43)36(44)20-33(42)40(3)17-6-5-9-31(47-4)28-13-10-25(28)21-41-22-35(23-48-32-15-11-26(36)19-30(32)41)16-7-8-24-18-27(37)12-14-29(24)35/h5,9,11-12,14-15,18-19,25,28,31,44H,6-8,10,13,16-17,20-23H2,1-4H3,(H,38,43)/b9-5+/t25-,28+,31-,35-,36+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514206
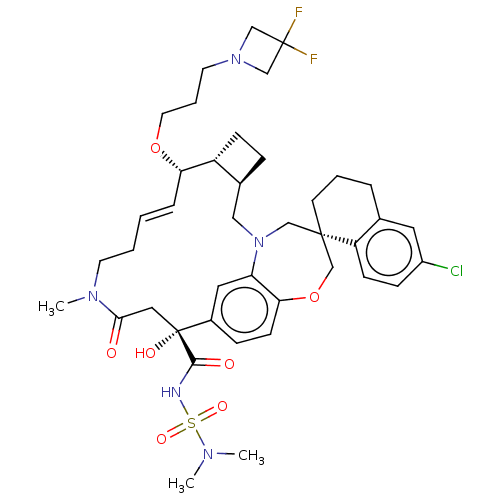
(CHEMBL4588330 | US11274105, Example 187)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCCN1CC(F)(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:19| Show InChI InChI=1S/C41H54ClF2N5O7S/c1-46(2)57(53,54)45-38(51)41(52)22-37(50)47(3)17-5-4-9-35(55-19-7-18-48-25-40(43,44)26-48)32-13-10-29(32)23-49-24-39(27-56-36-15-11-30(41)21-34(36)49)16-6-8-28-20-31(42)12-14-33(28)39/h4,9,11-12,14-15,20-21,29,32,35,52H,5-8,10,13,16-19,22-27H2,1-3H3,(H,45,51)/b9-4+/t29-,32+,35-,39-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514204
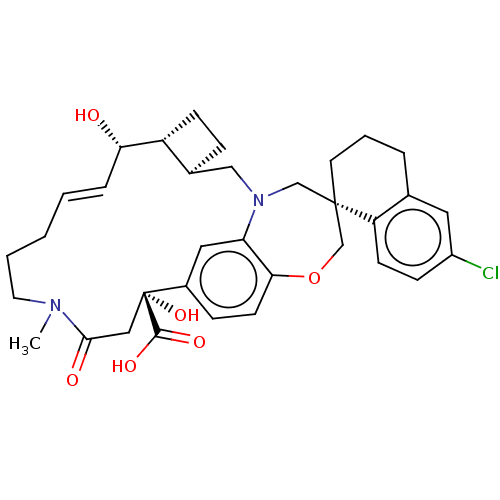
(CHEMBL4437832)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-2-3-7-29(38)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(42,32(40)41)18-31(36)39/h3,7,9-10,12-13,16-17,23,26,29,38,42H,2,4-6,8,11,14-15,18-21H2,1H3,(H,40,41)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514217

(CHEMBL4452794 | US11274105, Example 196)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCC(F)(F)CC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:20| Show InChI InChI=1S/C42H56ClF2N5O7S/c1-47(2)58(54,55)46-39(52)42(53)25-38(51)48(3)18-5-4-8-36(56-22-21-49-19-16-41(44,45)17-20-49)33-12-9-30(33)26-50-27-40(28-57-37-14-10-31(42)24-35(37)50)15-6-7-29-23-32(43)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,53H,5-7,9,12,15-22,25-28H2,1-3H3,(H,46,52)/b8-4+/t30-,33+,36-,40-,42+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514208
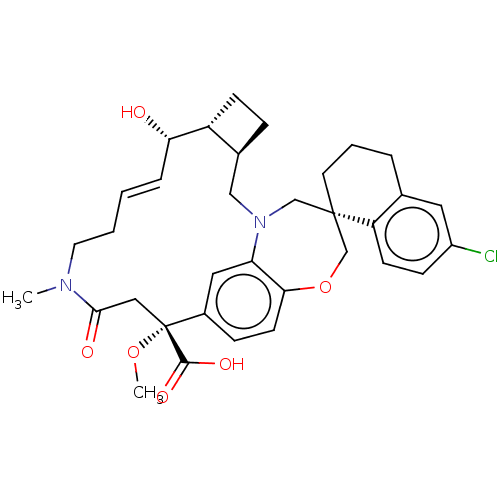
(CHEMBL4469850 | US11274105, Example 61)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCN(C)C(=O)C[C@](OC)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-3-7-29(38)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(42-2,32(40)41)18-31(36)39/h3,7,9-10,12-13,16-17,23,26,29,38H,4-6,8,11,14-15,18-21H2,1-2H3,(H,40,41)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514207

