 Found 39 hits with Last Name = 'cheng' and Initial = 'fc'
Found 39 hits with Last Name = 'cheng' and Initial = 'fc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50370582
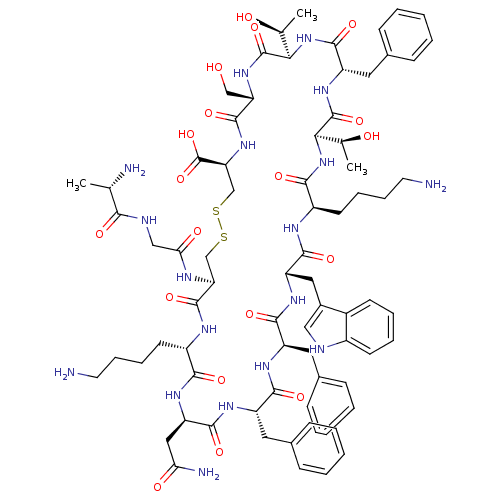
(CHEMBL1791306)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42-,43-,50-,51+,52-,53+,54-,55-,56+,57-,58-,59-,62+,63+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 2 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50370578
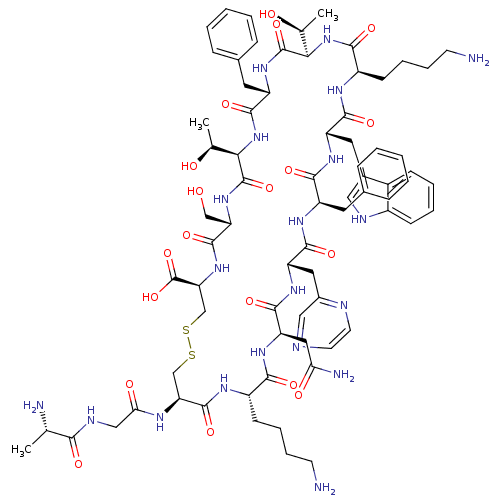
(CHEMBL1791304)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnccn2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C74H102N20O19S2/c1-39(77)62(100)82-35-59(99)83-56-37-114-115-38-57(74(112)113)92-70(108)55(36-95)91-73(111)61(41(3)97)94-69(107)51(29-43-18-8-5-9-19-43)90-72(110)60(40(2)96)93-64(102)49(23-13-15-25-76)84-66(104)52(30-44-33-81-47-21-11-10-20-46(44)47)87-65(103)50(28-42-16-6-4-7-17-42)86-67(105)53(31-45-34-79-26-27-80-45)88-68(106)54(32-58(78)98)89-63(101)48(85-71(56)109)22-12-14-24-75/h4-11,16-21,26-27,33-34,39-41,48-57,60-61,81,95-97H,12-15,22-25,28-32,35-38,75-77H2,1-3H3,(H2,78,98)(H,82,100)(H,83,99)(H,84,104)(H,85,109)(H,86,105)(H,87,103)(H,88,106)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t39-,40-,41-,48-,49+,50+,51-,52-,53-,54+,55-,56-,57-,60+,61+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 2 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50370577
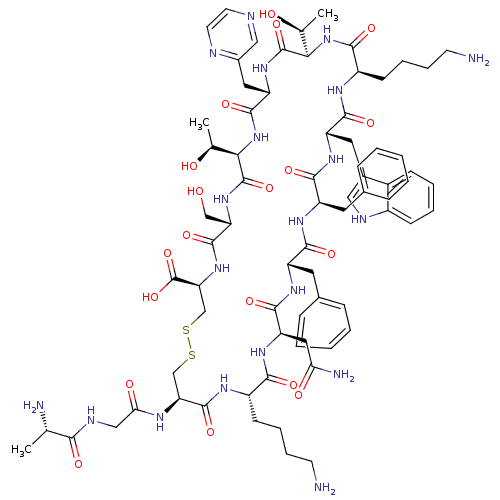
(CHEMBL1791312)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](Cc2cnccn2)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C74H102N20O19S2/c1-39(77)62(100)82-35-59(99)83-56-37-114-115-38-57(74(112)113)92-70(108)55(36-95)91-73(111)61(41(3)97)94-69(107)53(31-45-34-79-26-27-80-45)90-72(110)60(40(2)96)93-64(102)49(23-13-15-25-76)84-67(105)52(30-44-33-81-47-21-11-10-20-46(44)47)88-66(104)51(29-43-18-8-5-9-19-43)86-65(103)50(28-42-16-6-4-7-17-42)87-68(106)54(32-58(78)98)89-63(101)48(85-71(56)109)22-12-14-24-75/h4-11,16-21,26-27,33-34,39-41,48-57,60-61,81,95-97H,12-15,22-25,28-32,35-38,75-77H2,1-3H3,(H2,78,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t39-,40-,41-,48-,49+,50-,51+,52-,53-,54+,55-,56-,57-,60+,61+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 2 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50370582

