 Found 182 hits with Last Name = 'chiellini' and Initial = 'g'
Found 182 hits with Last Name = 'chiellini' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50388434
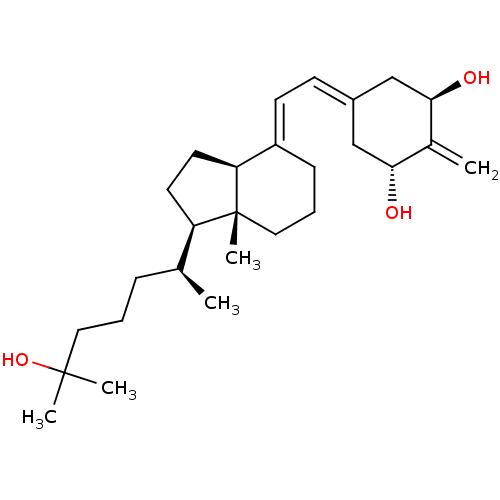
(CHEMBL605525)Show SMILES [#6]-[#6@@H](-[#6]-[#6]-[#6]C([#6])([#6])[#8])-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)22-12-13-23-21(9-7-15-27(22,23)5)11-10-20-16-24(28)19(2)25(29)17-20/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b21-11+/t18-,22+,23-,24+,25+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417515
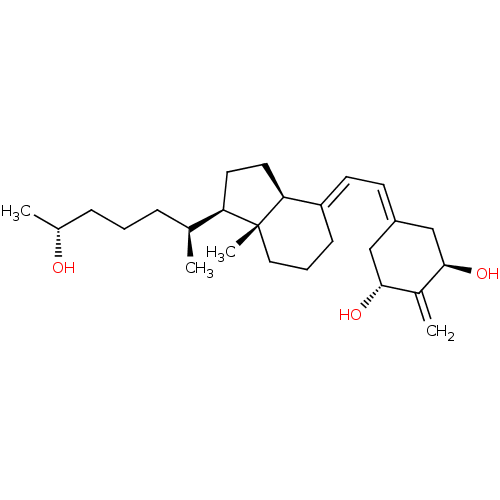
(CHEMBL1630755)Show SMILES [#6]-[#6@@H](-[#8])-[#6]-[#6]-[#6]-[#6@H](-[#6])-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C26H42O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-18,22-25,27-29H,3,5-9,12-16H2,1-2,4H3/b21-11+/t17-,18+,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417519
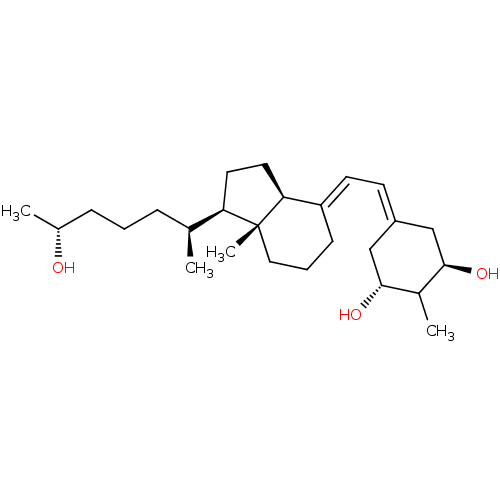
(CHEMBL1630759)Show SMILES C[C@@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,11.10,16.18,8.7,6.6,1.1,wD:26.28,(1.96,-41.79,;.6,-42.52,;.6,-44.06,;-.7,-41.71,;-2.06,-42.44,;-3.37,-41.62,;-4.73,-42.35,;-6.07,-41.6,;-4.71,-43.89,;-3.82,-45.14,;-4.73,-46.37,;-6.18,-45.89,;-7.5,-46.66,;-8.85,-45.89,;-8.84,-44.35,;-7.5,-43.58,;-6.17,-44.36,;-6.18,-42.82,;-7.5,-48.2,;-8.84,-48.97,;-8.84,-50.51,;-7.51,-51.29,;-7.51,-52.83,;-6.17,-53.6,;-8.84,-53.59,;-8.84,-55.13,;-10.17,-52.83,;-11.51,-53.6,;-10.17,-51.29,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18+,19?,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
Bioorg Med Chem 16: 8563-73 (2008)
Article DOI: 10.1016/j.bmc.2008.08.011
BindingDB Entry DOI: 10.7270/Q20001X4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417520

(CHEMBL1630753)Show SMILES C[C@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C(=C)[C@H](O)C1=C |r| Show InChI InChI=1S/C27H42O3/c1-17(8-6-9-18(2)28)23-13-14-24-21(10-7-15-27(23,24)5)11-12-22-16-25(29)20(4)26(30)19(22)3/h11-12,17-18,23-26,28-30H,3-4,6-10,13-16H2,1-2,5H3/b21-11+,22-12-/t17-,18-,23+,24-,25+,26+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417520

(CHEMBL1630753)Show SMILES C[C@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C(=C)[C@H](O)C1=C |r| Show InChI InChI=1S/C27H42O3/c1-17(8-6-9-18(2)28)23-13-14-24-21(10-7-15-27(23,24)5)11-12-22-16-25(29)20(4)26(30)19(22)3/h11-12,17-18,23-26,28-30H,3-4,6-10,13-16H2,1-2,5H3/b21-11+,22-12-/t17-,18-,23+,24-,25+,26+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417519

