 Found 1842 hits with Last Name = 'chessari' and Initial = 'g'
Found 1842 hits with Last Name = 'chessari' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50598056
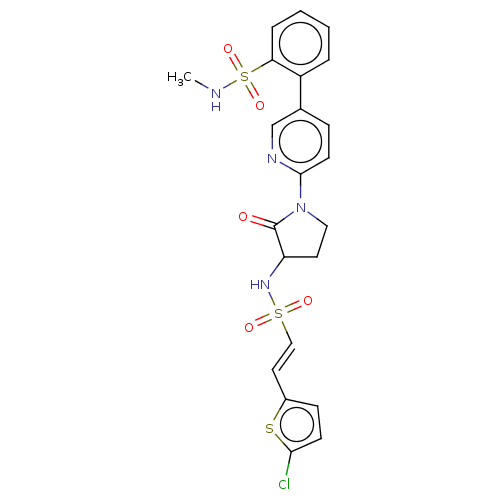
(CHEMBL403671)Show SMILES CNS(=O)(=O)c1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00164
BindingDB Entry DOI: 10.7270/Q24X5CTC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50598057
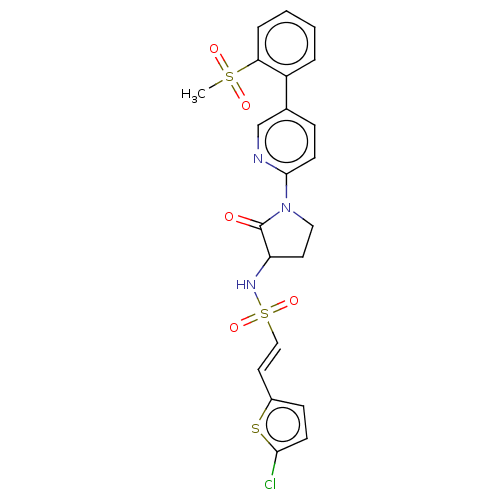
(CHEMBL254337)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00164
BindingDB Entry DOI: 10.7270/Q24X5CTC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228954
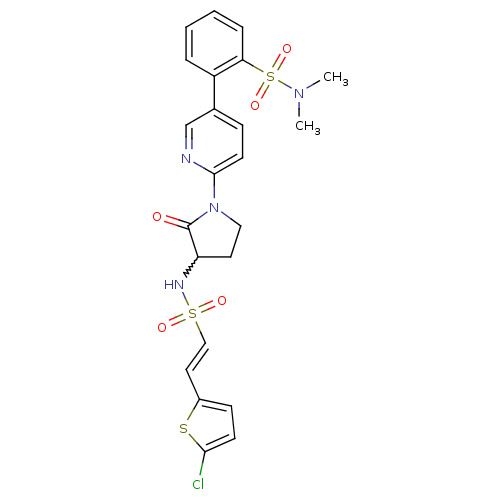
((E)-2-(6-(3-(2-(5-chlorothiophen-2-yl)vinylsulfona...)Show SMILES CN(C)S(=O)(=O)c1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |w:21.23| Show InChI InChI=1S/C23H23ClN4O5S3/c1-27(2)36(32,33)20-6-4-3-5-18(20)16-7-10-22(25-15-16)28-13-11-19(23(28)29)26-35(30,31)14-12-17-8-9-21(24)34-17/h3-10,12,14-15,19,26H,11,13H2,1-2H3/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00164
BindingDB Entry DOI: 10.7270/Q24X5CTC |
More data for this
Ligand-Target Pair | |
Bcl2-associated agonist of cell death
(Homo sapiens (Human)) | BDBM50270877
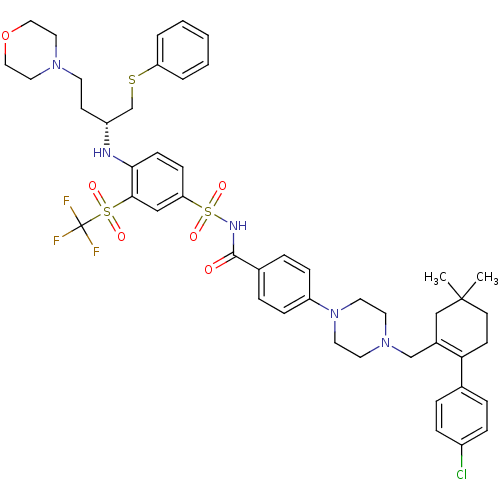
((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...)Show SMILES CC1(C)CCC(=C(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)C1)c1ccc(Cl)cc1 |r,t:5| Show InChI InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to BH3 binding groove of BclXL |
J Med Chem 51: 3661-80 (2008)
Article DOI: 10.1021/jm8000373
BindingDB Entry DOI: 10.7270/Q2N58M4H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228935
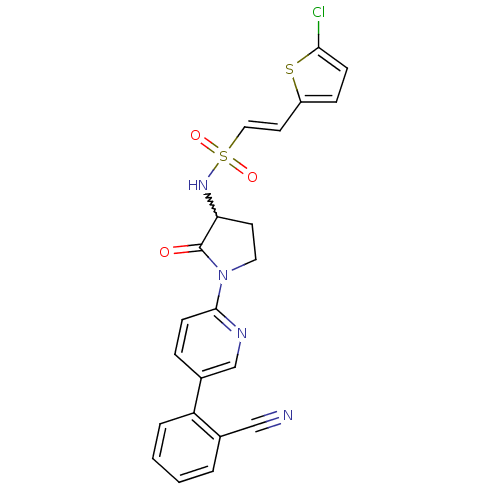
((E)-2-(5-chloro-thiophen-2-yl)-ethenesulfonic acid...)Show SMILES Clc1ccc(\C=C\S(=O)(=O)NC2CCN(C2=O)c2ccc(cn2)-c2ccccc2C#N)s1 |w:11.10| Show InChI InChI=1S/C22H17ClN4O3S2/c23-20-7-6-17(31-20)10-12-32(29,30)26-19-9-11-27(22(19)28)21-8-5-16(14-25-21)18-4-2-1-3-15(18)13-24/h1-8,10,12,14,19,26H,9,11H2/b12-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00164
BindingDB Entry DOI: 10.7270/Q24X5CTC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221972
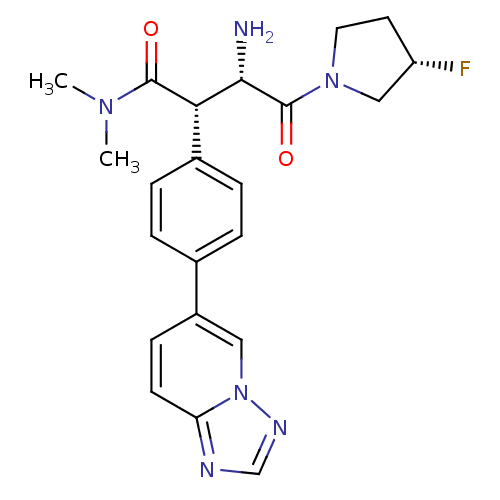
((2S,3S)-2-(4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)p...)Show SMILES CN(C)C(=O)[C@H]([C@H](N)C(=O)N1CC[C@H](F)C1)c1ccc(cc1)-c1ccc2ncnn2c1 |r| Show InChI InChI=1S/C22H25FN6O2/c1-27(2)21(30)19(20(24)22(31)28-10-9-17(23)12-28)15-5-3-14(4-6-15)16-7-8-18-25-13-26-29(18)11-16/h3-8,11,13,17,19-20H,9-10,12,24H2,1-2H3/t17-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to DPP4 |
J Med Chem 51: 3661-80 (2008)
Article DOI: 10.1021/jm8000373
BindingDB Entry DOI: 10.7270/Q2N58M4H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228946
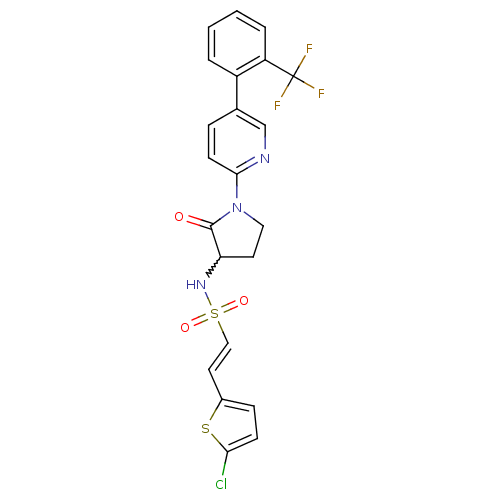
((E)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(5-(2-(tr...)Show SMILES FC(F)(F)c1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |w:19.21| Show InChI InChI=1S/C22H17ClF3N3O3S2/c23-19-7-6-15(33-19)10-12-34(31,32)28-18-9-11-29(21(18)30)20-8-5-14(13-27-20)16-3-1-2-4-17(16)22(24,25)26/h1-8,10,12-13,18,28H,9,11H2/b12-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00164
BindingDB Entry DOI: 10.7270/Q24X5CTC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228933
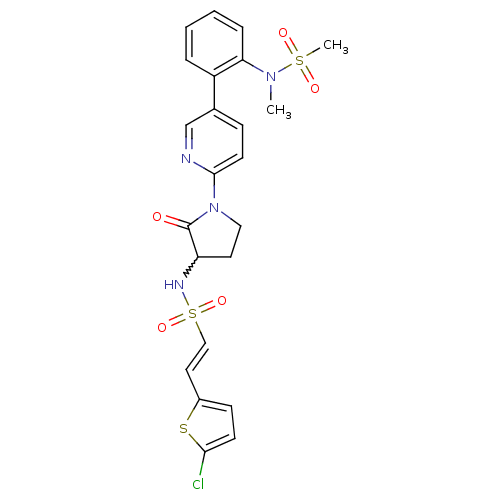
((E)-2-(5-chlorothiophen-2-yl)-N-(1-(5-(2-(N-methyl...)Show SMILES CN(c1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)S(C)(=O)=O |w:17.19| Show InChI InChI=1S/C23H23ClN4O5S3/c1-27(35(2,30)31)20-6-4-3-5-18(20)16-7-10-22(25-15-16)28-13-11-19(23(28)29)26-36(32,33)14-12-17-8-9-21(24)34-17/h3-10,12,14-15,19,26H,11,13H2,1-2H3/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00164
BindingDB Entry DOI: 10.7270/Q24X5CTC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228939
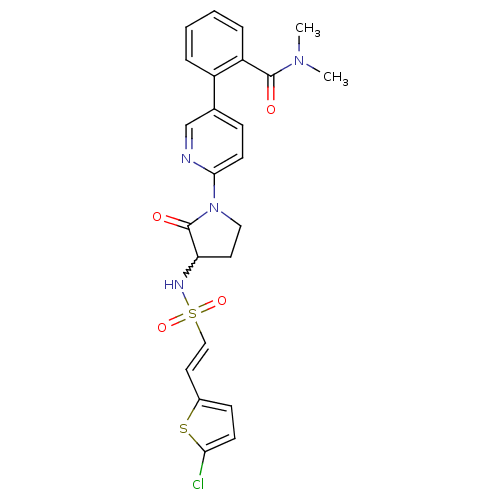
((E)-2-(6-(3-(2-(5-chlorothiophen-2-yl)vinylsulfona...)Show SMILES CN(C)C(=O)c1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |w:20.22| Show InChI InChI=1S/C24H23ClN4O4S2/c1-28(2)23(30)19-6-4-3-5-18(19)16-7-10-22(26-15-16)29-13-11-20(24(29)31)27-35(32,33)14-12-17-8-9-21(25)34-17/h3-10,12,14-15,20,27H,11,13H2,1-2H3/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00164
BindingDB Entry DOI: 10.7270/Q24X5CTC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228937
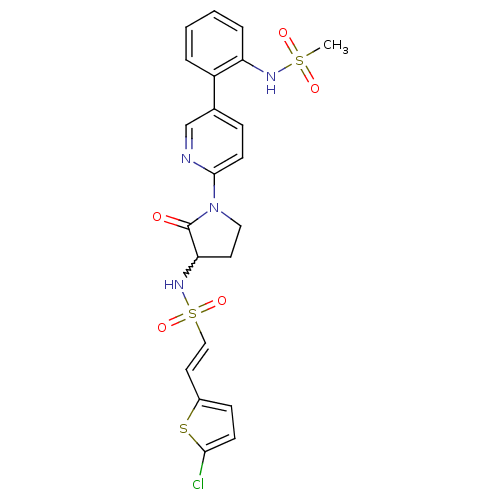
((E)-2-(5-chloro-thiophen-2-yl)-ethenesulfonic acid...)Show SMILES CS(=O)(=O)Nc1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |w:20.22| Show InChI InChI=1S/C22H21ClN4O5S3/c1-34(29,30)25-18-5-3-2-4-17(18)15-6-9-21(24-14-15)27-12-10-19(22(27)28)26-35(31,32)13-11-16-7-8-20(23)33-16/h2-9,11,13-14,19,25-26H,10,12H2,1H3/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00164
BindingDB Entry DOI: 10.7270/Q24X5CTC |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228929
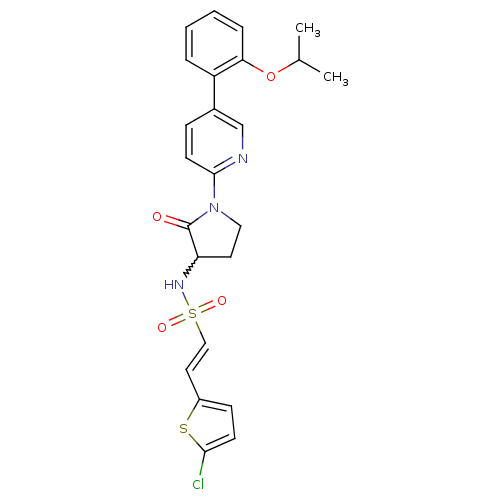
((E)-2-(5-chlorothiophen-2-yl)-N-(1-(5-(2-isopropox...)Show SMILES CC(C)Oc1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |w:19.21| Show InChI InChI=1S/C24H24ClN3O4S2/c1-16(2)32-21-6-4-3-5-19(21)17-7-10-23(26-15-17)28-13-11-20(24(28)29)27-34(30,31)14-12-18-8-9-22(25)33-18/h3-10,12,14-16,20,27H,11,13H2,1-2H3/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00164
BindingDB Entry DOI: 10.7270/Q24X5CTC |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase beta
(Homo sapiens (Human)) | BDBM50270587