(CHEMBL1791306)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42-,43-,50-,51+,52-,53+,54-,55-,56+,57-,58-,59-,62+,63+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 4 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50370580
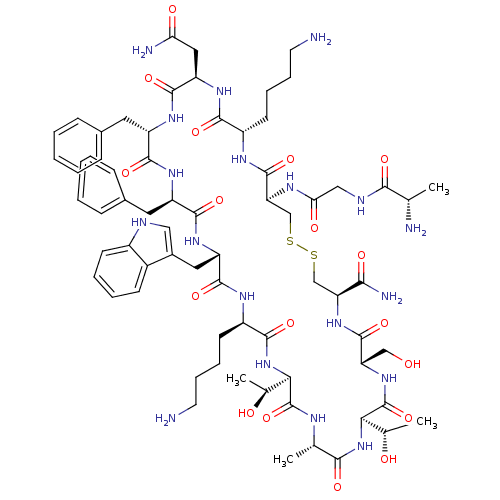
(CHEMBL1791307)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](C)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(N)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C70H101N19O18S2/c1-36(73)59(96)77-32-55(94)79-53-35-109-108-34-52(58(75)95)87-67(104)51(33-90)86-70(107)57(39(4)92)88-60(97)37(2)78-69(106)56(38(3)91)89-62(99)46(24-14-16-26-72)80-65(102)49(29-42-31-76-44-22-12-11-21-43(42)44)84-64(101)48(28-41-19-9-6-10-20-41)82-63(100)47(27-40-17-7-5-8-18-40)83-66(103)50(30-54(74)93)85-61(98)45(81-68(53)105)23-13-15-25-71/h5-12,17-22,31,36-39,45-53,56-57,76,90-92H,13-16,23-30,32-35,71-73H2,1-4H3,(H2,74,93)(H2,75,95)(H,77,96)(H,78,106)(H,79,94)(H,80,102)(H,81,105)(H,82,100)(H,83,103)(H,84,101)(H,85,98)(H,86,107)(H,87,104)(H,88,97)(H,89,99)/t36-,37-,38-,39-,45-,46+,47-,48+,49-,50+,51-,52-,53-,56+,57+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 2 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50370578

(CHEMBL1791304)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnccn2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C74H102N20O19S2/c1-39(77)62(100)82-35-59(99)83-56-37-114-115-38-57(74(112)113)92-70(108)55(36-95)91-73(111)61(41(3)97)94-69(107)51(29-43-18-8-5-9-19-43)90-72(110)60(40(2)96)93-64(102)49(23-13-15-25-76)84-66(104)52(30-44-33-81-47-21-11-10-20-46(44)47)87-65(103)50(28-42-16-6-4-7-17-42)86-67(105)53(31-45-34-79-26-27-80-45)88-68(106)54(32-58(78)98)89-63(101)48(85-71(56)109)22-12-14-24-75/h4-11,16-21,26-27,33-34,39-41,48-57,60-61,81,95-97H,12-15,22-25,28-32,35-38,75-77H2,1-3H3,(H2,78,98)(H,82,100)(H,83,99)(H,84,104)(H,85,109)(H,86,105)(H,87,103)(H,88,106)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t39-,40-,41-,48-,49+,50+,51-,52-,53-,54+,55-,56-,57-,60+,61+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 2 (n=6) |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50370581
(CHEMBL1791310)Show SMILES CC(O)[C@H]1NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](C)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@H](C)O)C(N)=O)NC(=O)CNC(=O)[C@H](C)N Show InChI InChI=1S/C70H101N19O18S2/c1-36(73)59(96)77-32-55(94)79-53-35-109-108-34-52(58(75)95)87-67(104)51(33-90)86-70(107)57(39(4)92)89-66(103)48(28-41-19-9-6-10-20-41)85-69(106)56(38(3)91)88-62(99)46(24-14-16-26-72)80-65(102)49(29-42-31-76-44-22-12-11-21-43(42)44)83-64(101)47(27-40-17-7-5-8-18-40)82-60(97)37(2)78-63(100)50(30-54(74)93)84-61(98)45(81-68(53)105)23-13-15-25-71/h5-12,17-22,31,36-39,45-53,56-57,76,90-92H,13-16,23-30,32-35,71-73H2,1-4H3,(H2,74,93)(H2,75,95)(H,77,96)(H,78,100)(H,79,94)(H,80,102)(H,81,105)(H,82,97)(H,83,101)(H,84,98)(H,85,106)(H,86,107)(H,87,104)(H,88,99)(H,89,103)/t36-,37-,38?,39-,45-,46+,47+,48-,49-,50+,51-,52-,53-,56+,57+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 2 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50370583
(CHEMBL1791311)Show SMILES CC(O)[C@H]1NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@H](C)O)C(N)=O)NC(=O)CNC(=O)[C@H](C)N Show InChI InChI=1S/C70H101N19O18S2/c1-36(73)59(96)77-32-55(94)79-53-35-109-108-34-52(58(75)95)87-67(104)51(33-90)86-70(107)57(39(4)92)89-66(103)48(28-41-19-9-6-10-20-41)85-69(106)56(38(3)91)88-62(99)46(24-14-16-26-72)80-64(101)49(29-42-31-76-44-22-12-11-21-43(42)44)82-60(97)37(2)78-63(100)47(27-40-17-7-5-8-18-40)83-65(102)50(30-54(74)93)84-61(98)45(81-68(53)105)23-13-15-25-71/h5-12,17-22,31,36-39,45-53,56-57,76,90-92H,13-16,23-30,32-35,71-73H2,1-4H3,(H2,74,93)(H2,75,95)(H,77,96)(H,78,100)(H,79,94)(H,80,101)(H,81,105)(H,82,97)(H,83,102)(H,84,98)(H,85,106)(H,86,107)(H,87,104)(H,88,99)(H,89,103)/t36-,37+,38?,39-,45-,46+,47-,48-,49-,50+,51-,52-,53-,56+,57+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 2 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50370578

