 Found 7472 hits with Last Name = 'chin' and Initial = 'j'
Found 7472 hits with Last Name = 'chin' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50266003
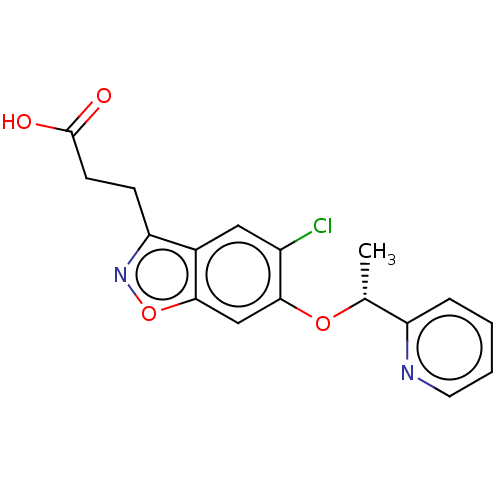
(CHEMBL4091152)Show SMILES C[C@@H](Oc1cc2onc(CCC(O)=O)c2cc1Cl)c1ccccn1 |r| Show InChI InChI=1S/C17H15ClN2O4/c1-10(13-4-2-3-7-19-13)23-16-9-15-11(8-12(16)18)14(20-24-15)5-6-17(21)22/h2-4,7-10H,5-6H2,1H3,(H,21,22)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length human GST-tagged KMO expressed in baculovirus infected Sf9 insect cell membranes using kynurenine as substrate ... |
J Med Chem 60: 3383-3404 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00055
BindingDB Entry DOI: 10.7270/Q26112SB |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50266041

(CHEMBL4070212)Show SMILES C[C@@H](Oc1cc2oc(=O)n(CCC(O)=O)c2cc1Cl)c1ccccn1 |r| Show InChI InChI=1S/C17H15ClN2O5/c1-10(12-4-2-3-6-19-12)24-14-9-15-13(8-11(14)18)20(17(23)25-15)7-5-16(21)22/h2-4,6,8-10H,5,7H2,1H3,(H,21,22)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length human GST-tagged KMO expressed in baculovirus infected Sf9 insect cell membranes using kynurenine as substrate measured aft... |
J Med Chem 60: 3383-3404 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00055
BindingDB Entry DOI: 10.7270/Q26112SB |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642632

(US20230416245, Compound 70)Show SMILES CCNc1cc(nc2n(cnc12)[C@@H]1[C@H]2C[C@@]2([C@@H](O)[C@H]1O)C(=O)NC)C#N |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642621
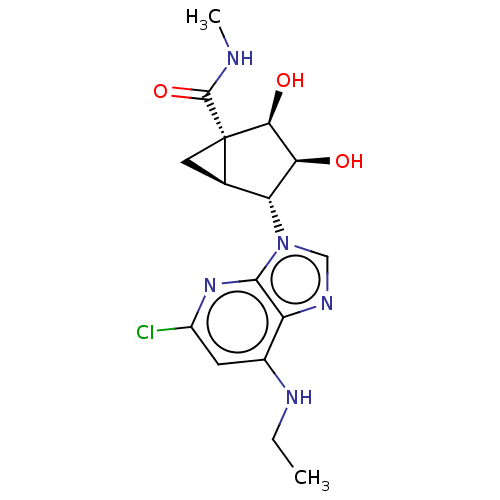
(US20230416245, Compound 59)Show SMILES CCNc1cc(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@]2([C@@H](O)[C@H]1O)C(=O)NC |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642624
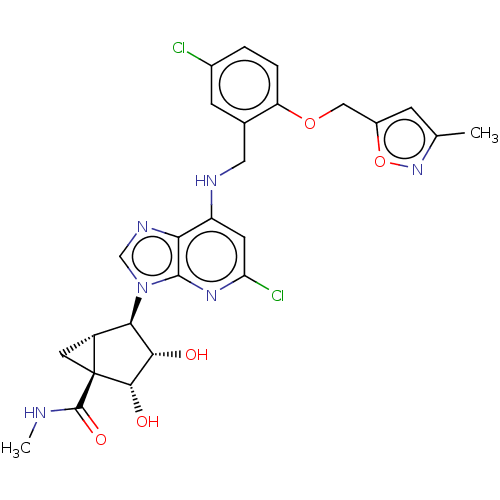
(US20230416245, Compound 62)Show SMILES CNC(=O)[C@@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCc3cc(Cl)ccc3OCc3cc(C)no3)cc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642626
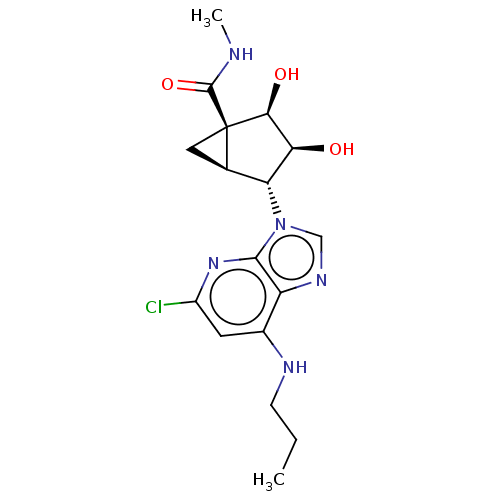
(US20230416245, Compound 64)Show SMILES CCCNc1cc(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@@]2([C@@H](O)[C@H]1O)C(=O)NC |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642625
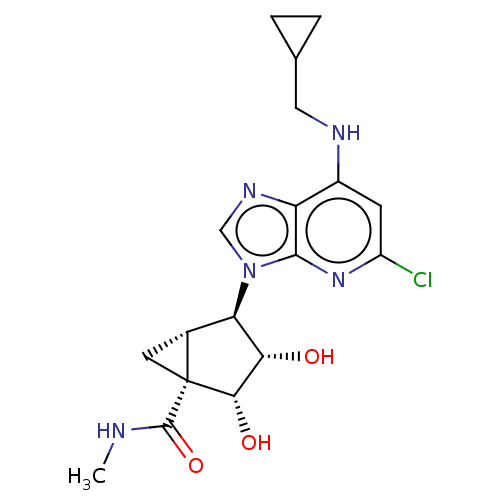
(US20230416245, Compound 63)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCC3CC3)cc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50121205