(5-(3-Cyclohexyl-4'-fluoro-2'-trifluoromethyl-biphe...)Show SMILES OC(=O)c1cc(on1)-c1ccc(cc1C1CCCCC1)-c1ccc(F)cc1C(F)(F)F Show InChI InChI=1S/C23H19F4NO3/c24-15-7-9-16(19(11-15)23(25,26)27)14-6-8-17(21-12-20(22(29)30)28-31-21)18(10-14)13-4-2-1-3-5-13/h6-13H,1-5H2,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PtpB |
J Med Chem 51: 3661-80 (2008)
Article DOI: 10.1021/jm8000373
BindingDB Entry DOI: 10.7270/Q2N58M4H |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50178428
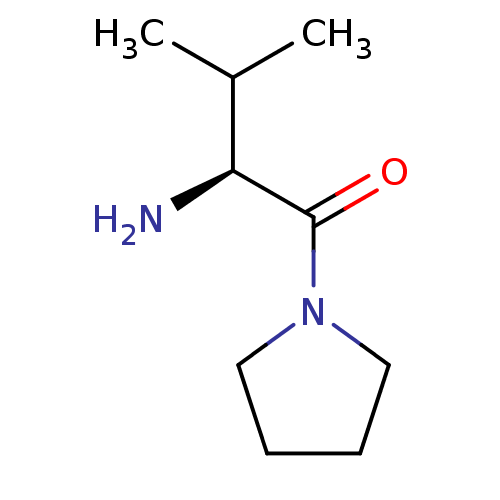
((S)-2-amino-3-methyl-1-(pyrrolidin-1-yl)butan-1-on...)Show InChI InChI=1S/C9H18N2O/c1-7(2)8(10)9(12)11-5-3-4-6-11/h7-8H,3-6,10H2,1-2H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to DPP4 |
J Med Chem 51: 3661-80 (2008)
Article DOI: 10.1021/jm8000373
BindingDB Entry DOI: 10.7270/Q2N58M4H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Queuine tRNA-ribosyltransferase catalytic subunit 1
(Homo sapiens (Human)) | BDBM50240341