(CHEMBL1791304)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnccn2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C74H102N20O19S2/c1-39(77)62(100)82-35-59(99)83-56-37-114-115-38-57(74(112)113)92-70(108)55(36-95)91-73(111)61(41(3)97)94-69(107)51(29-43-18-8-5-9-19-43)90-72(110)60(40(2)96)93-64(102)49(23-13-15-25-76)84-66(104)52(30-44-33-81-47-21-11-10-20-46(44)47)87-65(103)50(28-42-16-6-4-7-17-42)86-67(105)53(31-45-34-79-26-27-80-45)88-68(106)54(32-58(78)98)89-63(101)48(85-71(56)109)22-12-14-24-75/h4-11,16-21,26-27,33-34,39-41,48-57,60-61,81,95-97H,12-15,22-25,28-32,35-38,75-77H2,1-3H3,(H2,78,98)(H,82,100)(H,83,99)(H,84,104)(H,85,109)(H,86,105)(H,87,103)(H,88,106)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t39-,40-,41-,48-,49+,50+,51-,52-,53-,54+,55-,56-,57-,60+,61+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 4 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50370578

(CHEMBL1791304)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnccn2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C74H102N20O19S2/c1-39(77)62(100)82-35-59(99)83-56-37-114-115-38-57(74(112)113)92-70(108)55(36-95)91-73(111)61(41(3)97)94-69(107)51(29-43-18-8-5-9-19-43)90-72(110)60(40(2)96)93-64(102)49(23-13-15-25-76)84-66(104)52(30-44-33-81-47-21-11-10-20-46(44)47)87-65(103)50(28-42-16-6-4-7-17-42)86-67(105)53(31-45-34-79-26-27-80-45)88-68(106)54(32-58(78)98)89-63(101)48(85-71(56)109)22-12-14-24-75/h4-11,16-21,26-27,33-34,39-41,48-57,60-61,81,95-97H,12-15,22-25,28-32,35-38,75-77H2,1-3H3,(H2,78,98)(H,82,100)(H,83,99)(H,84,104)(H,85,109)(H,86,105)(H,87,103)(H,88,106)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t39-,40-,41-,48-,49+,50+,51-,52-,53-,54+,55-,56-,57-,60+,61+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 4 (n=5) |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50370581

(CHEMBL1791310)Show SMILES CC(O)[C@H]1NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](C)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@H](C)O)C(N)=O)NC(=O)CNC(=O)[C@H](C)N Show InChI InChI=1S/C70H101N19O18S2/c1-36(73)59(96)77-32-55(94)79-53-35-109-108-34-52(58(75)95)87-67(104)51(33-90)86-70(107)57(39(4)92)89-66(103)48(28-41-19-9-6-10-20-41)85-69(106)56(38(3)91)88-62(99)46(24-14-16-26-72)80-65(102)49(29-42-31-76-44-22-12-11-21-43(42)44)83-64(101)47(27-40-17-7-5-8-18-40)82-60(97)37(2)78-63(100)50(30-54(74)93)84-61(98)45(81-68(53)105)23-13-15-25-71/h5-12,17-22,31,36-39,45-53,56-57,76,90-92H,13-16,23-30,32-35,71-73H2,1-4H3,(H2,74,93)(H2,75,95)(H,77,96)(H,78,100)(H,79,94)(H,80,102)(H,81,105)(H,82,97)(H,83,101)(H,84,98)(H,85,106)(H,86,107)(H,87,104)(H,88,99)(H,89,103)/t36-,37-,38?,39-,45-,46+,47+,48-,49-,50+,51-,52-,53-,56+,57+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 4 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50370577