(CHEMBL1630759)Show SMILES C[C@@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,11.10,16.18,8.7,6.6,1.1,wD:26.28,(1.96,-41.79,;.6,-42.52,;.6,-44.06,;-.7,-41.71,;-2.06,-42.44,;-3.37,-41.62,;-4.73,-42.35,;-6.07,-41.6,;-4.71,-43.89,;-3.82,-45.14,;-4.73,-46.37,;-6.18,-45.89,;-7.5,-46.66,;-8.85,-45.89,;-8.84,-44.35,;-7.5,-43.58,;-6.17,-44.36,;-6.18,-42.82,;-7.5,-48.2,;-8.84,-48.97,;-8.84,-50.51,;-7.51,-51.29,;-7.51,-52.83,;-6.17,-53.6,;-8.84,-53.59,;-8.84,-55.13,;-10.17,-52.83,;-11.51,-53.6,;-10.17,-51.29,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18+,19?,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417517

(CHEMBL1630757)Show SMILES C[C@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,11.10,16.18,8.7,6.6,wD:26.28,1.1,(14.49,-28.73,;13.13,-29.45,;13.13,-30.99,;11.83,-28.64,;10.47,-29.37,;9.16,-28.56,;7.8,-29.29,;6.46,-28.53,;7.82,-30.83,;8.71,-32.07,;7.8,-33.31,;6.35,-32.82,;5.03,-33.59,;3.68,-32.83,;3.69,-31.28,;5.03,-30.51,;6.36,-31.29,;6.35,-29.75,;5.03,-35.13,;3.69,-35.9,;3.69,-37.44,;5.02,-38.22,;5.02,-39.76,;6.36,-40.53,;3.69,-40.52,;3.69,-42.06,;2.36,-39.76,;1.03,-40.53,;2.36,-38.22,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18-,19?,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417515

(CHEMBL1630755)Show SMILES [#6]-[#6@@H](-[#8])-[#6]-[#6]-[#6]-[#6@H](-[#6])-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C26H42O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-18,22-25,27-29H,3,5-9,12-16H2,1-2,4H3/b21-11+/t17-,18+,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417517

(CHEMBL1630757)Show SMILES C[C@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,11.10,16.18,8.7,6.6,wD:26.28,1.1,(14.49,-28.73,;13.13,-29.45,;13.13,-30.99,;11.83,-28.64,;10.47,-29.37,;9.16,-28.56,;7.8,-29.29,;6.46,-28.53,;7.82,-30.83,;8.71,-32.07,;7.8,-33.31,;6.35,-32.82,;5.03,-33.59,;3.68,-32.83,;3.69,-31.28,;5.03,-30.51,;6.36,-31.29,;6.35,-29.75,;5.03,-35.13,;3.69,-35.9,;3.69,-37.44,;5.02,-38.22,;5.02,-39.76,;6.36,-40.53,;3.69,-40.52,;3.69,-42.06,;2.36,-39.76,;1.03,-40.53,;2.36,-38.22,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18-,19?,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417514
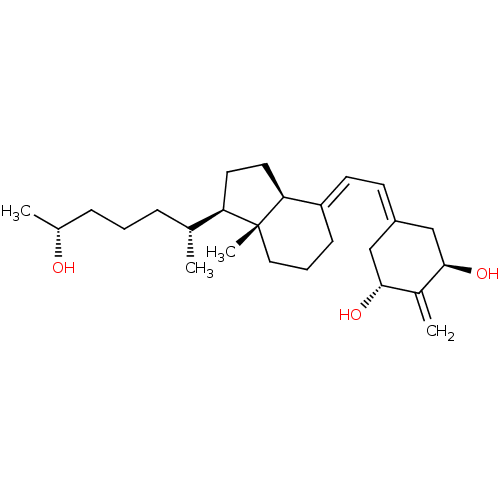
(CHEMBL1630754)Show SMILES [#6]-[#6@@H](-[#8])-[#6]-[#6]-[#6]-[#6@@H](-[#6])-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C26H42O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-18,22-25,27-29H,3,5-9,12-16H2,1-2,4H3/b21-11+/t17-,18-,22-,23+,24-,25-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417513
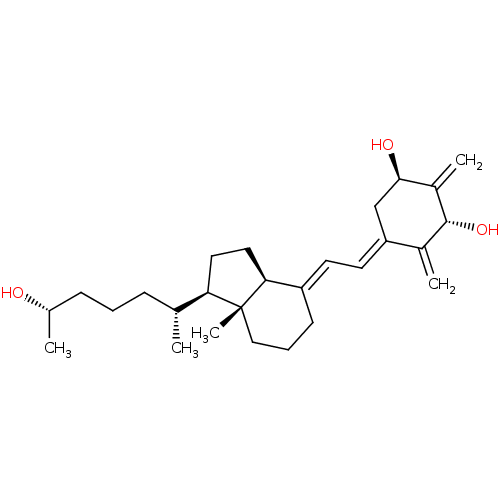
(CHEMBL1630752)Show SMILES C[C@H](O)CCC[C@@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C(=C)[C@H](O)C1=C |r| Show InChI InChI=1S/C27H42O3/c1-17(8-6-9-18(2)28)23-13-14-24-21(10-7-15-27(23,24)5)11-12-22-16-25(29)20(4)26(30)19(22)3/h11-12,17-18,23-26,28-30H,3-4,6-10,13-16H2,1-2,5H3/b21-11+,22-12-/t17-,18+,23-,24+,25-,26-,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50388434