(2-AMINOQUINAZOLIN-4(3H)-ONE | 2-Amino-quinazolin-4...)Show InChI InChI=1S/C8H7N3O/c9-8-10-6-4-2-1-3-5(6)7(12)11-8/h1-4H,(H3,9,10,11,12) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of TGT |
J Med Chem 51: 3661-80 (2008)
Article DOI: 10.1021/jm8000373
BindingDB Entry DOI: 10.7270/Q2N58M4H |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228942
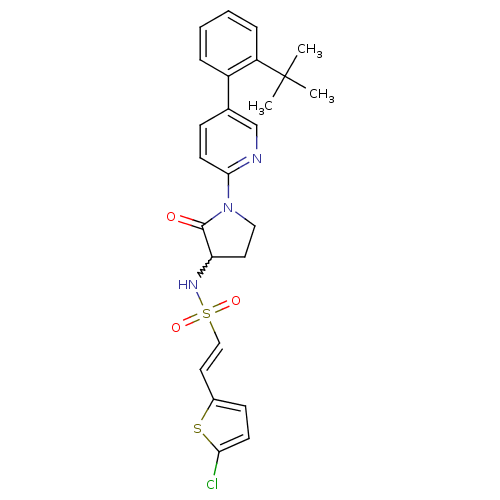
((E)-N-(1-(5-(2-tert-butylphenyl)pyridin-2-yl)-2-ox...)Show SMILES CC(C)(C)c1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |w:19.21| Show InChI InChI=1S/C25H26ClN3O3S2/c1-25(2,3)20-7-5-4-6-19(20)17-8-11-23(27-16-17)29-14-12-21(24(29)30)28-34(31,32)15-13-18-9-10-22(26)33-18/h4-11,13,15-16,21,28H,12,14H2,1-3H3/b15-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00164
BindingDB Entry DOI: 10.7270/Q24X5CTC |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase catalytic subunit 1
(Homo sapiens (Human)) | BDBM50271082
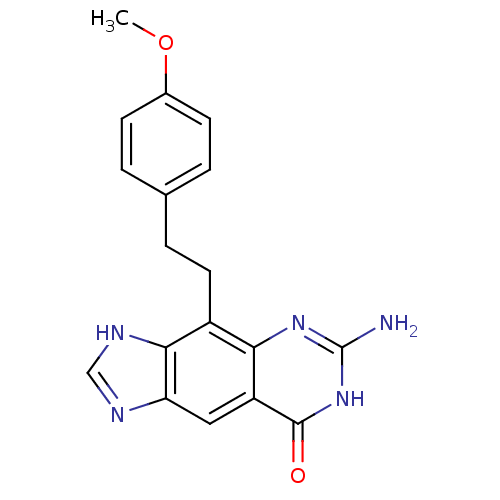
(4-(4-methoxyphenethyl)-6-amino-3H-imidazo[4,5-g]qu...)Show SMILES COc1ccc(CCc2c3[nH]cnc3cc3c2nc(N)[nH]c3=O)cc1 Show InChI InChI=1S/C18H17N5O2/c1-25-11-5-2-10(3-6-11)4-7-12-15-13(17(24)23-18(19)22-15)8-14-16(12)21-9-20-14/h2-3,5-6,8-9H,4,7H2,1H3,(H,20,21)(H3,19,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of TGT |
J Med Chem 51: 3661-80 (2008)
Article DOI: 10.1021/jm8000373
BindingDB Entry DOI: 10.7270/Q2N58M4H |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50226527