(CHEMBL1791312)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](Cc2cnccn2)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C74H102N20O19S2/c1-39(77)62(100)82-35-59(99)83-56-37-114-115-38-57(74(112)113)92-70(108)55(36-95)91-73(111)61(41(3)97)94-69(107)53(31-45-34-79-26-27-80-45)90-72(110)60(40(2)96)93-64(102)49(23-13-15-25-76)84-67(105)52(30-44-33-81-47-21-11-10-20-46(44)47)88-66(104)51(29-43-18-8-5-9-19-43)86-65(103)50(28-42-16-6-4-7-17-42)87-68(106)54(32-58(78)98)89-63(101)48(85-71(56)109)22-12-14-24-75/h4-11,16-21,26-27,33-34,39-41,48-57,60-61,81,95-97H,12-15,22-25,28-32,35-38,75-77H2,1-3H3,(H2,78,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t39-,40-,41-,48-,49+,50-,51+,52-,53-,54+,55-,56-,57-,60+,61+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 4 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50370579
(CHEMBL1791305)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2cnccn2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C74H102N20O19S2/c1-39(77)62(100)82-35-59(99)83-56-37-114-115-38-57(74(112)113)92-70(108)55(36-95)91-73(111)61(41(3)97)94-69(107)51(29-43-18-8-5-9-19-43)90-72(110)60(40(2)96)93-64(102)49(23-13-15-25-76)84-66(104)52(30-44-33-81-47-21-11-10-20-46(44)47)87-67(105)53(31-45-34-79-26-27-80-45)88-65(103)50(28-42-16-6-4-7-17-42)86-68(106)54(32-58(78)98)89-63(101)48(85-71(56)109)22-12-14-24-75/h4-11,16-21,26-27,33-34,39-41,48-57,60-61,81,95-97H,12-15,22-25,28-32,35-38,75-77H2,1-3H3,(H2,78,98)(H,82,100)(H,83,99)(H,84,104)(H,85,109)(H,86,106)(H,87,105)(H,88,103)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t39-,40-,41-,48-,49+,50-,51-,52-,53+,54+,55-,56-,57-,60+,61+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 4 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50370579

(CHEMBL1791305)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2cnccn2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C74H102N20O19S2/c1-39(77)62(100)82-35-59(99)83-56-37-114-115-38-57(74(112)113)92-70(108)55(36-95)91-73(111)61(41(3)97)94-69(107)51(29-43-18-8-5-9-19-43)90-72(110)60(40(2)96)93-64(102)49(23-13-15-25-76)84-66(104)52(30-44-33-81-47-21-11-10-20-46(44)47)87-67(105)53(31-45-34-79-26-27-80-45)88-65(103)50(28-42-16-6-4-7-17-42)86-68(106)54(32-58(78)98)89-63(101)48(85-71(56)109)22-12-14-24-75/h4-11,16-21,26-27,33-34,39-41,48-57,60-61,81,95-97H,12-15,22-25,28-32,35-38,75-77H2,1-3H3,(H2,78,98)(H,82,100)(H,83,99)(H,84,104)(H,85,109)(H,86,106)(H,87,105)(H,88,103)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t39-,40-,41-,48-,49+,50-,51-,52-,53+,54+,55-,56-,57-,60+,61+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 2 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM22966
(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NCCc3ccccc3)c2c1 Show InChI InChI=1S/C27H25ClN2O3/c1-18-23(17-26(31)29-15-14-19-6-4-3-5-7-19)24-16-22(33-2)12-13-25(24)30(18)27(32)20-8-10-21(28)11-9-20/h3-13,16H,14-15,17H2,1-2H3,(H,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem 18: 597-604 (2010)
Article DOI: 10.1016/j.bmc.2009.12.008
BindingDB Entry DOI: 10.7270/Q2RF5V4Z |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50310340
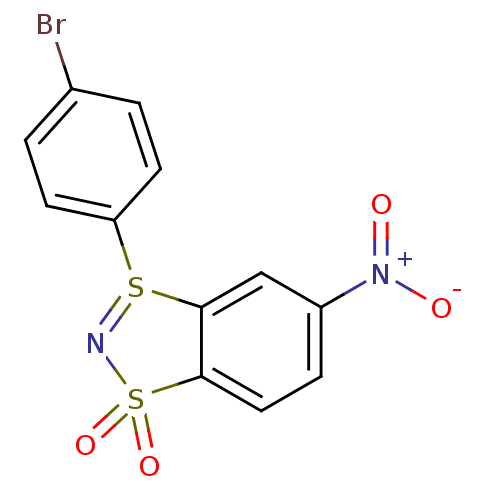
(3-(4-bromophenyl)-5-nitrobenzo[1.3.2]dithiazolium-...)Show SMILES [O-][N+](=O)c1ccc2c(c1)S(=NS2(=O)=O)c1ccc(Br)cc1 |c:10| Show InChI InChI=1S/C12H7BrN2O4S2/c13-8-1-4-10(5-2-8)20-11-7-9(15(16)17)3-6-12(11)21(18,19)14-20/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of LOX5 in human PMBL by enzyme immunoassay |
Bioorg Med Chem 18: 597-604 (2010)
Article DOI: 10.1016/j.bmc.2009.12.008
BindingDB Entry DOI: 10.7270/Q2RF5V4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem 18: 597-604 (2010)
Article DOI: 10.1016/j.bmc.2009.12.008
BindingDB Entry DOI: 10.7270/Q2RF5V4Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50310339