(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 4 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50266064
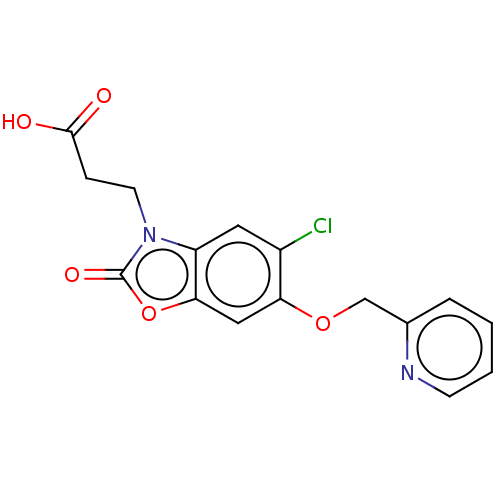
(CHEMBL4104310)Show InChI InChI=1S/C16H13ClN2O5/c17-11-7-12-14(24-16(22)19(12)6-4-15(20)21)8-13(11)23-9-10-3-1-2-5-18-10/h1-3,5,7-8H,4,6,9H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full length human GST-tagged KMO expressed in baculovirus infected Sf9 insect cell membranes using kynurenine as substrate measured aft... |
J Med Chem 60: 3383-3404 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00055
BindingDB Entry DOI: 10.7270/Q26112SB |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642618
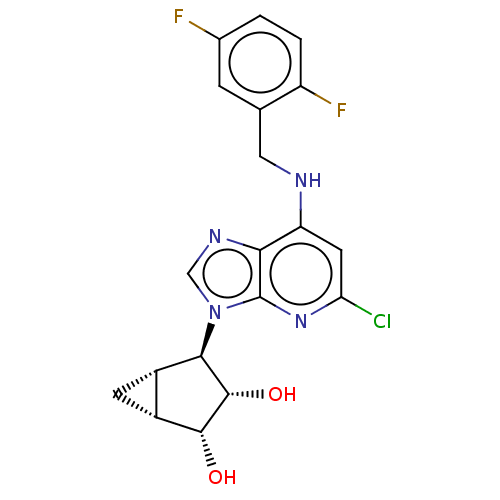
(US20230416245, Compound 45)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NCc3cc(F)ccc3F)cc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642635