(CHEMBL107251 | N-(3-(AMINOMETHYL)BENZYL)ACETAMIDIN...)Show InChI InChI=1S/C10H15N3/c1-8(12)13-7-10-4-2-3-9(5-10)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity nNOS |
J Med Chem 51: 3661-80 (2008)
Article DOI: 10.1021/jm8000373
BindingDB Entry DOI: 10.7270/Q2N58M4H |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50239422
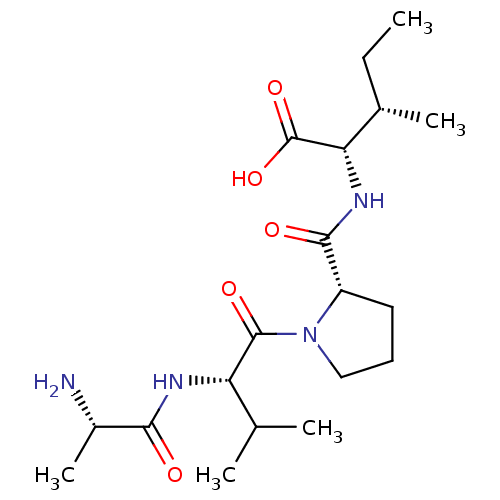
(CHEMBL234346)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)N)C(C)C)C(O)=O |r| Show InChI InChI=1S/C19H34N4O5/c1-6-11(4)15(19(27)28)22-17(25)13-8-7-9-23(13)18(26)14(10(2)3)21-16(24)12(5)20/h10-15H,6-9,20H2,1-5H3,(H,21,24)(H,22,25)(H,27,28)/t11-,12-,13-,14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SMAC-derived peptide abuRPFK (5 and 6FAM)-amide interaction with XIAP BIR3 domain (unknown origin) by fluorescence polarization assay |
J Med Chem 60: 4611-4625 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01877
BindingDB Entry DOI: 10.7270/Q2KK9DX7 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450049

(CHEMBL4166057)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nc(CO)c(Cc4ccc(F)cc4)cc23)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C31H44FN5O3/c1-20-13-35(26(12-33-20)14-36-21(2)17-40-18-22(36)3)15-29(39)37-19-31(4,5)30-28(37)11-24(27(16-38)34-30)10-23-6-8-25(32)9-7-23/h6-9,11,20-22,26,33,38H,10,12-19H2,1-5H3/t20-,21-,22-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450044

(CHEMBL4167141)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3[nH]c(=O)c(Cc4ccc(F)cc4)cc23)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O3/c1-19-13-34(25(12-32-19)14-35-20(2)16-39-17-21(35)3)15-27(37)36-18-30(4,5)28-26(36)11-23(29(38)33-28)10-22-6-8-24(31)9-7-22/h6-9,11,19-21,25,32H,10,12-18H2,1-5H3,(H,33,38)/t19-,20-,21-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450038
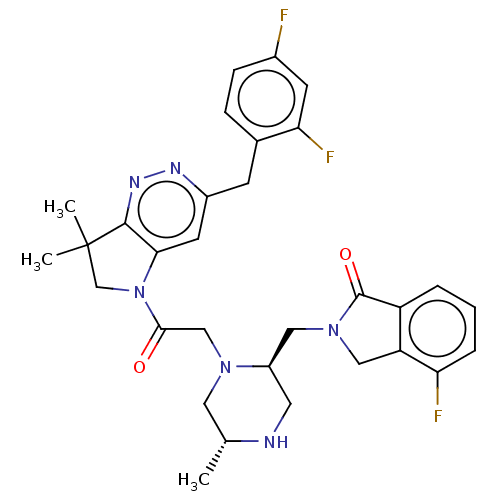
(CHEMBL4171490)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nnc(Cc4ccc(F)cc4F)cc23)[C@@H](CN2Cc3c(cccc3F)C2=O)CN1 |r| Show InChI InChI=1S/C31H33F3N6O2/c1-18-13-38(22(12-35-18)14-39-15-24-23(30(39)42)5-4-6-25(24)33)16-28(41)40-17-31(2,3)29-27(40)11-21(36-37-29)9-19-7-8-20(32)10-26(19)34/h4-8,10-11,18,22,35H,9,12-17H2,1-3H3/t18-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450047

(CHEMBL4169478)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3c2cc(Cc2ccc(F)cc2)c(=O)n3C)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C31H44FN5O3/c1-20-14-35(26(13-33-20)15-36-21(2)17-40-18-22(36)3)16-28(38)37-19-31(4,5)29-27(37)12-24(30(39)34(29)6)11-23-7-9-25(32)10-8-23/h7-10,12,20-22,26,33H,11,13-19H2,1-6H3/t20-,21-,22-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450043
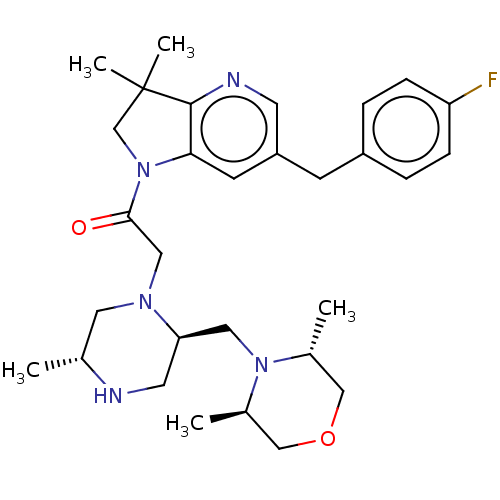
(CHEMBL4166607)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3ncc(Cc4ccc(F)cc4)cc23)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O2/c1-20-14-34(26(13-32-20)15-35-21(2)17-38-18-22(35)3)16-28(37)36-19-30(4,5)29-27(36)11-24(12-33-29)10-23-6-8-25(31)9-7-23/h6-9,11-12,20-22,26,32H,10,13-19H2,1-5H3/t20-,21-,22-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450037
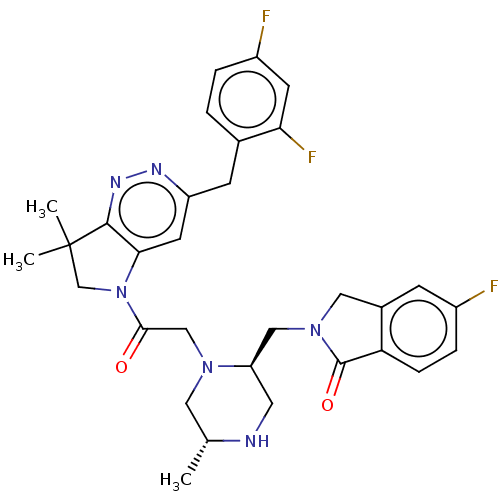
(CHEMBL4164271)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nnc(Cc4ccc(F)cc4F)cc23)[C@@H](CN2Cc3cc(F)ccc3C2=O)CN1 |r| Show InChI InChI=1S/C31H33F3N6O2/c1-18-13-38(24(12-35-18)15-39-14-20-8-21(32)6-7-25(20)30(39)42)16-28(41)40-17-31(2,3)29-27(40)11-23(36-37-29)9-19-4-5-22(33)10-26(19)34/h4-8,10-11,18,24,35H,9,12-17H2,1-3H3/t18-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450046
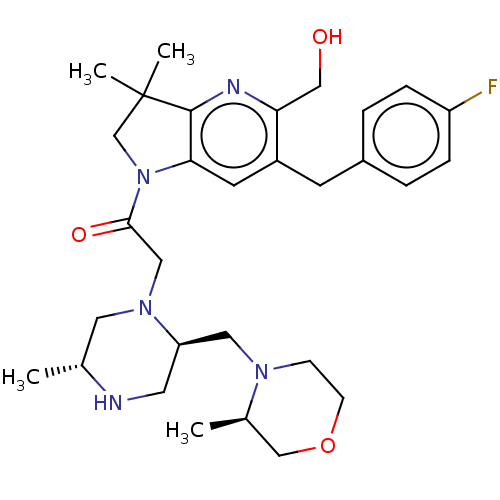
(CHEMBL4173974)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nc(CO)c(Cc4ccc(F)cc4)cc23)[C@@H](CN2CCOC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O3/c1-20-14-35(25(13-32-20)15-34-9-10-39-18-21(34)2)16-28(38)36-19-30(3,4)29-27(36)12-23(26(17-37)33-29)11-22-5-7-24(31)8-6-22/h5-8,12,20-21,25,32,37H,9-11,13-19H2,1-4H3/t20-,21-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50239422