(3-(4-bromophenyl)-6-nitrobenzo[1.3.2]dithiazolium-...)Show SMILES [O-][N+](=O)c1ccc2c(c1)S(=O)(=O)N=S2c1ccc(Br)cc1 |c:13| Show InChI InChI=1S/C12H7BrN2O4S2/c13-8-1-4-10(5-2-8)20-11-6-3-9(15(16)17)7-12(11)21(18,19)14-20/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of LOX5 in human PMBL by enzyme immunoassay |
Bioorg Med Chem 18: 597-604 (2010)
Article DOI: 10.1016/j.bmc.2009.12.008
BindingDB Entry DOI: 10.7270/Q2RF5V4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50310340

(3-(4-bromophenyl)-5-nitrobenzo[1.3.2]dithiazolium-...)Show SMILES [O-][N+](=O)c1ccc2c(c1)S(=NS2(=O)=O)c1ccc(Br)cc1 |c:10| Show InChI InChI=1S/C12H7BrN2O4S2/c13-8-1-4-10(5-2-8)20-11-7-9(15(16)17)3-6-12(11)21(18,19)14-20/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem 18: 597-604 (2010)
Article DOI: 10.1016/j.bmc.2009.12.008
BindingDB Entry DOI: 10.7270/Q2RF5V4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50310339

(3-(4-bromophenyl)-6-nitrobenzo[1.3.2]dithiazolium-...)Show SMILES [O-][N+](=O)c1ccc2c(c1)S(=O)(=O)N=S2c1ccc(Br)cc1 |c:13| Show InChI InChI=1S/C12H7BrN2O4S2/c13-8-1-4-10(5-2-8)20-11-6-3-9(15(16)17)7-12(11)21(18,19)14-20/h1-7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect Sf9 cells by enzyme immunoassay |
Bioorg Med Chem 18: 597-604 (2010)
Article DOI: 10.1016/j.bmc.2009.12.008
BindingDB Entry DOI: 10.7270/Q2RF5V4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50310339