(CHEMBL4562159)Show SMILES [H][C@@]12CC[C@@]1([H])[C@H](CCCCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1)OC |r| Show InChI InChI=1S/C36H49ClN4O7S/c1-39(2)49(45,46)38-34(43)36(44)20-33(42)40(3)17-6-5-9-31(47-4)28-13-10-25(28)21-41-22-35(23-48-32-15-11-26(36)19-30(32)41)16-7-8-24-18-27(37)12-14-29(24)35/h11-12,14-15,18-19,25,28,31,44H,5-10,13,16-17,20-23H2,1-4H3,(H,38,43)/t25-,28+,31-,35-,36+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514197
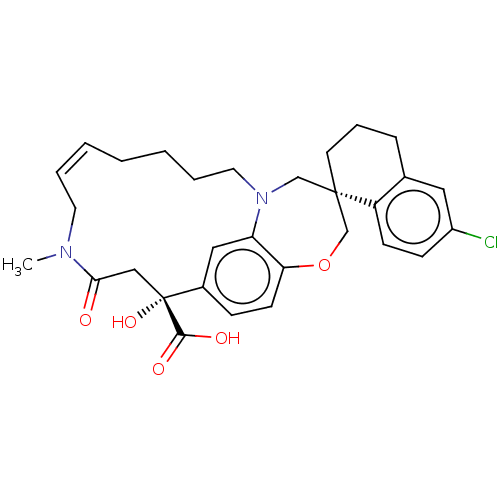
(CHEMBL4561691 | US11274105, Example 63)Show SMILES CN1C\C=C\CCCCN2C[C@@]3(CCCc4cc(Cl)ccc34)COc3ccc(cc23)[C@](O)(CC1=O)C(O)=O |r,t:3| Show InChI InChI=1S/C30H35ClN2O5/c1-32-14-5-3-2-4-6-15-33-19-29(13-7-8-21-16-23(31)10-11-24(21)29)20-38-26-12-9-22(17-25(26)33)30(37,28(35)36)18-27(32)34/h3,5,9-12,16-17,37H,2,4,6-8,13-15,18-20H2,1H3,(H,35,36)/b5-3+/t29-,30+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514205

(CHEMBL4452403)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C32H37ClN2O6/c1-34-13-3-5-27(36)24-9-6-21(24)17-35-18-31(12-2-4-20-14-23(33)8-10-25(20)31)19-41-28-11-7-22(15-26(28)35)32(40,30(38)39)16-29(34)37/h3,5,7-8,10-11,14-15,21,24,27,36,40H,2,4,6,9,12-13,16-19H2,1H3,(H,38,39)/b5-3+/t21-,24+,27-,31-,32+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514209

(CHEMBL4460664)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)CCCCN(C)C(=O)C[C@@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r| Show InChI InChI=1S/C33H41ClN2O6/c1-35-14-3-2-6-28(37)25-10-7-22(25)18-36-19-32(13-4-5-21-15-24(34)9-11-26(21)32)20-42-29-12-8-23(16-27(29)36)33(41,31(39)40)17-30(35)38/h8-9,11-12,15-16,22,25,28,37,41H,2-7,10,13-14,17-20H2,1H3,(H,39,40)/t22-,25+,28-,32-,33-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50343835

((S)-1-(4-(4-(3-(2-Cyclohexylethyl)phenyl)oxazol-2-...)Show SMILES NC(=N)[C@@H]1CCCN1C(=O)c1ccc(cc1)-c1nc(co1)-c1cccc(CCC2CCCCC2)c1 |r| Show InChI InChI=1S/C29H34N4O2/c30-27(31)26-10-5-17-33(26)29(34)23-15-13-22(14-16-23)28-32-25(19-35-28)24-9-4-8-21(18-24)12-11-20-6-2-1-3-7-20/h4,8-9,13-16,18-20,26H,1-3,5-7,10-12,17H2,(H3,30,31)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified human SphK1 assessed as inhibition of formation of [33P]-S1P after 50 mins by scintillation counting |
Bioorg Med Chem Lett 23: 4608-16 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.030
BindingDB Entry DOI: 10.7270/Q2736SB3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032720