(CHEMBL234346)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)N)C(C)C)C(O)=O |r| Show InChI InChI=1S/C19H34N4O5/c1-6-11(4)15(19(27)28)22-17(25)13-8-7-9-23(13)18(26)14(10(2)3)21-16(24)12(5)20/h10-15H,6-9,20H2,1-5H3,(H,21,24)(H,22,25)(H,27,28)/t11-,12-,13-,14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SMAC-derived peptide abuRPFK (5 and 6FAM)-amide interaction with cIAP1 BIR3 domain (unknown origin) by fluorescence polarization assay |
J Med Chem 60: 4611-4625 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01877
BindingDB Entry DOI: 10.7270/Q2KK9DX7 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50239425
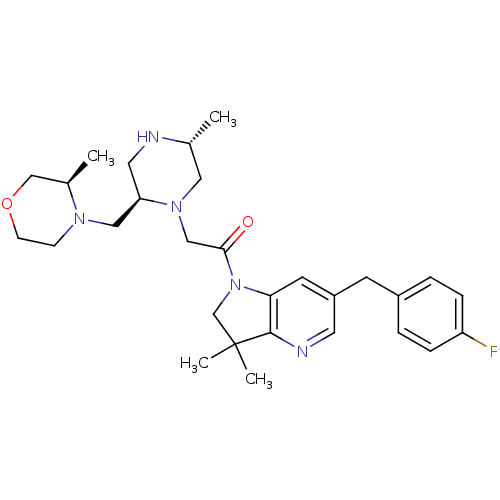
(CHEMBL4064619)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3ncc(Cc4ccc(F)cc4)cc23)[C@@H](CN2CCOC[C@H]2C)CN1 |r| Show InChI InChI=1S/C29H40FN5O2/c1-20-15-34(25(14-31-20)16-33-9-10-37-18-21(33)2)17-27(36)35-19-29(3,4)28-26(35)12-23(13-32-28)11-22-5-7-24(30)8-6-22/h5-8,12-13,20-21,25,31H,9-11,14-19H2,1-4H3/t20-,21-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450039

(CHEMBL4160872)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3nnc(Cc4ccc(F)cc4F)cc23)[C@@H](CN2Cc3ccccc3C2=O)CN1 |r| Show InChI InChI=1S/C31H34F2N6O2/c1-19-14-37(24(13-34-19)16-38-15-21-6-4-5-7-25(21)30(38)41)17-28(40)39-18-31(2,3)29-27(39)12-23(35-36-29)10-20-8-9-22(32)11-26(20)33/h4-9,11-12,19,24,34H,10,13-18H2,1-3H3/t19-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50441356
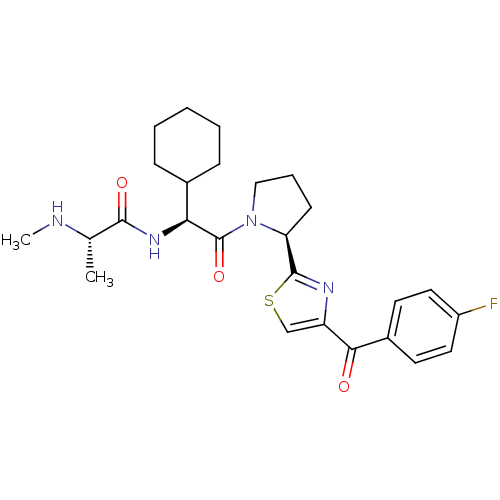
(CHEMBL2431768)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1c1nc(cs1)C(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H33FN4O3S/c1-16(28-2)24(33)30-22(17-7-4-3-5-8-17)26(34)31-14-6-9-21(31)25-29-20(15-35-25)23(32)18-10-12-19(27)13-11-18/h10-13,15-17,21-22,28H,3-9,14H2,1-2H3,(H,30,33)/t16-,21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM535783
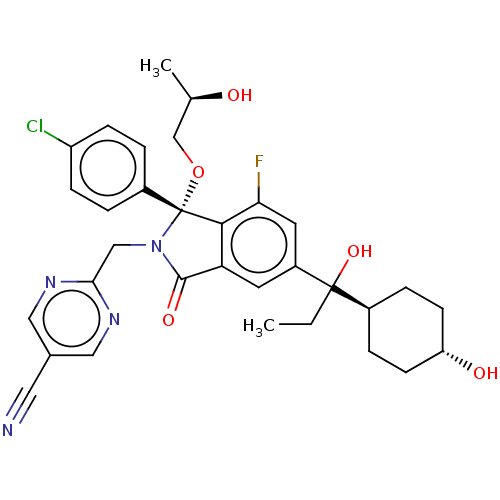
(2-{[(1R)-1-(4-chlorophenyl)-7-fluoro- 5-{1-hydroxy...)Show SMILES CCC(O)([C@H]1CC[C@H](O)CC1)c1cc2C(=O)N(Cc3ncc(cn3)C#N)[C@](OC[C@@H](C)O)(c2c(F)c1)c1ccc(Cl)cc1 |r,wU:4.3,26.28,29.32,wD:7.7,(-5.52,-2.86,;-3.98,-2.86,;-3.21,-1.53,;-2.44,-2.86,;-4.55,-.76,;-5.88,-1.53,;-7.21,-.76,;-7.21,.78,;-8.55,1.55,;-5.88,1.55,;-4.55,.78,;-1.88,-.76,;-.55,-1.53,;.79,-.76,;2.25,-1.23,;2.73,-2.7,;3.16,.01,;4.7,.01,;5.47,-1.32,;4.7,-2.65,;5.47,-3.99,;7.04,-4.04,;7.78,-2.65,;7.01,-1.32,;7.81,-5.37,;8.58,-6.7,;2.25,1.26,;1.48,2.59,;1.88,4.08,;.79,5.17,;-.7,4.77,;1.19,6.66,;.79,.78,;-.55,1.55,;-.55,3.09,;-1.88,.78,;3.59,2.03,;4.92,1.26,;6.25,2.03,;6.25,3.57,;7.59,4.34,;4.92,4.34,;3.59,3.57,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 ul per well of 1 μg ml−1 biotinylated IP3 pep... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XD14WT |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM427143