(CHEMBL605525)Show SMILES [#6]-[#6@@H](-[#6]-[#6]-[#6]C([#6])([#6])[#8])-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)22-12-13-23-21(9-7-15-27(22,23)5)11-10-20-16-24(28)19(2)25(29)17-20/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b21-11+/t18-,22+,23-,24+,25+,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417514

(CHEMBL1630754)Show SMILES [#6]-[#6@@H](-[#8])-[#6]-[#6]-[#6]-[#6@@H](-[#6])-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C26H42O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-18,22-25,27-29H,3,5-9,12-16H2,1-2,4H3/b21-11+/t17-,18-,22-,23+,24-,25-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417516

(CHEMBL1630756)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1)[C@H](C)CCC[C@H](C)O |r,wU:16.18,10.12,18.20,wD:20.22,4.4,1.0,23.26,28.32,@:18,(-2.52,-29.03,;-4,-29.43,;-3.11,-30.68,;-4.02,-31.92,;-5.47,-31.43,;-5.48,-32.97,;-6.8,-32.2,;-8.14,-31.43,;-8.13,-29.89,;-6.79,-29.12,;-5.46,-29.9,;-5.48,-28.36,;-6.8,-33.74,;-8.13,-34.51,;-8.13,-36.05,;-6.8,-36.83,;-6.8,-38.37,;-5.47,-39.14,;-8.13,-39.13,;-8.13,-40.67,;-9.46,-38.37,;-10.79,-39.14,;-9.46,-36.83,;-4.02,-27.89,;-5.36,-27.14,;-2.66,-27.16,;-1.35,-27.98,;0,-27.25,;1.31,-28.06,;2.67,-27.33,;1.31,-29.6,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18+,19?,22-,23+,24-,25-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417518
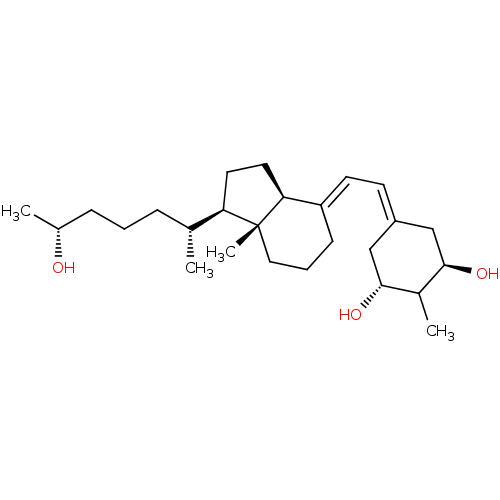
(CHEMBL1630758)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1)[C@H](C)CCC[C@@H](C)O |r,wU:16.18,10.12,18.20,28.32,wD:20.22,4.4,1.0,23.26,@:18,(22,-29.84,;20.52,-30.25,;21.41,-31.5,;20.5,-32.73,;19.05,-32.25,;19.04,-33.79,;17.73,-33.02,;16.38,-32.25,;16.39,-30.71,;17.73,-29.94,;19.06,-30.72,;19.05,-29.17,;17.73,-34.56,;16.39,-35.33,;16.39,-36.87,;17.72,-37.64,;17.72,-39.18,;19.06,-39.96,;16.39,-39.95,;16.39,-41.49,;15.07,-39.18,;13.73,-39.96,;15.07,-37.64,;20.5,-28.71,;19.16,-27.96,;21.86,-27.98,;23.17,-28.79,;24.53,-28.07,;25.84,-28.88,;27.19,-28.15,;25.83,-30.41,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18-,19?,22-,23+,24-,25-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417518

(CHEMBL1630758)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1)[C@H](C)CCC[C@@H](C)O |r,wU:16.18,10.12,18.20,28.32,wD:20.22,4.4,1.0,23.26,@:18,(22,-29.84,;20.52,-30.25,;21.41,-31.5,;20.5,-32.73,;19.05,-32.25,;19.04,-33.79,;17.73,-33.02,;16.38,-32.25,;16.39,-30.71,;17.73,-29.94,;19.06,-30.72,;19.05,-29.17,;17.73,-34.56,;16.39,-35.33,;16.39,-36.87,;17.72,-37.64,;17.72,-39.18,;19.06,-39.96,;16.39,-39.95,;16.39,-41.49,;15.07,-39.18,;13.73,-39.96,;15.07,-37.64,;20.5,-28.71,;19.16,-27.96,;21.86,-27.98,;23.17,-28.79,;24.53,-28.07,;25.84,-28.88,;27.19,-28.15,;25.83,-30.41,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18-,19?,22-,23+,24-,25-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417513

(CHEMBL1630752)Show SMILES C[C@H](O)CCC[C@@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C(=C)[C@H](O)C1=C |r| Show InChI InChI=1S/C27H42O3/c1-17(8-6-9-18(2)28)23-13-14-24-21(10-7-15-27(23,24)5)11-12-22-16-25(29)20(4)26(30)19(22)3/h11-12,17-18,23-26,28-30H,3-4,6-10,13-16H2,1-2,5H3/b21-11+,22-12-/t17-,18+,23-,24+,25-,26-,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417519