(CHEMBL3354696)Show SMILES NC1=N[C@]2(CO1)c1cc(OCC3CCCCC3)ccc1Oc1ccc(cc21)-c1cncnc1 |r,t:1| Show InChI InChI=1S/C26H26N4O3/c27-25-30-26(15-32-25)21-10-18(19-12-28-16-29-13-19)6-8-23(21)33-24-9-7-20(11-22(24)26)31-14-17-4-2-1-3-5-17/h6-13,16-17H,1-5,14-15H2,(H2,27,30)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032730
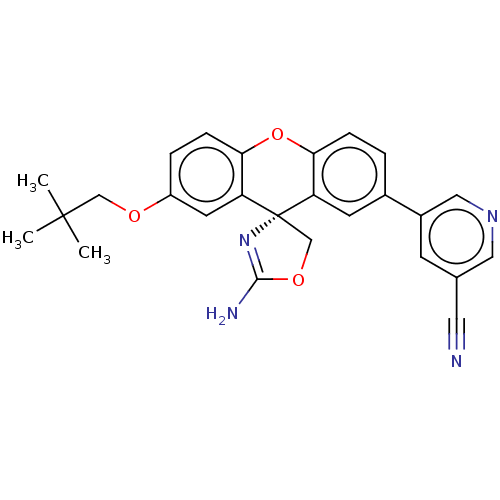
(CHEMBL3354706)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncc(c1)C#N |r,c:22| Show InChI InChI=1S/C26H24N4O3/c1-25(2,3)14-31-19-5-7-23-21(10-19)26(15-32-24(28)30-26)20-9-17(4-6-22(20)33-23)18-8-16(11-27)12-29-13-18/h4-10,12-13H,14-15H2,1-3H3,(H2,28,30)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032713
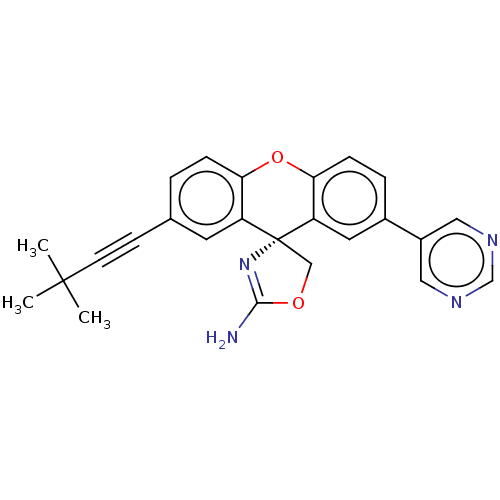
(CHEMBL3354689)Show SMILES CC(C)(C)C#Cc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C25H22N4O2/c1-24(2,3)9-8-16-4-6-21-19(10-16)25(14-30-23(26)29-25)20-11-17(5-7-22(20)31-21)18-12-27-15-28-13-18/h4-7,10-13,15H,14H2,1-3H3,(H2,26,29)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50438113
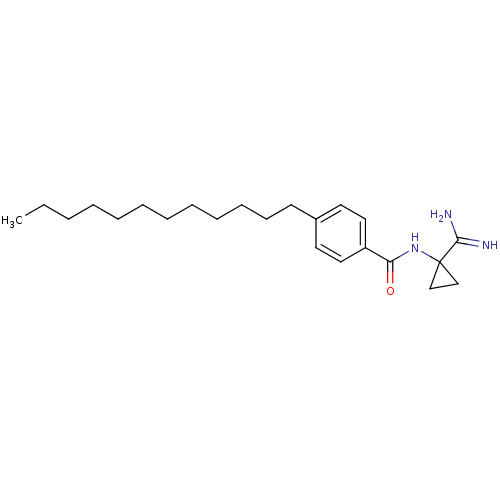
(CHEMBL1092481)Show InChI InChI=1S/C23H37N3O/c1-2-3-4-5-6-7-8-9-10-11-12-19-13-15-20(16-14-19)21(27)26-23(17-18-23)22(24)25/h13-16H,2-12,17-18H2,1H3,(H3,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified human SphK1 assessed as inhibition of formation of [33P]-S1P after 50 mins by scintillation counting |
Bioorg Med Chem Lett 23: 4608-16 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.030
BindingDB Entry DOI: 10.7270/Q2736SB3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031614