(3-(4-bromophenyl)-6-nitrobenzo[1.3.2]dithiazolium-...)Show SMILES [O-][N+](=O)c1ccc2c(c1)S(=O)(=O)N=S2c1ccc(Br)cc1 |c:13| Show InChI InChI=1S/C12H7BrN2O4S2/c13-8-1-4-10(5-2-8)20-11-6-3-9(15(16)17)7-12(11)21(18,19)14-20/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem 18: 597-604 (2010)
Article DOI: 10.1016/j.bmc.2009.12.008
BindingDB Entry DOI: 10.7270/Q2RF5V4Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50105987
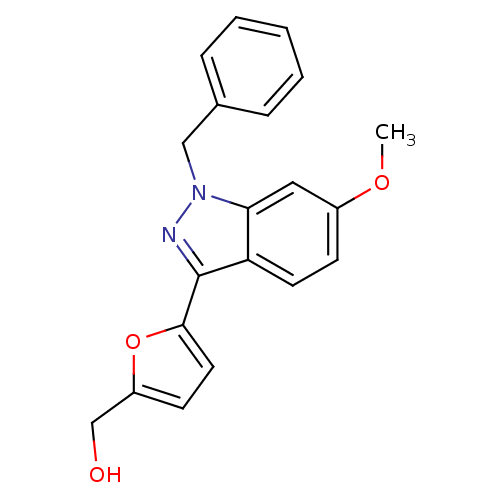
(CHEMBL125441 | [5-(1-Benzyl-6-methoxy-1H-indazol-3...)Show InChI InChI=1S/C20H18N2O3/c1-24-15-7-9-17-18(11-15)22(12-14-5-3-2-4-6-14)21-20(17)19-10-8-16(13-23)25-19/h2-11,23H,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from platelets |
J Med Chem 44: 3746-9 (2001)
BindingDB Entry DOI: 10.7270/Q2H13196 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50105986
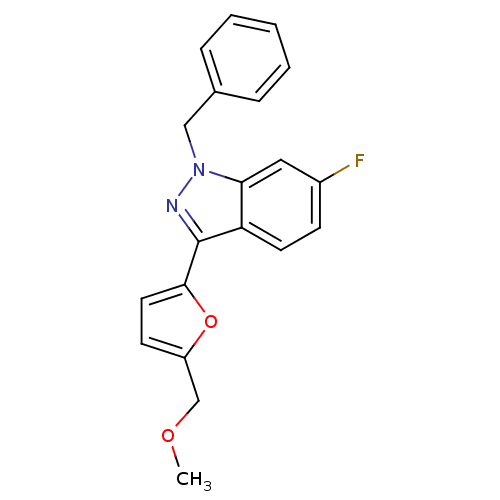
(1-Benzyl-6-fluoro-3-(5-methoxymethyl-furan-2-yl)-1...)Show InChI InChI=1S/C20H17FN2O2/c1-24-13-16-8-10-19(25-16)20-17-9-7-15(21)11-18(17)23(22-20)12-14-5-3-2-4-6-14/h2-11H,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from platelets |
J Med Chem 44: 3746-9 (2001)
BindingDB Entry DOI: 10.7270/Q2H13196 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50310339

(3-(4-bromophenyl)-6-nitrobenzo[1.3.2]dithiazolium-...)Show SMILES [O-][N+](=O)c1ccc2c(c1)S(=O)(=O)N=S2c1ccc(Br)cc1 |c:13| Show InChI InChI=1S/C12H7BrN2O4S2/c13-8-1-4-10(5-2-8)20-11-6-3-9(15(16)17)7-12(11)21(18,19)14-20/h1-7H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem 18: 597-604 (2010)
Article DOI: 10.1016/j.bmc.2009.12.008
BindingDB Entry DOI: 10.7270/Q2RF5V4Z |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50310339

(3-(4-bromophenyl)-6-nitrobenzo[1.3.2]dithiazolium-...)Show SMILES [O-][N+](=O)c1ccc2c(c1)S(=O)(=O)N=S2c1ccc(Br)cc1 |c:13| Show InChI InChI=1S/C12H7BrN2O4S2/c13-8-1-4-10(5-2-8)20-11-6-3-9(15(16)17)7-12(11)21(18,19)14-20/h1-7H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX1 in human platelets by enzyme immunoassay |
Bioorg Med Chem 18: 597-604 (2010)
Article DOI: 10.1016/j.bmc.2009.12.008
BindingDB Entry DOI: 10.7270/Q2RF5V4Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50105994

(CHEMBL440716 | [5-(1-Benzyl-6-methyl-1H-indazol-3-...)Show InChI InChI=1S/C20H18N2O2/c1-14-7-9-17-18(11-14)22(12-15-5-3-2-4-6-15)21-20(17)19-10-8-16(13-23)24-19/h2-11,23H,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from platelets |
J Med Chem 44: 3746-9 (2001)
BindingDB Entry DOI: 10.7270/Q2H13196 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50106010

(1-Benzyl-3-(5-methoxymethyl-furan-2-yl)-6-methyl-1...)Show InChI InChI=1S/C21H20N2O2/c1-15-8-10-18-19(12-15)23(13-16-6-4-3-5-7-16)22-21(18)20-11-9-17(25-20)14-24-2/h3-12H,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from platelets |
J Med Chem 44: 3746-9 (2001)
BindingDB Entry DOI: 10.7270/Q2H13196 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50105983

(1-Benzyl-3-(5-methoxymethyl-furan-2-yl)-1H-indazol...)Show InChI InChI=1S/C20H18N2O2/c1-23-14-16-11-12-19(24-16)20-17-9-5-6-10-18(17)22(21-20)13-15-7-3-2-4-8-15/h2-12H,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from platelets |
J Med Chem 44: 3746-9 (2001)
BindingDB Entry DOI: 10.7270/Q2H13196 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50105999