(CHEMBL1630759)Show SMILES C[C@@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,11.10,16.18,8.7,6.6,1.1,wD:26.28,(1.96,-41.79,;.6,-42.52,;.6,-44.06,;-.7,-41.71,;-2.06,-42.44,;-3.37,-41.62,;-4.73,-42.35,;-6.07,-41.6,;-4.71,-43.89,;-3.82,-45.14,;-4.73,-46.37,;-6.18,-45.89,;-7.5,-46.66,;-8.85,-45.89,;-8.84,-44.35,;-7.5,-43.58,;-6.17,-44.36,;-6.18,-42.82,;-7.5,-48.2,;-8.84,-48.97,;-8.84,-50.51,;-7.51,-51.29,;-7.51,-52.83,;-6.17,-53.6,;-8.84,-53.59,;-8.84,-55.13,;-10.17,-52.83,;-11.51,-53.6,;-10.17,-51.29,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18+,19?,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417517

(CHEMBL1630757)Show SMILES C[C@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,11.10,16.18,8.7,6.6,wD:26.28,1.1,(14.49,-28.73,;13.13,-29.45,;13.13,-30.99,;11.83,-28.64,;10.47,-29.37,;9.16,-28.56,;7.8,-29.29,;6.46,-28.53,;7.82,-30.83,;8.71,-32.07,;7.8,-33.31,;6.35,-32.82,;5.03,-33.59,;3.68,-32.83,;3.69,-31.28,;5.03,-30.51,;6.36,-31.29,;6.35,-29.75,;5.03,-35.13,;3.69,-35.9,;3.69,-37.44,;5.02,-38.22,;5.02,-39.76,;6.36,-40.53,;3.69,-40.52,;3.69,-42.06,;2.36,-39.76,;1.03,-40.53,;2.36,-38.22,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18-,19?,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417516

(CHEMBL1630756)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1)[C@H](C)CCC[C@H](C)O |r,wU:16.18,10.12,18.20,wD:20.22,4.4,1.0,23.26,28.32,@:18,(-2.52,-29.03,;-4,-29.43,;-3.11,-30.68,;-4.02,-31.92,;-5.47,-31.43,;-5.48,-32.97,;-6.8,-32.2,;-8.14,-31.43,;-8.13,-29.89,;-6.79,-29.12,;-5.46,-29.9,;-5.48,-28.36,;-6.8,-33.74,;-8.13,-34.51,;-8.13,-36.05,;-6.8,-36.83,;-6.8,-38.37,;-5.47,-39.14,;-8.13,-39.13,;-8.13,-40.67,;-9.46,-38.37,;-10.79,-39.14,;-9.46,-36.83,;-4.02,-27.89,;-5.36,-27.14,;-2.66,-27.16,;-1.35,-27.98,;0,-27.25,;1.31,-28.06,;2.67,-27.33,;1.31,-29.6,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18+,19?,22-,23+,24-,25-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50556532
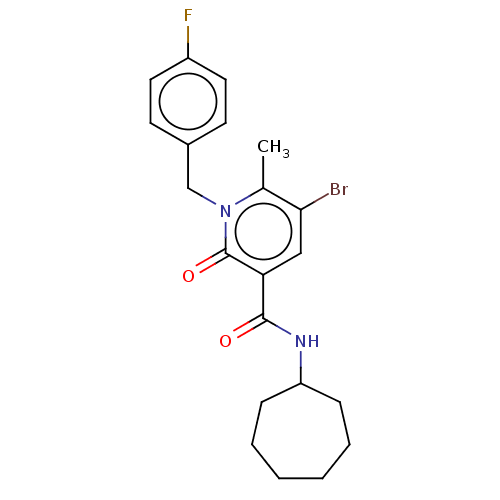
(CHEMBL4753891)Show SMILES Cc1c(Br)cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50292629
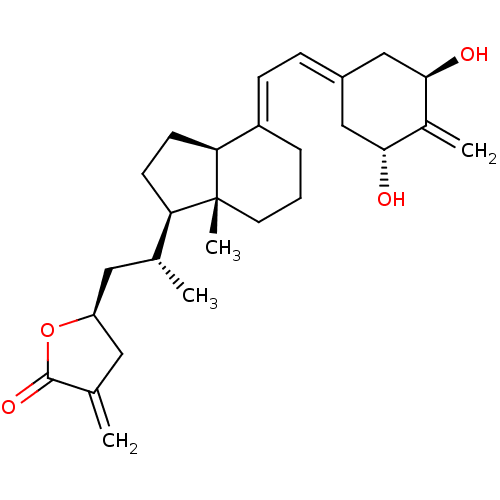
((23S)-25-dehydro-2-methylene-19-nor-1alpha-hydroxy...)Show SMILES [#6]-[#6@H](-[#6]-[#6@H]-1-[#6]-[#6](=[#6])-[#6](=O)-[#8]-1)-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C27H38O4/c1-16(12-21-13-17(2)26(30)31-21)22-9-10-23-20(6-5-11-27(22,23)4)8-7-19-14-24(28)18(3)25(29)15-19/h7-8,16,21-25,28-29H,2-3,5-6,9-15H2,1,4H3/b20-8+/t16-,21+,22-,23+,24-,25-,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
Bioorg Med Chem 16: 8563-73 (2008)
Article DOI: 10.1016/j.bmc.2008.08.011
BindingDB Entry DOI: 10.7270/Q20001X4 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417519