(CHEMBL3359758)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1ncc(cc21)-c1ccc(cc1)C#N)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C26H16FN5O2/c27-23-19(2-1-9-30-23)17-7-8-22-20(10-17)26(14-33-25(29)32-26)21-11-18(13-31-24(21)34-22)16-5-3-15(12-28)4-6-16/h1-11,13H,14H2,(H2,29,32)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis |
J Med Chem 57: 9811-31 (2014)
Article DOI: 10.1021/jm5012676
BindingDB Entry DOI: 10.7270/Q20G3MRW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032729
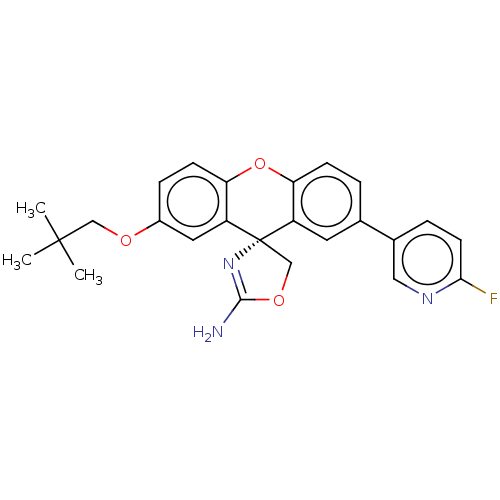
(CHEMBL3354705)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1ccc(F)nc1 |r,c:22| Show InChI InChI=1S/C25H24FN3O3/c1-24(2,3)13-30-17-6-8-21-19(11-17)25(14-31-23(27)29-25)18-10-15(4-7-20(18)32-21)16-5-9-22(26)28-12-16/h4-12H,13-14H2,1-3H3,(H2,27,29)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032731
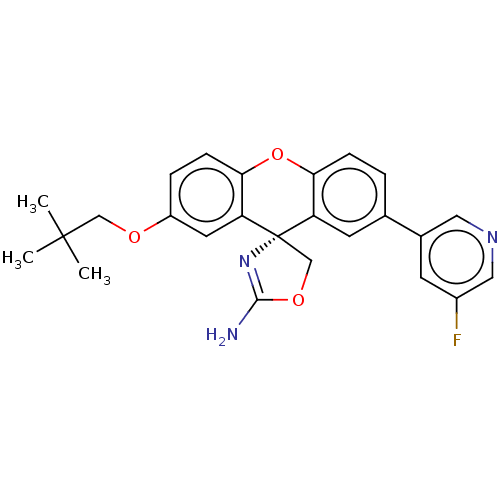
(CHEMBL3354707)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncc(F)c1 |r,c:22| Show InChI InChI=1S/C25H24FN3O3/c1-24(2,3)13-30-18-5-7-22-20(10-18)25(14-31-23(27)29-25)19-9-15(4-6-21(19)32-22)16-8-17(26)12-28-11-16/h4-12H,13-14H2,1-3H3,(H2,27,29)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032714
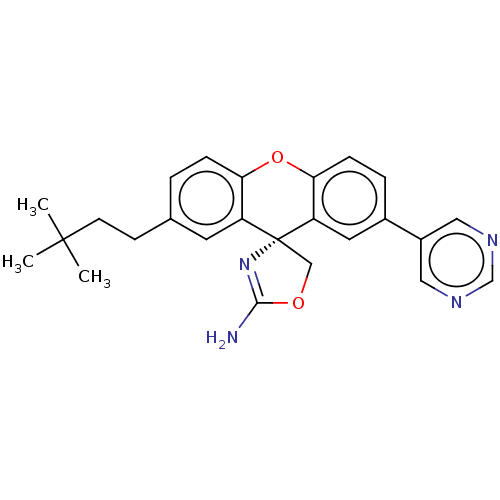
(CHEMBL3354690)Show SMILES CC(C)(C)CCc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C25H26N4O2/c1-24(2,3)9-8-16-4-6-21-19(10-16)25(14-30-23(26)29-25)20-11-17(5-7-22(20)31-21)18-12-27-15-28-13-18/h4-7,10-13,15H,8-9,14H2,1-3H3,(H2,26,29)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032726
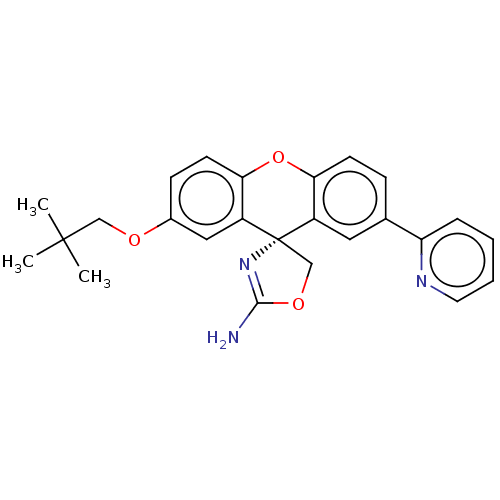
(CHEMBL3354702)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1ccccn1 |r,c:22| Show InChI InChI=1S/C25H25N3O3/c1-24(2,3)14-29-17-8-10-22-19(13-17)25(15-30-23(26)28-25)18-12-16(7-9-21(18)31-22)20-6-4-5-11-27-20/h4-13H,14-15H2,1-3H3,(H2,26,28)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 496 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50312869