(1-({[(1R)-2-[(4-chloro-2-methanesulfonylphenyl)met...)Show SMILES C[C@@](O)(C1CCOCC1)c1cc2C(=O)N(Cc3ccc(Cl)cc3S(C)(=O)=O)[C@](OCC3(CC3)C(N)=O)(c2c(F)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C34H35Cl2FN2O7S/c1-32(42,21-9-13-45-14-10-21)23-15-26-29(27(37)16-23)34(22-4-7-24(35)8-5-22,46-19-33(11-12-33)31(38)41)39(30(26)40)18-20-3-6-25(36)17-28(20)47(2,43)44/h3-8,15-17,21,42H,9-14,18-19H2,1-2H3,(H2,38,41)/t32-,34-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... |
US Patent US10981898 (2021)
BindingDB Entry DOI: 10.7270/Q2154M5V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM427143

(1-({[(1R)-2-[(4-chloro-2-methanesulfonylphenyl)met...)Show SMILES C[C@@](O)(C1CCOCC1)c1cc2C(=O)N(Cc3ccc(Cl)cc3S(C)(=O)=O)[C@](OCC3(CC3)C(N)=O)(c2c(F)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C34H35Cl2FN2O7S/c1-32(42,21-9-13-45-14-10-21)23-15-26-29(27(37)16-23)34(22-4-7-24(35)8-5-22,46-19-33(11-12-33)31(38)41)39(30(26)40)18-20-3-6-25(36)17-28(20)47(2,43)44/h3-8,15-17,21,42H,9-14,18-19H2,1-2H3,(H2,38,41)/t32-,34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... |
US Patent US10544132 (2020)
BindingDB Entry DOI: 10.7270/Q27H1N06 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM535250
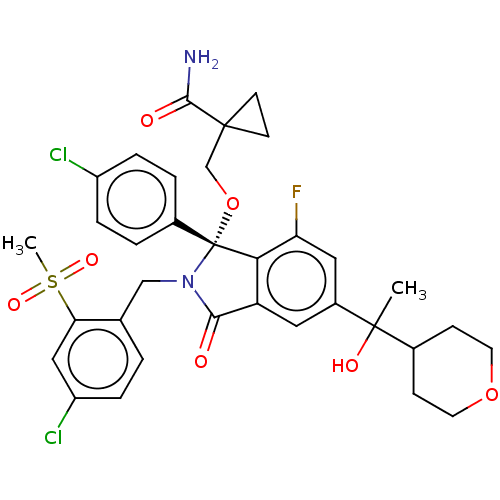
(US11236047, Example 85 | US11236047, Example 86 | ...)Show SMILES CC(O)(C1CCOCC1)c1cc2C(=O)N(Cc3ccc(Cl)cc3S(C)(=O)=O)[C@](OCC3(CC3)C(N)=O)(c2c(F)c1)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 ul per well of 1 μg ml−1 biotinylated IP3 pep... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XD14WT |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM427127

(1-({[(1R)-2-[(4-chloro-2-methanesulfonylphenyl)met...)Show SMILES Cn1cc(cn1)[C@](C)(O)c1cc2C(=O)N(Cc3ccc(Cl)cc3S(C)(=O)=O)[C@](OCC3(CC3)C(N)=O)(c2c(F)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C33H31Cl2FN4O6S/c1-31(43,22-15-38-39(2)17-22)21-12-25-28(26(36)13-21)33(20-5-8-23(34)9-6-20,46-18-32(10-11-32)30(37)42)40(29(25)41)16-19-4-7-24(35)14-27(19)47(3,44)45/h4-9,12-15,17,43H,10-11,16,18H2,1-3H3,(H2,37,42)/t31-,33-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... |
US Patent US10981898 (2021)
BindingDB Entry DOI: 10.7270/Q2154M5V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM427127

(1-({[(1R)-2-[(4-chloro-2-methanesulfonylphenyl)met...)Show SMILES Cn1cc(cn1)[C@](C)(O)c1cc2C(=O)N(Cc3ccc(Cl)cc3S(C)(=O)=O)[C@](OCC3(CC3)C(N)=O)(c2c(F)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C33H31Cl2FN4O6S/c1-31(43,22-15-38-39(2)17-22)21-12-25-28(26(36)13-21)33(20-5-8-23(34)9-6-20,46-18-32(10-11-32)30(37)42)40(29(25)41)16-19-4-7-24(35)14-27(19)47(3,44)45/h4-9,12-15,17,43H,10-11,16,18H2,1-3H3,(H2,37,42)/t31-,33-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... |
US Patent US10544132 (2020)
BindingDB Entry DOI: 10.7270/Q27H1N06 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM535242

(6-{[(1R)-1-(4-chlorophenyl)-7-fluoro- 1-{[2- (hydr...)Show SMILES Cn1cc(cn1)C(C)(O)c1cc2C(=O)N(Cc3ccc(Cl)cc3S(C)(=O)=O)[C@](OCC3(CC3)C(N)=O)(c2c(F)c1)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 ul per well of 1 μg ml−1 biotinylated IP3 pep... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XD14WT |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM535752