(CHEMBL1630759)Show SMILES C[C@@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,11.10,16.18,8.7,6.6,1.1,wD:26.28,(1.96,-41.79,;.6,-42.52,;.6,-44.06,;-.7,-41.71,;-2.06,-42.44,;-3.37,-41.62,;-4.73,-42.35,;-6.07,-41.6,;-4.71,-43.89,;-3.82,-45.14,;-4.73,-46.37,;-6.18,-45.89,;-7.5,-46.66,;-8.85,-45.89,;-8.84,-44.35,;-7.5,-43.58,;-6.17,-44.36,;-6.18,-42.82,;-7.5,-48.2,;-8.84,-48.97,;-8.84,-50.51,;-7.51,-51.29,;-7.51,-52.83,;-6.17,-53.6,;-8.84,-53.59,;-8.84,-55.13,;-10.17,-52.83,;-11.51,-53.6,;-10.17,-51.29,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18+,19?,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50601131

(CHEMBL5193398)Show SMILES Cc1c(Br)cn(CCCc2cn(CCCCCn3c(C)c(Br)cc(C(=O)NC4CCCCCC4)c3=O)nn2)c(=O)c1NC(=O)C1CCCCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50601132

(CHEMBL5204176)Show SMILES Cc1nc(OCCCCCn2cc(CCCn3cc(Br)c(C)c(NC(=O)C4CCCCCC4)c3=O)nn2)c(cc1Br)C(=O)NC1CCCCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50292630
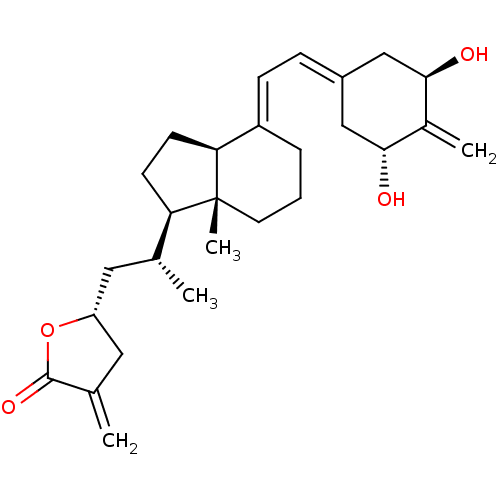
((23R)-25-dehydro-2-methylene-19-nor-1alpha-hydroxy...)Show SMILES [#6]-[#6@H](-[#6]-[#6@@H]-1-[#6]-[#6](=[#6])-[#6](=O)-[#8]-1)-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r| Show InChI InChI=1S/C27H38O4/c1-16(12-21-13-17(2)26(30)31-21)22-9-10-23-20(6-5-11-27(22,23)4)8-7-19-14-24(28)18(3)25(29)15-19/h7-8,16,21-25,28-29H,2-3,5-6,9-15H2,1,4H3/b20-8+/t16-,21-,22-,23+,24-,25-,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
Bioorg Med Chem 16: 8563-73 (2008)
Article DOI: 10.1016/j.bmc.2008.08.011
BindingDB Entry DOI: 10.7270/Q20001X4 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417518

(CHEMBL1630758)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1)[C@H](C)CCC[C@@H](C)O |r,wU:16.18,10.12,18.20,28.32,wD:20.22,4.4,1.0,23.26,@:18,(22,-29.84,;20.52,-30.25,;21.41,-31.5,;20.5,-32.73,;19.05,-32.25,;19.04,-33.79,;17.73,-33.02,;16.38,-32.25,;16.39,-30.71,;17.73,-29.94,;19.06,-30.72,;19.05,-29.17,;17.73,-34.56,;16.39,-35.33,;16.39,-36.87,;17.72,-37.64,;17.72,-39.18,;19.06,-39.96,;16.39,-39.95,;16.39,-41.49,;15.07,-39.18,;13.73,-39.96,;15.07,-37.64,;20.5,-28.71,;19.16,-27.96,;21.86,-27.98,;23.17,-28.79,;24.53,-28.07,;25.84,-28.88,;27.19,-28.15,;25.83,-30.41,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18-,19?,22-,23+,24-,25-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50029051
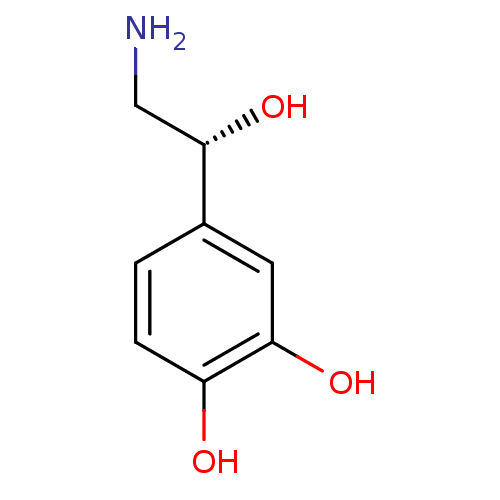
((-)-arterenol | (-)-noradrenaline | (-)-norepineph...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2/t8-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Tested for binding affinity against alpha-2 adrenergic receptor in rat brain using [3H]- rauwolscine as radioligand |
J Med Chem 36: 3077-86 (1993)
BindingDB Entry DOI: 10.7270/Q2PG1SB1 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417516