(4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...)Show InChI InChI=1S/C15H11ClN2OS/c16-11-3-1-10(2-4-11)14-9-20-15(18-14)17-12-5-7-13(19)8-6-12/h1-9,19H,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified human SphK1 assessed as inhibition of formation of [33P]-S1P after 50 mins by scintillation counting |
Bioorg Med Chem Lett 23: 4608-16 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.030
BindingDB Entry DOI: 10.7270/Q2736SB3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50438113

(CHEMBL1092481)Show InChI InChI=1S/C23H37N3O/c1-2-3-4-5-6-7-8-9-10-11-12-19-13-15-20(16-14-19)21(27)26-23(17-18-23)22(24)25/h13-16H,2-12,17-18H2,1H3,(H3,24,25)(H,26,27) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified human SphK2 assessed as inhibition of formation of [33P]-S1P after 50 mins by scintillation counting |
Bioorg Med Chem Lett 23: 4608-16 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.030
BindingDB Entry DOI: 10.7270/Q2736SB3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032727

(CHEMBL3354703)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1ccncc1 |r,c:22| Show InChI InChI=1S/C25H25N3O3/c1-24(2,3)14-29-18-5-7-22-20(13-18)25(15-30-23(26)28-25)19-12-17(4-6-21(19)31-22)16-8-10-27-11-9-16/h4-13H,14-15H2,1-3H3,(H2,26,28)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 624 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031612
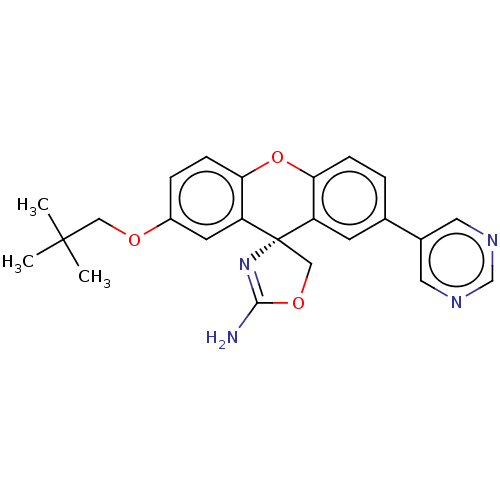
(CHEMBL3354688)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C24H24N4O3/c1-23(2,3)12-29-17-5-7-21-19(9-17)24(13-30-22(25)28-24)18-8-15(4-6-20(18)31-21)16-10-26-14-27-11-16/h4-11,14H,12-13H2,1-3H3,(H2,25,28)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031612