(6-{[(1R)-1-(4-chlorophenyl)-7-fluoro- 5-[1-hydroxy...)Show SMILES CCC(O)(c1cnn(C)c1)c1cc2C(=O)N(Cc3ccc(cn3)C#N)[C@](O[C@@H]3C[C@H](O)C3)(c2c(F)c1)c1ccc(Cl)cc1 |r,wU:25.27,27.28,29.31,(-3.23,-4.78,;-2.46,-3.45,;-3.23,-2.11,;-4.32,-3.2,;-4.56,-1.34,;-4.72,.19,;-6.23,.51,;-7,-.83,;-8.53,-.99,;-5.97,-1.97,;-1.9,-1.34,;-.56,-2.11,;.77,-1.34,;2.24,-1.82,;2.71,-3.28,;3.14,-.57,;4.68,-.57,;5.45,-1.91,;4.68,-3.24,;5.45,-4.58,;7.02,-4.62,;7.76,-3.24,;6.99,-1.91,;7.79,-5.96,;8.56,-7.29,;2.24,.67,;1.43,1.98,;1.43,3.52,;2.52,4.61,;1.43,5.7,;1.43,7.24,;.34,4.61,;.77,.2,;-.56,.97,;-.56,2.51,;-1.9,.2,;3.33,1.76,;4.81,1.36,;5.9,2.45,;5.5,3.94,;6.59,5.03,;4.02,4.34,;2.93,3.25,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 ul per well of 1 μg ml−1 biotinylated IP3 pep... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XD14WT |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM535770
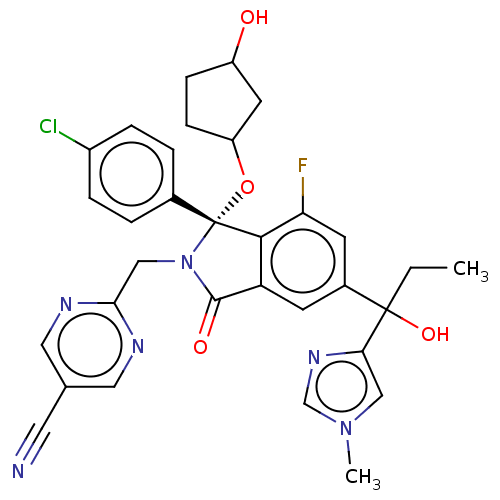
(2-{[(1R)-1-(4-chlorophenyl)-7-fluoro- 5-[1-hydroxy...)Show SMILES CCC(O)(c1cn(C)cn1)c1cc2C(=O)N(Cc3ncc(cn3)C#N)[C@](OC3CCC(O)C3)(c2c(F)c1)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 ul per well of 1 μg ml−1 biotinylated IP3 pep... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XD14WT |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM535777

((3R)-3-(4-chlorophenyl)-2-[(5- chloropyrimidin-2-y...)Show SMILES CC(O)(c1cc2C(=O)N(Cc3ncc(Cl)cn3)[C@](O[C@H]3CCOC3)(c2c(F)c1)c1ccc(Cl)cc1)C1(F)CCOCC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 ul per well of 1 μg ml−1 biotinylated IP3 pep... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XD14WT |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM427195
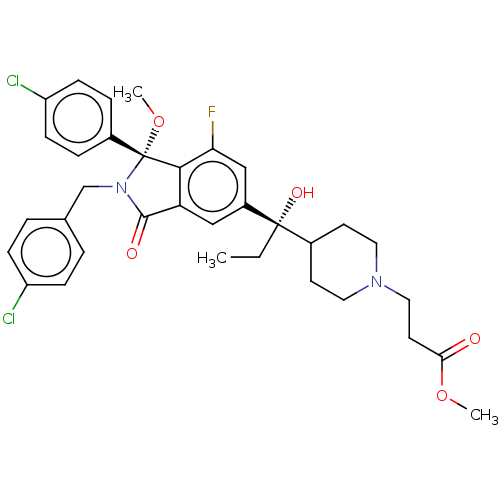
(US10544132, Example 136 | US10981898, Example 137 ...)Show SMILES CC[C@](O)(C1CCN(CCC(=O)OC)CC1)c1cc2C(=O)N(Cc3ccc(Cl)cc3)[C@](OC)(c2c(F)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C34H37Cl2FN2O5/c1-4-33(42,23-13-16-38(17-14-23)18-15-30(40)43-2)25-19-28-31(29(37)20-25)34(44-3,24-7-11-27(36)12-8-24)39(32(28)41)21-22-5-9-26(35)10-6-22/h5-12,19-20,23,42H,4,13-18,21H2,1-3H3/t33-,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... |
US Patent US10981898 (2021)
BindingDB Entry DOI: 10.7270/Q2154M5V |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM535265

(3-{4-[(1S)-1-[(1R)-1-(4- chlorophenyl)-2-[(4- chlo...)Show SMILES CC[C@](O)(C1CCN(CCC(O)=O)CC1)c1cc2C(=O)N(Cc3ccc(Cl)cc3)[C@](OC)(c2c(F)c1)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 ul per well of 1 μg ml−1 biotinylated IP3 pep... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XD14WT |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
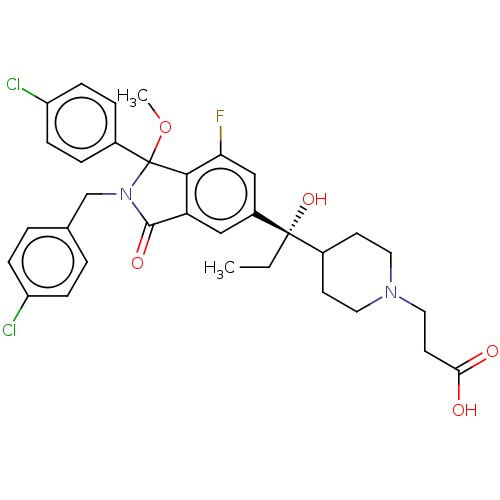
(Homo sapiens (Human)) | BDBM427196
(US10544132, Example 137)Show SMILES CC[C@](O)(C1CCN(CCC(O)=O)CC1)c1cc2C(=O)N(Cc3ccc(Cl)cc3)C(OC)(c2c(F)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C33H35Cl2FN2O5/c1-3-32(42,22-12-15-37(16-13-22)17-14-29(39)40)24-18-27-30(28(36)19-24)33(43-2,23-6-10-26(35)11-7-23)38(31(27)41)20-21-4-8-25(34)9-5-21/h4-11,18-19,22,42H,3,12-17,20H2,1-2H3,(H,39,40)/t32-,33?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTEX THERAPEUTICS LIMITED; CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 μl per well of 1 μg ml−1 biotinylated IP... |
US Patent US10544132 (2020)
BindingDB Entry DOI: 10.7270/Q27H1N06 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450054