(CHEMBL1630756)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1)[C@H](C)CCC[C@H](C)O |r,wU:16.18,10.12,18.20,wD:20.22,4.4,1.0,23.26,28.32,@:18,(-2.52,-29.03,;-4,-29.43,;-3.11,-30.68,;-4.02,-31.92,;-5.47,-31.43,;-5.48,-32.97,;-6.8,-32.2,;-8.14,-31.43,;-8.13,-29.89,;-6.79,-29.12,;-5.46,-29.9,;-5.48,-28.36,;-6.8,-33.74,;-8.13,-34.51,;-8.13,-36.05,;-6.8,-36.83,;-6.8,-38.37,;-5.47,-39.14,;-8.13,-39.13,;-8.13,-40.67,;-9.46,-38.37,;-10.79,-39.14,;-9.46,-36.83,;-4.02,-27.89,;-5.36,-27.14,;-2.66,-27.16,;-1.35,-27.98,;0,-27.25,;1.31,-28.06,;2.67,-27.33,;1.31,-29.6,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18+,19?,22-,23+,24-,25-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50556532

(CHEMBL4753891)Show SMILES Cc1c(Br)cc(C(=O)NC2CCCCCC2)c(=O)n1Cc1ccc(F)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417517

(CHEMBL1630757)Show SMILES C[C@H](O)CCC[C@H](C)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1 |r,wU:22.24,11.10,16.18,8.7,6.6,wD:26.28,1.1,(14.49,-28.73,;13.13,-29.45,;13.13,-30.99,;11.83,-28.64,;10.47,-29.37,;9.16,-28.56,;7.8,-29.29,;6.46,-28.53,;7.82,-30.83,;8.71,-32.07,;7.8,-33.31,;6.35,-32.82,;5.03,-33.59,;3.68,-32.83,;3.69,-31.28,;5.03,-30.51,;6.36,-31.29,;6.35,-29.75,;5.03,-35.13,;3.69,-35.9,;3.69,-37.44,;5.02,-38.22,;5.02,-39.76,;6.36,-40.53,;3.69,-40.52,;3.69,-42.06,;2.36,-39.76,;1.03,-40.53,;2.36,-38.22,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18-,19?,22+,23-,24+,25+,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50010891

(1-Aminomethyl-isochroman-5,6-diol | 1-Aminomethyl-...)Show InChI InChI=1S/C10H13NO3/c11-5-9-6-1-2-8(12)10(13)7(6)3-4-14-9/h1-2,9,12-13H,3-5,11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Tested for binding affinity against alpha-2 adrenergic receptor in rat brain using [3H]- rauwolscine as radioligand |
J Med Chem 36: 3077-86 (1993)
BindingDB Entry DOI: 10.7270/Q2PG1SB1 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50570253

(CHEMBL4467500)Show SMILES Cc1c(Br)cn(Cc2ccc(F)cc2)c(=O)c1NC(=O)C1CCCCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417518

(CHEMBL1630758)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1)[C@H](C)CCC[C@@H](C)O |r,wU:16.18,10.12,18.20,28.32,wD:20.22,4.4,1.0,23.26,@:18,(22,-29.84,;20.52,-30.25,;21.41,-31.5,;20.5,-32.73,;19.05,-32.25,;19.04,-33.79,;17.73,-33.02,;16.38,-32.25,;16.39,-30.71,;17.73,-29.94,;19.06,-30.72,;19.05,-29.17,;17.73,-34.56,;16.39,-35.33,;16.39,-36.87,;17.72,-37.64,;17.72,-39.18,;19.06,-39.96,;16.39,-39.95,;16.39,-41.49,;15.07,-39.18,;13.73,-39.96,;15.07,-37.64,;20.5,-28.71,;19.16,-27.96,;21.86,-27.98,;23.17,-28.79,;24.53,-28.07,;25.84,-28.88,;27.19,-28.15,;25.83,-30.41,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18-,19?,22-,23+,24-,25-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM92778
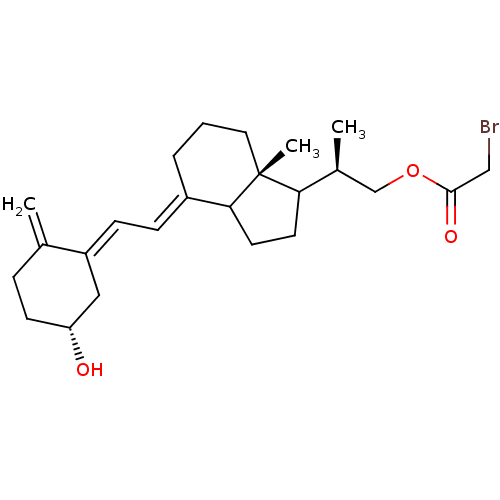
(AB47)Show SMILES C[C@@H](COC(=O)CBr)C1CCC2\C(CCC[C@]12C)=C\C=C1\C[C@H](O)CCC1=C |r| Show InChI InChI=1S/C24H35BrO3/c1-16-6-9-20(26)13-19(16)8-7-18-5-4-12-24(3)21(10-11-22(18)24)17(2)15-28-23(27)14-25/h7-8,17,20-22,26H,1,4-6,9-15H2,2-3H3/b18-7+,19-8-/t17-,20+,21?,22?,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin
| Assay Description
Inhibition assay using CYP24A1 and CYP27B1. |
Biochemistry 49: 10403-11 (2010)
Article DOI: 10.1021/bi101488p
BindingDB Entry DOI: 10.7270/Q2CC0Z80 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM92780
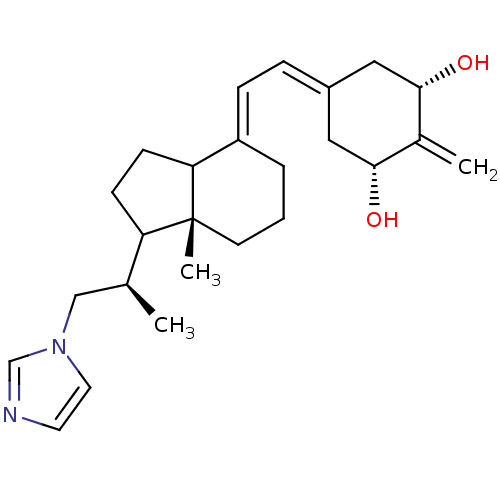
(VIMI)Show SMILES C[C@@H](Cn1ccnc1)C1CCC2\C(CCC[C@]12C)=C\C=C1\C[C@H](O)C(=C)[C@H](O)C1 |r| Show InChI InChI=1S/C25H36N2O2/c1-17(15-27-12-11-26-16-27)21-8-9-22-20(5-4-10-25(21,22)3)7-6-19-13-23(28)18(2)24(29)14-19/h6-7,11-12,16-17,21-24,28-29H,2,4-5,8-10,13-15H2,1,3H3/b19-6-,20-7+/t17-,21?,22?,23-,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin
| Assay Description
Inhibition assay using CYP24A1 and CYP27B1. |
Biochemistry 49: 10403-11 (2010)
Article DOI: 10.1021/bi101488p
BindingDB Entry DOI: 10.7270/Q2CC0Z80 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin
| Assay Description
Inhibition assay using CYP24A1 and CYP27B1. |
Biochemistry 49: 10403-11 (2010)
Article DOI: 10.1021/bi101488p
BindingDB Entry DOI: 10.7270/Q2CC0Z80 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Rattus norvegicus) | BDBM50417516