(CHEMBL3354688)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C24H24N4O3/c1-23(2,3)12-29-17-5-7-21-19(9-17)24(13-30-22(25)28-24)18-8-15(4-6-20(18)31-21)16-10-26-14-27-11-16/h4-11,14H,12-13H2,1-3H3,(H2,25,28)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis |
J Med Chem 57: 9811-31 (2014)
Article DOI: 10.1021/jm5012676
BindingDB Entry DOI: 10.7270/Q20G3MRW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032728
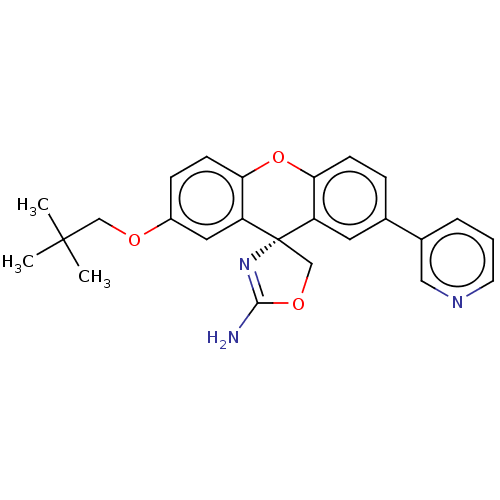
(CHEMBL3354704)Show SMILES CC(C)(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cccnc1 |r,c:22| Show InChI InChI=1S/C25H25N3O3/c1-24(2,3)14-29-18-7-9-22-20(12-18)25(15-30-23(26)28-25)19-11-16(6-8-21(19)31-22)17-5-4-10-27-13-17/h4-13H,14-15H2,1-3H3,(H2,26,28)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 797 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031617

(CHEMBL3359760)Show SMILES CC(C)(C)C#Cc1cnc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cccnc1F |r,c:22| Show InChI InChI=1S/C25H21FN4O2/c1-24(2,3)9-8-15-11-19-22(29-13-15)32-20-7-6-16(17-5-4-10-28-21(17)26)12-18(20)25(19)14-31-23(27)30-25/h4-7,10-13H,14H2,1-3H3,(H2,27,30)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis |
J Med Chem 57: 9811-31 (2014)
Article DOI: 10.1021/jm5012676
BindingDB Entry DOI: 10.7270/Q20G3MRW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031619

(CHEMBL3359757)Show SMILES Cc1ccc(cc1)-c1cnc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cccnc1F |r,c:24| Show InChI InChI=1S/C26H19FN4O2/c1-15-4-6-16(7-5-15)18-12-21-24(30-13-18)33-22-9-8-17(19-3-2-10-29-23(19)27)11-20(22)26(21)14-32-25(28)31-26/h2-13H,14H2,1H3,(H2,28,31)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis |
J Med Chem 57: 9811-31 (2014)
Article DOI: 10.1021/jm5012676
BindingDB Entry DOI: 10.7270/Q20G3MRW |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50299323