(US20230416245, Compound 83)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCC(C)C)cc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642631
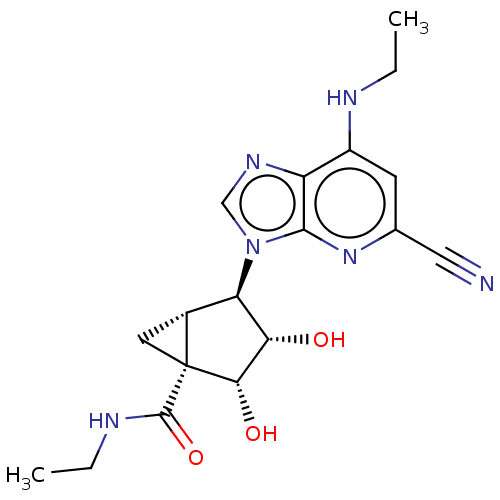
(US20230416245, Compound 69)Show SMILES CCNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCC)cc(nc12)C#N |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642620
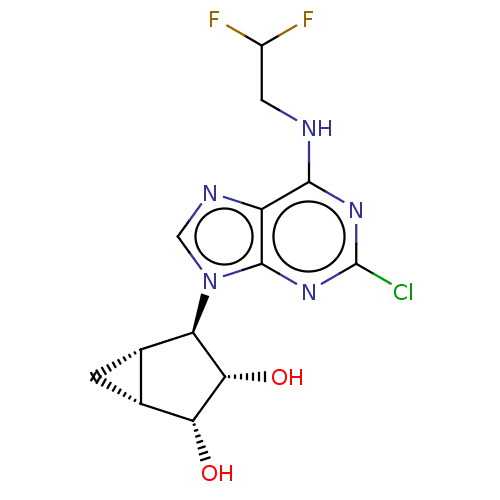
(US20230416245, Compound 35)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NCC(F)F)nc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642615

(US20230416245, Compound 40)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NCC(F)F)cc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642636
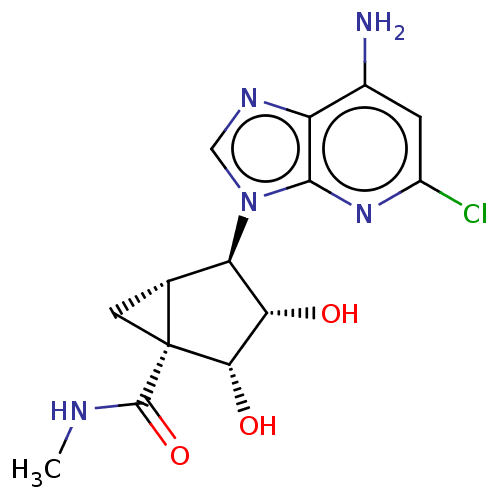
(US20230416245, Compound 90)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(N)cc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50121205

(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642639
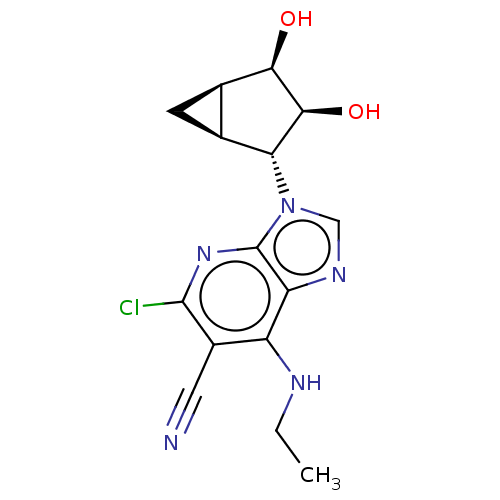
(US20230416245, Compound 93)Show SMILES CCNc1c(C#N)c(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@H]2[C@@H](O)[C@H]1O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642627
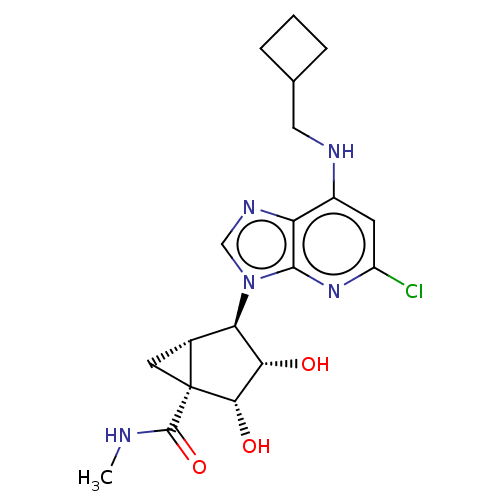
(US20230416245, Compound 65)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCC3CCC3)cc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552436