(CHEMBL1630756)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1C[C@@H](O)C(C)[C@H](O)C1)[C@H](C)CCC[C@H](C)O |r,wU:16.18,10.12,18.20,wD:20.22,4.4,1.0,23.26,28.32,@:18,(-2.52,-29.03,;-4,-29.43,;-3.11,-30.68,;-4.02,-31.92,;-5.47,-31.43,;-5.48,-32.97,;-6.8,-32.2,;-8.14,-31.43,;-8.13,-29.89,;-6.79,-29.12,;-5.46,-29.9,;-5.48,-28.36,;-6.8,-33.74,;-8.13,-34.51,;-8.13,-36.05,;-6.8,-36.83,;-6.8,-38.37,;-5.47,-39.14,;-8.13,-39.13,;-8.13,-40.67,;-9.46,-38.37,;-10.79,-39.14,;-9.46,-36.83,;-4.02,-27.89,;-5.36,-27.14,;-2.66,-27.16,;-1.35,-27.98,;0,-27.25,;1.31,-28.06,;2.67,-27.33,;1.31,-29.6,)| Show InChI InChI=1S/C26H44O3/c1-17(7-5-8-18(2)27)22-12-13-23-21(9-6-14-26(22,23)4)11-10-20-15-24(28)19(3)25(29)16-20/h10-11,17-19,22-25,27-29H,5-9,12-16H2,1-4H3/b20-10-,21-11+/t17-,18+,19?,22-,23+,24-,25-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison
Curated by ChEMBL
| Assay Description
Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... |
J Med Chem 53: 8642-9 (2010)
Article DOI: 10.1021/jm1010447
BindingDB Entry DOI: 10.7270/Q23B61D2 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin
| Assay Description
Inhibition assay using CYP24A1 and CYP27B1. |
Biochemistry 49: 10403-11 (2010)
Article DOI: 10.1021/bi101488p
BindingDB Entry DOI: 10.7270/Q2CC0Z80 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00582
BindingDB Entry DOI: 10.7270/Q2NV9PB9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM92779
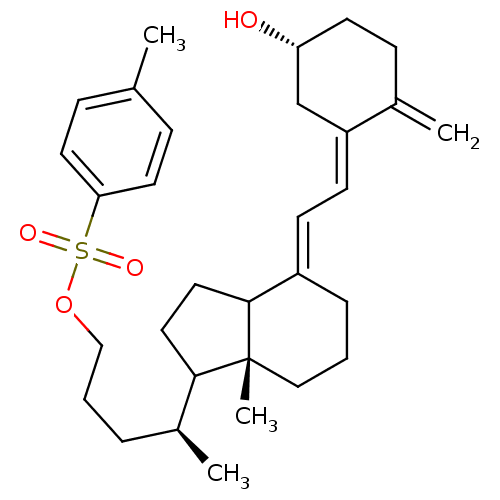
(TS17)Show SMILES C[C@@H](CCCOS(=O)(=O)c1ccc(C)cc1)C1CCC2\C(CCC[C@]12C)=C\C=C1\C[C@H](O)CCC1=C |r| Show InChI InChI=1S/C31H44O4S/c1-22-9-15-28(16-10-22)36(33,34)35-20-6-7-24(3)29-17-18-30-25(8-5-19-31(29,30)4)12-13-26-21-27(32)14-11-23(26)2/h9-10,12-13,15-16,24,27,29-30,32H,2,5-8,11,14,17-21H2,1,3-4H3/b25-12+,26-13-/t24-,27+,29?,30?,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin
| Assay Description
Inhibition assay using CYP24A1 and CYP27B1. |
Biochemistry 49: 10403-11 (2010)
Article DOI: 10.1021/bi101488p
BindingDB Entry DOI: 10.7270/Q2CC0Z80 |
More data for this
Ligand-Target Pair | |
1,25-dihydroxyvitamin D(3) 24-hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM92781
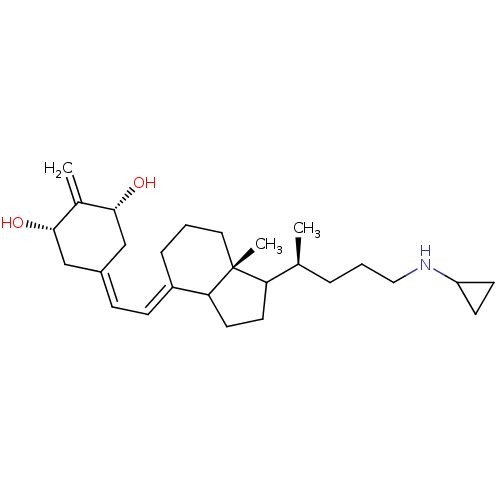