((S)-2-amino-4-hydroxy-N-(4-octylphenyl)butanamide ...)Show InChI InChI=1S/C18H30N2O2/c1-2-3-4-5-6-7-8-15-9-11-16(12-10-15)20-18(22)17(19)13-14-21/h9-12,17,21H,2-8,13-14,19H2,1H3,(H,20,22)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified human SphK1 assessed as inhibition of formation of [33P]-S1P after 50 mins by scintillation counting |
Bioorg Med Chem Lett 23: 4608-16 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.030
BindingDB Entry DOI: 10.7270/Q2736SB3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032716
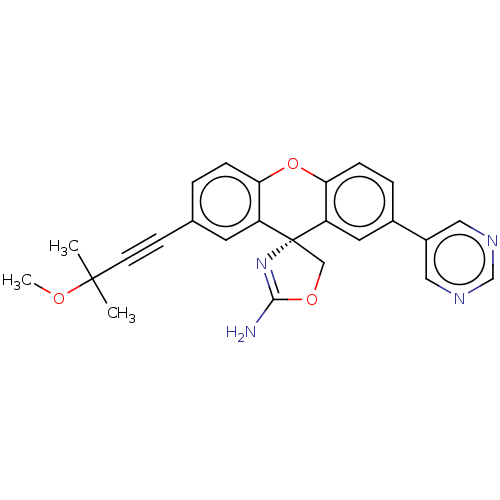
(CHEMBL3354692)Show SMILES COC(C)(C)C#Cc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:23| Show InChI InChI=1S/C25H22N4O3/c1-24(2,30-3)9-8-16-4-6-21-19(10-16)25(14-31-23(26)29-25)20-11-17(5-7-22(20)32-21)18-12-27-15-28-13-18/h4-7,10-13,15H,14H2,1-3H3,(H2,26,29)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50398311
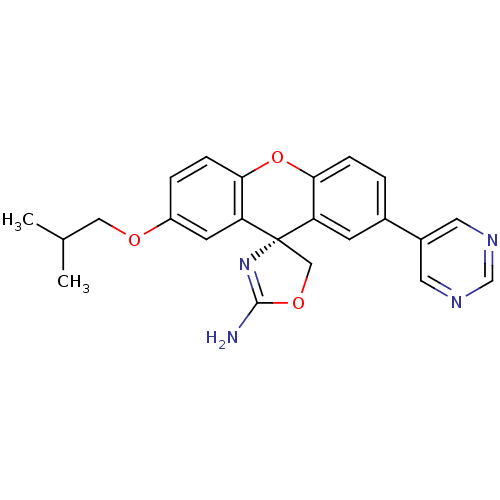
(CHEMBL2177343)Show SMILES CC(C)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:21| Show InChI InChI=1S/C23H22N4O3/c1-14(2)11-28-17-4-6-21-19(8-17)23(12-29-22(24)27-23)18-7-15(3-5-20(18)30-21)16-9-25-13-26-10-16/h3-10,13-14H,11-12H2,1-2H3,(H2,24,27)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032712
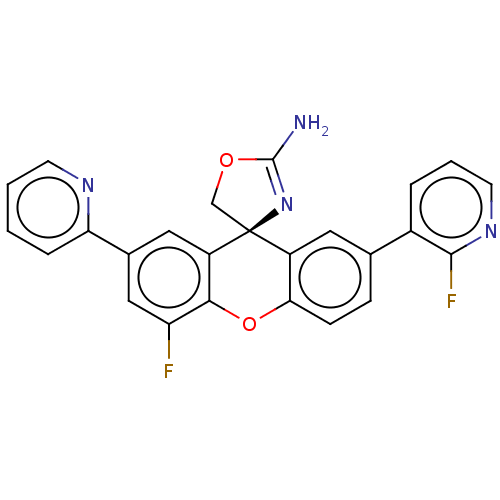
(CHEMBL3355143)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)cc(cc21)-c1ccccn1)-c1cccnc1F |r,t:1| Show InChI InChI=1S/C25H16F2N4O2/c26-19-12-15(20-5-1-2-8-29-20)11-18-22(19)33-21-7-6-14(16-4-3-9-30-23(16)27)10-17(21)25(18)13-32-24(28)31-25/h1-12H,13H2,(H2,28,31)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031625

(CHEMBL3359751)Show SMILES COC(C)(C)COc1cnc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cccnc1F |r,c:23| Show InChI InChI=1S/C24H23FN4O4/c1-23(2,30-3)12-31-15-10-18-21(28-11-15)33-19-7-6-14(16-5-4-8-27-20(16)25)9-17(19)24(18)13-32-22(26)29-24/h4-11H,12-13H2,1-3H3,(H2,26,29)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis |
J Med Chem 57: 9811-31 (2014)
Article DOI: 10.1021/jm5012676
BindingDB Entry DOI: 10.7270/Q20G3MRW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031627
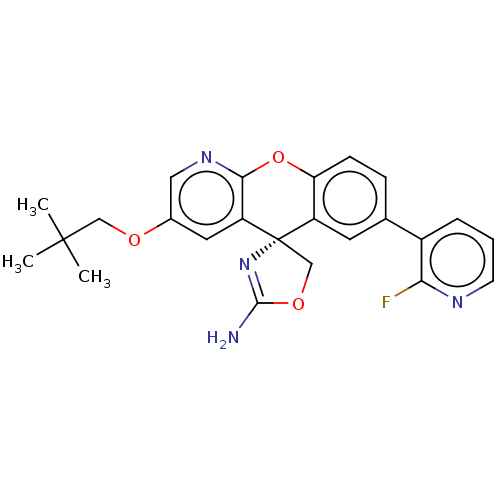
(CHEMBL3359749)Show SMILES CC(C)(C)COc1cnc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cccnc1F |r,c:22| Show InChI InChI=1S/C24H23FN4O3/c1-23(2,3)12-30-15-10-18-21(28-11-15)32-19-7-6-14(16-5-4-8-27-20(16)25)9-17(19)24(18)13-31-22(26)29-24/h4-11H,12-13H2,1-3H3,(H2,26,29)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis |
J Med Chem 57: 9811-31 (2014)
Article DOI: 10.1021/jm5012676
BindingDB Entry DOI: 10.7270/Q20G3MRW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032737