(CHEMBL4159232)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3ncc(Cc4ccc(F)cc4O)cc23)[C@@H](CN2[C@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O3/c1-19-13-34(25(12-32-19)14-35-20(2)16-39-17-21(35)3)15-28(38)36-18-30(4,5)29-26(36)9-22(11-33-29)8-23-6-7-24(31)10-27(23)37/h6-7,9-11,19-21,25,32,37H,8,12-18H2,1-5H3/t19-,20-,21-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM535793
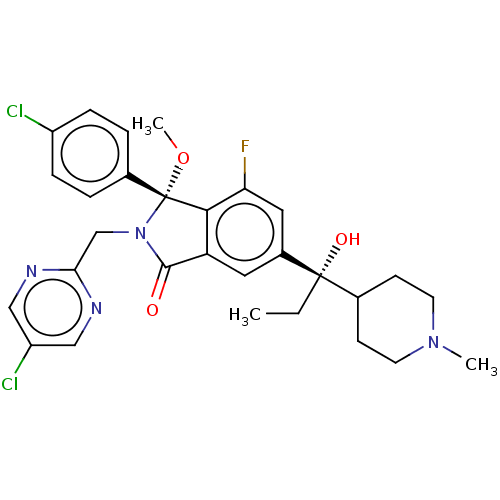
((3R)-3-(4-chlorophenyl)-2-[(5- chloropyrimidin-2-y...)Show SMILES CC[C@](O)(C1CCN(C)CC1)c1cc2C(=O)N(Cc3ncc(Cl)cn3)[C@](OC)(c2c(F)c1)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 ul per well of 1 μg ml−1 biotinylated IP3 pep... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XD14WT |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM240596
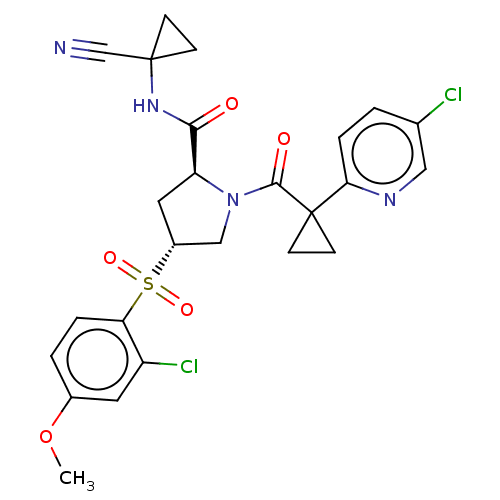
(US9409882, 1)Show SMILES COc1ccc(c(Cl)c1)S(=O)(=O)[C@@H]1C[C@H](N(C1)C(=O)C1(CC1)c1ccc(Cl)cn1)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H24Cl2N4O5S/c1-36-16-3-4-20(18(27)10-16)37(34,35)17-11-19(22(32)30-24(14-28)6-7-24)31(13-17)23(33)25(8-9-25)21-5-2-15(26)12-29-21/h2-5,10,12,17,19H,6-9,11,13H2,1H3,(H,30,32)/t17-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00164
BindingDB Entry DOI: 10.7270/Q24X5CTC |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM535770

(2-{[(1R)-1-(4-chlorophenyl)-7-fluoro- 5-[1-hydroxy...)Show SMILES CCC(O)(c1cn(C)cn1)c1cc2C(=O)N(Cc3ncc(cn3)C#N)[C@](OC3CCC(O)C3)(c2c(F)c1)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 ul per well of 1 μg ml−1 biotinylated IP3 pep... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XD14WT |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM535751
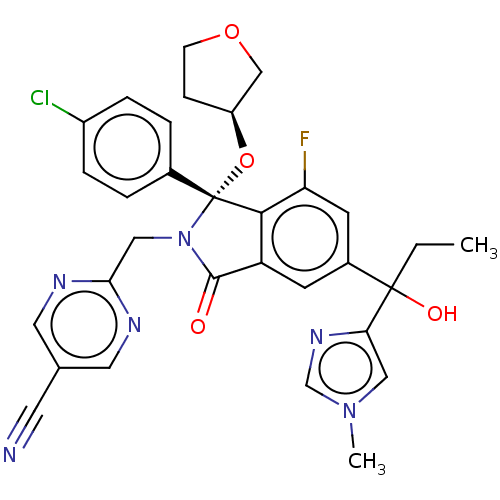
(2-{[(1R)-1-(4-chlorophenyl)-7-fluoro- 5-[1-hydroxy...)Show SMILES CCC(O)(c1cn(C)cn1)c1cc2C(=O)N(Cc3ncc(cn3)C#N)[C@](O[C@H]3CCOC3)(c2c(F)c1)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 ul per well of 1 μg ml−1 biotinylated IP3 pep... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XD14WT |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM535794

((3R)-3-(4-chlorophenyl)-2-[(5- chloropyrimidin-2-y...)Show SMILES CC[C@](O)(C1CCN(C)CC1)c1cc2C(=O)N(Cc3ncc(Cl)cn3)[C@](OCCO)(c2c(F)c1)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 ul per well of 1 μg ml−1 biotinylated IP3 pep... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XD14WT |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50450042
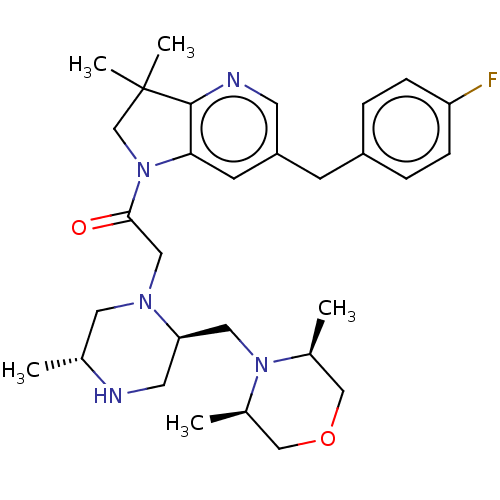
(CHEMBL4168197)Show SMILES C[C@@H]1CN(CC(=O)N2CC(C)(C)c3ncc(Cc4ccc(F)cc4)cc23)[C@@H](CN2[C@@H](C)COC[C@H]2C)CN1 |r| Show InChI InChI=1S/C30H42FN5O2/c1-20-14-34(26(13-32-20)15-35-21(2)17-38-18-22(35)3)16-28(37)36-19-30(4,5)29-27(36)11-24(12-33-29)10-23-6-8-25(31)9-7-23/h6-9,11-12,20-22,26,32H,10,13-19H2,1-5H3/t20-,21-,22+,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Induction of intracellular cIAP1 degradation in human MDA-MB-231 cells after 2 hrs |
J Med Chem 61: 7314-7329 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00900
BindingDB Entry DOI: 10.7270/Q2TT4THH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM535394

(US11236047, Example 197 | US11236047, Example 198 ...)Show SMILES Cc1nc(cn1C)C(C)(O)c1cc2C(=O)N(Cc3ccc(Cl)cn3)[C@](OCC3(CC3)C(N)=O)(c2c(F)c1)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The ELISA assay was performed in streptavidin coated plates which were preincubated with 200 ul per well of 1 μg ml−1 biotinylated IP3 pep... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XD14WT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data