(CPA1)Show SMILES C[C@@H](CCCNC1CC1)C1CCC2\C(CCC[C@]12C)=C\C=C1\C[C@H](O)C(=C)[C@H](O)C1 |r| Show InChI InChI=1S/C27H43NO2/c1-18(6-5-15-28-22-10-11-22)23-12-13-24-21(7-4-14-27(23,24)3)9-8-20-16-25(29)19(2)26(30)17-20/h8-9,18,22-26,28-30H,2,4-7,10-17H2,1,3H3/b20-8-,21-9+/t18-,23?,24?,25-,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin
| Assay Description
Inhibition assay using CYP24A1 and CYP27B1. |
Biochemistry 49: 10403-11 (2010)
Article DOI: 10.1021/bi101488p
BindingDB Entry DOI: 10.7270/Q2CC0Z80 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50036835
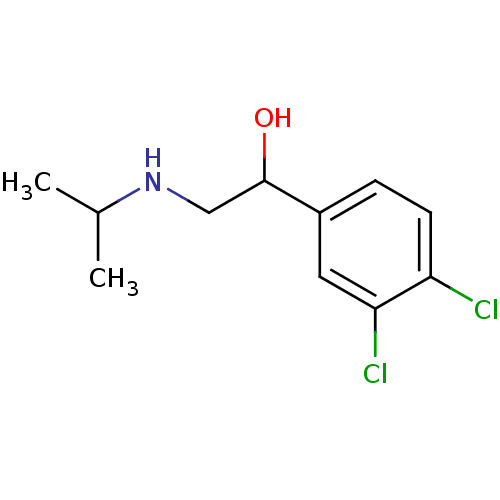
(1-(3,4-Dichloro-phenyl)-2-isopropylamino-ethanol |...)Show InChI InChI=1S/C11H15Cl2NO/c1-7(2)14-6-11(15)8-3-4-9(12)10(13)5-8/h3-5,7,11,14-15H,6H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitá di Pisa
Curated by ChEMBL
| Assay Description
Binding affinity against beta1-adrenergic receptor in rat brain |
J Med Chem 37: 1518-25 (1994)
BindingDB Entry DOI: 10.7270/Q2V69HM4 |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin
| Assay Description
Inhibition assay using CYP24A1 and CYP27B1. |
Biochemistry 49: 10403-11 (2010)
Article DOI: 10.1021/bi101488p
BindingDB Entry DOI: 10.7270/Q2CC0Z80 |
More data for this
Ligand-Target Pair | |
25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial
(Homo sapiens (Human)) | BDBM92778

(AB47)Show SMILES C[C@@H](COC(=O)CBr)C1CCC2\C(CCC[C@]12C)=C\C=C1\C[C@H](O)CCC1=C |r| Show InChI InChI=1S/C24H35BrO3/c1-16-6-9-20(26)13-19(16)8-7-18-5-4-12-24(3)21(10-11-22(18)24)17(2)15-28-23(27)14-25/h7-8,17,20-22,26H,1,4-6,9-15H2,2-3H3/b18-7+,19-8-/t17-,20+,21?,22?,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin
| Assay Description
Inhibition assay using CYP24A1 and CYP27B1. |
Biochemistry 49: 10403-11 (2010)
Article DOI: 10.1021/bi101488p
BindingDB Entry DOI: 10.7270/Q2CC0Z80 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM25392

(4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...)Show InChI InChI=1S/C11H17NO3/c1-7(2)12-6-11(15)8-3-4-9(13)10(14)5-8/h3-5,7,11-15H,6H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Tested for binding affinity against beta-1 adrenergic receptor in rat brain using [3H]-CGP- 26505 as radioligand |
J Med Chem 36: 3077-86 (1993)
BindingDB Entry DOI: 10.7270/Q2PG1SB1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data