(CHEMBL4749654) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642630

(US20230416245, Compound 68)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NCc3cc(Cl)ccc3Cl)cc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642634
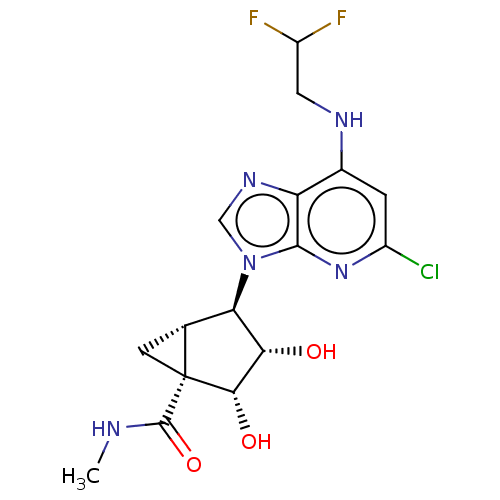
(US20230416245, Compound 82)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCC(F)F)cc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642637
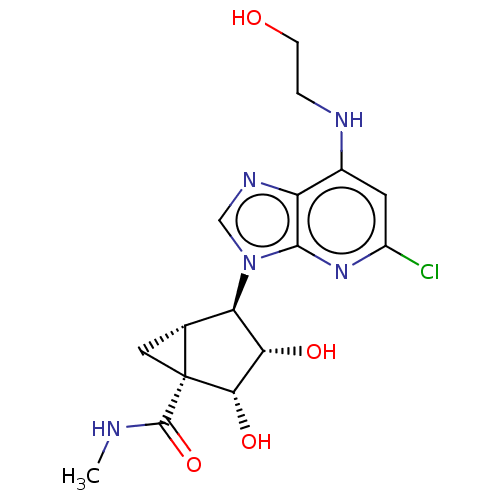
(US20230416245, Compound 91)Show SMILES CNC(=O)[C@]12C[C@@H]1[C@H]([C@H](O)[C@@H]2O)n1cnc2c(NCCO)cc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552434
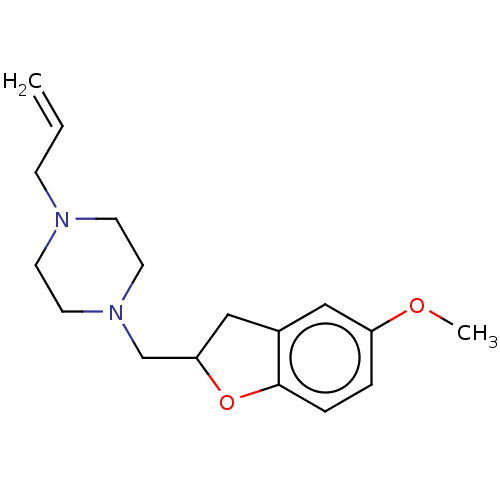
(CHEMBL4747180) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642623

(US20230416245, Compound 61)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NCC(F)F)c(F)c(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 7.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552435
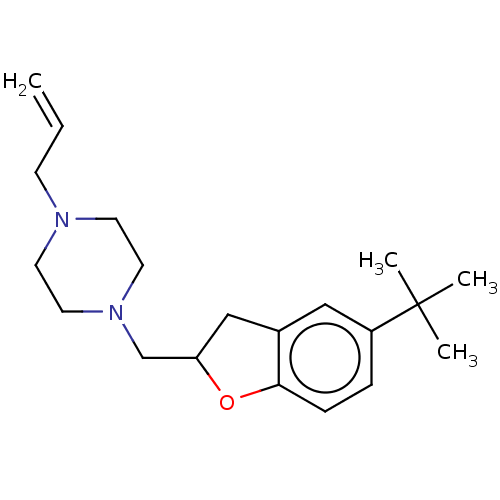
(CHEMBL4756432) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642619
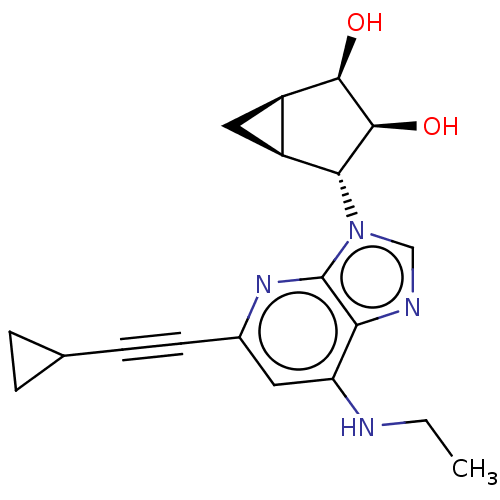
(US20230416245, Compound 46)Show SMILES CCNc1cc(nc2n(cnc12)[C@@H]1[C@H]2C[C@H]2[C@@H](O)[C@H]1O)C#CC1CC1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 21.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642638
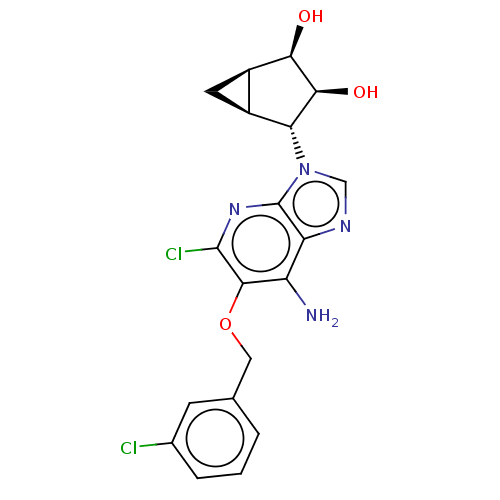
(US20230416245, Compound 92)Show SMILES Nc1c(OCc2cccc(Cl)c2)c(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@H]2[C@@H](O)[C@H]1O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 26.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642617