(CHEMBL123034 | [5-(1-Benzyl-6-fluoro-1H-indazol-3-...)Show InChI InChI=1S/C19H15FN2O2/c20-14-6-8-16-17(10-14)22(11-13-4-2-1-3-5-13)21-19(16)18-9-7-15(12-23)24-18/h1-10,23H,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from platelets |
J Med Chem 44: 3746-9 (2001)
BindingDB Entry DOI: 10.7270/Q2H13196 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50310340

(3-(4-bromophenyl)-5-nitrobenzo[1.3.2]dithiazolium-...)Show SMILES [O-][N+](=O)c1ccc2c(c1)S(=NS2(=O)=O)c1ccc(Br)cc1 |c:10| Show InChI InChI=1S/C12H7BrN2O4S2/c13-8-1-4-10(5-2-8)20-11-7-9(15(16)17)3-6-12(11)21(18,19)14-20/h1-7H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem 18: 597-604 (2010)
Article DOI: 10.1016/j.bmc.2009.12.008
BindingDB Entry DOI: 10.7270/Q2RF5V4Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50105989
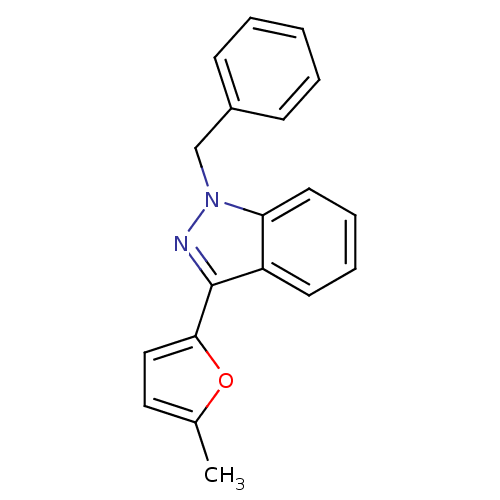
(1-Benzyl-3-(5-methyl-furan-2-yl)-1H-indazole | CHE...)Show InChI InChI=1S/C19H16N2O/c1-14-11-12-18(22-14)19-16-9-5-6-10-17(16)21(20-19)13-15-7-3-2-4-8-15/h2-12H,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from platelets |
J Med Chem 44: 3746-9 (2001)
BindingDB Entry DOI: 10.7270/Q2H13196 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50095469
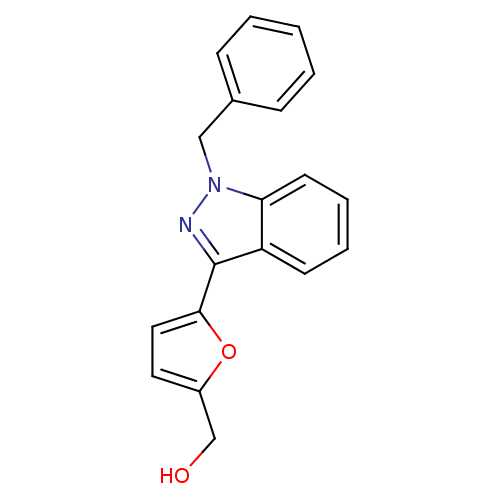
((5-(1-benzyl-1H-indazol-3-yl)furan-2-yl)methanol |...)Show InChI InChI=1S/C19H16N2O2/c22-13-15-10-11-18(23-15)19-16-8-4-5-9-17(16)21(20-19)12-14-6-2-1-3-7-14/h1-11,22H,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from platelets |
J Med Chem 44: 3746-9 (2001)
BindingDB Entry DOI: 10.7270/Q2H13196 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50106006
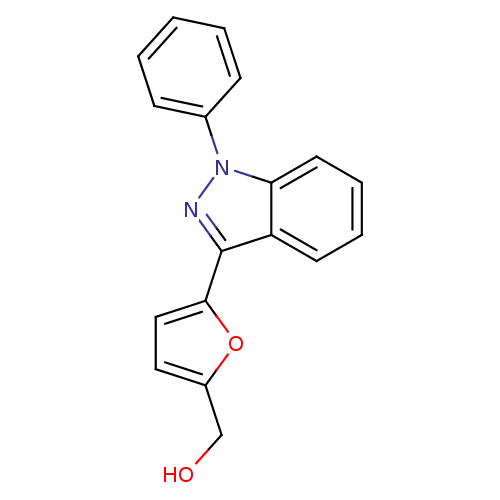
(CHEMBL340558 | [5-(1-Phenyl-1H-indazol-3-yl)-furan...)Show InChI InChI=1S/C18H14N2O2/c21-12-14-10-11-17(22-14)18-15-8-4-5-9-16(15)20(19-18)13-6-2-1-3-7-13/h1-11,21H,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from platelets |
J Med Chem 44: 3746-9 (2001)
BindingDB Entry DOI: 10.7270/Q2H13196 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM22966