(CHEMBL3354718)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)cc(cc21)C1=CCOCC1)-c1cccnc1F |r,t:1,24| Show InChI InChI=1S/C25H19F2N3O3/c26-20-12-16(14-5-8-31-9-6-14)11-19-22(20)33-21-4-3-15(17-2-1-7-29-23(17)27)10-18(21)25(19)13-32-24(28)30-25/h1-5,7,10-12H,6,8-9,13H2,(H2,28,30)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032737

(CHEMBL3354718)Show SMILES NC1=N[C@@]2(CO1)c1cc(ccc1Oc1c(F)cc(cc21)C1=CCOCC1)-c1cccnc1F |r,t:1,24| Show InChI InChI=1S/C25H19F2N3O3/c26-20-12-16(14-5-8-31-9-6-14)11-19-22(20)33-21-4-3-15(17-2-1-7-29-23(17)27)10-18(21)25(19)13-32-24(28)30-25/h1-5,7,10-12H,6,8-9,13H2,(H2,28,30)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 25: 767-74 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.092
BindingDB Entry DOI: 10.7270/Q21G0NXQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031626
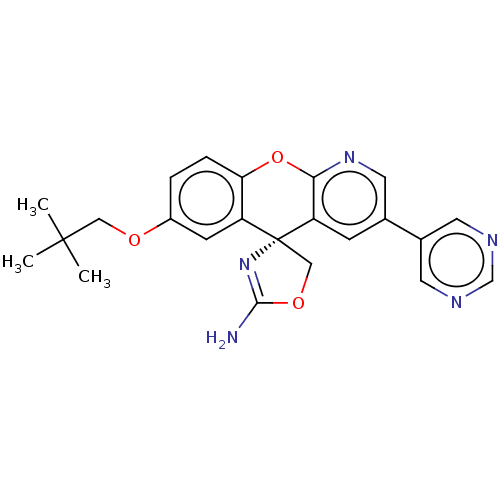
(CHEMBL3359750)Show SMILES CC(C)(C)COc1ccc2Oc3ncc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C23H23N5O3/c1-22(2,3)11-29-16-4-5-19-17(7-16)23(12-30-21(24)28-23)18-6-14(10-27-20(18)31-19)15-8-25-13-26-9-15/h4-10,13H,11-12H2,1-3H3,(H2,24,28)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells assessed as [3H]-dofetilide binding after 90 mins by liquid scintillation counting analysis |
J Med Chem 57: 9811-31 (2014)
Article DOI: 10.1021/jm5012676
BindingDB Entry DOI: 10.7270/Q20G3MRW |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 2
(Homo sapiens (Human)) | BDBM50343835

((S)-1-(4-(4-(3-(2-Cyclohexylethyl)phenyl)oxazol-2-...)Show SMILES NC(=N)[C@@H]1CCCN1C(=O)c1ccc(cc1)-c1nc(co1)-c1cccc(CCC2CCCCC2)c1 |r| Show InChI InChI=1S/C29H34N4O2/c30-27(31)26-10-5-17-33(26)29(34)23-15-13-22(14-16-23)28-32-25(19-35-28)24-9-4-8-21(18-24)12-11-20-6-2-1-3-7-20/h4,8-9,13-16,18-20,26H,1-3,5-7,10-12,17H2,(H3,30,31)/t26-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of purified human SphK2 assessed as inhibition of formation of [33P]-S1P after 50 mins by scintillation counting |
Bioorg Med Chem Lett 23: 4608-16 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.030
BindingDB Entry DOI: 10.7270/Q2736SB3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50032723

(CHEMBL3354699)Show SMILES CC(C)(F)COc1ccc2Oc3ccc(cc3[C@@]3(COC(N)=N3)c2c1)-c1cncnc1 |r,c:22| Show InChI InChI=1S/C23H21FN4O3/c1-22(2,24)11-29-16-4-6-20-18(8-16)23(12-30-21(25)28-23)17-7-14(3-5-19(17)31-20)15-9-26-13-27-10-15/h3-10,13H,11-12H2,1-2H3,(H2,25,28)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressing HEK293 cells by [3H]dofetilide binding assay |
J Med Chem 57: 9796-810 (2014)
Article DOI: 10.1021/jm501266w
BindingDB Entry DOI: 10.7270/Q28S4RHC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data