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NCCc3ccccc3)c2c1 Show InChI InChI=1S/C27H25ClN2O3/c1-18-23(17-26(31)29-15-14-19-6-4-3-5-7-19)24-16-22(33-2)12-13-25(24)30(18)27(32)20-8-10-21(28)11-9-20/h3-13,16H,14-15,17H2,1-2H3,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem 18: 597-604 (2010)
Article DOI: 10.1016/j.bmc.2009.12.008
BindingDB Entry DOI: 10.7270/Q2RF5V4Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50105996
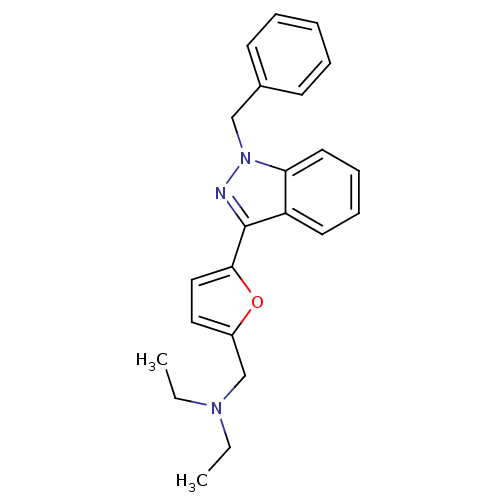
(CHEMBL121831 | [5-(1-Benzyl-1H-indazol-3-yl)-furan...)Show InChI InChI=1S/C23H25N3O/c1-3-25(4-2)17-19-14-15-22(27-19)23-20-12-8-9-13-21(20)26(24-23)16-18-10-6-5-7-11-18/h5-15H,3-4,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from platelets |
J Med Chem 44: 3746-9 (2001)
BindingDB Entry DOI: 10.7270/Q2H13196 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM15336
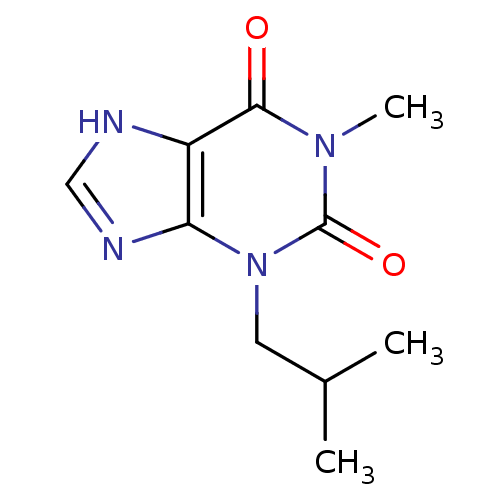
(1-methyl-3-(2-methylpropyl)-2,3,6,7-tetrahydro-1H-...)Show InChI InChI=1S/C10H14N4O2/c1-6(2)4-14-8-7(11-5-12-8)9(15)13(3)10(14)16/h5-6H,4H2,1-3H3,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmaceutical Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 from platelets |
J Med Chem 44: 3746-9 (2001)
BindingDB Entry DOI: 10.7270/Q2H13196 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in mouse RAW264.7 cells by enzyme immunoassay |
Bioorg Med Chem 18: 597-604 (2010)
Article DOI: 10.1016/j.bmc.2009.12.008
BindingDB Entry DOI: 10.7270/Q2RF5V4Z |
More data for this
Ligand-Target Pair | |
Isoform A1-A of Heterogeneous nuclear ribonucleoprotein A1 (A1-A) 68-320]
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | 4.0 | n/a |
National Taiwan University
| Assay Description
Recombinant full-length hnRNPA1 (aa 1-320) and truncated versions of hnRNPA1, including the N-terminal RNA binding domain (aa 1-196), the middle regi... |
J Biol Chem 289: 22078-89 (2014)
Article DOI: 10.1074/jbc.M114.553248
BindingDB Entry DOI: 10.7270/Q26Q1W4N |
More data for this
Ligand-Target Pair | |
Isoform A1-A of Heterogeneous nuclear ribonucleoprotein A1 (A1-A)
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | 4.0 | n/a |
National Taiwan University
| Assay Description
Recombinant full-length hnRNPA1 (aa 1-320) and truncated versions of hnRNPA1, including the N-terminal RNA binding domain (aa 1-196), the middle regi... |
J Biol Chem 289: 22078-89 (2014)
Article DOI: 10.1074/jbc.M114.553248
BindingDB Entry DOI: 10.7270/Q26Q1W4N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data