(US20230416245, Compound 44)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NC3CCC3)cc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 28.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552430

(CHEMBL4798369) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Rattus norvegicus) | BDBM50266003

(CHEMBL4091152)Show SMILES C[C@@H](Oc1cc2onc(CCC(O)=O)c2cc1Cl)c1ccccn1 |r| Show InChI InChI=1S/C17H15ClN2O4/c1-10(13-4-2-3-7-19-13)23-16-9-15-11(8-12(16)18)14(20-24-15)5-6-17(21)22/h2-4,7-10H,5-6H2,1H3,(H,21,22)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of rat KMO expressed in HEK293 cells using kynurenine as substrate measured after 20 hrs by LC-MS/MS analysis |
J Med Chem 60: 3383-3404 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00055
BindingDB Entry DOI: 10.7270/Q26112SB |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50552439

(CHEMBL4568949) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 4 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642629
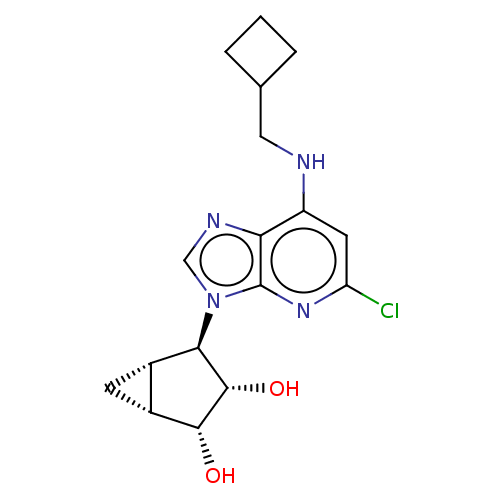
(US20230416245, Compound 67)Show SMILES O[C@@H]1[C@@H]2C[C@@H]2[C@H]([C@@H]1O)n1cnc2c(NCC3CCC3)cc(Cl)nc12 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 94.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642628

(US20230416245, Compound 66)Show SMILES CCCNc1cc(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@H]2[C@@H](O)[C@H]1O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
All-trans-retinol dehydrogenase [NAD(+)] ADH1B
(Homo sapiens (Human)) | BDBM50064288

(CHEMBL46293 | N-(phenylmethyl)formamide | N-BENZYL...)Show InChI InChI=1S/C8H9NO/c10-7-9-6-8-4-2-1-3-5-8/h1-5,7H,6H2,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of human alcohol dehydrogenase beta 1 activity |
J Med Chem 41: 1696-701 (1998)
Article DOI: 10.1021/jm9707380
BindingDB Entry DOI: 10.7270/Q2VM4BC5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
All-trans-retinol dehydrogenase [NAD(+)] ADH1B
(Homo sapiens (Human)) | BDBM50064266
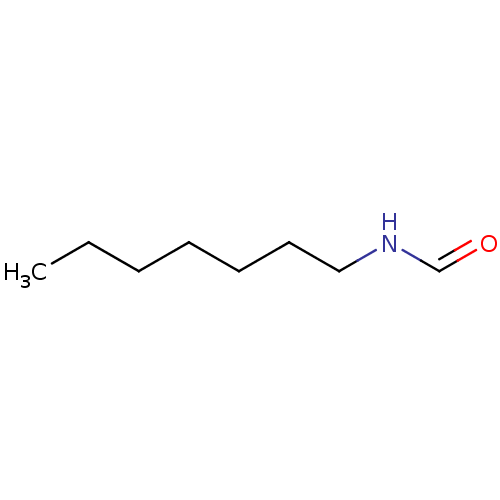
(CHEMBL43719 | HEPTYLFORMAMIDE | N-Heptyl-formamide)Show InChI InChI=1S/C8H17NO/c1-2-3-4-5-6-7-9-8-10/h8H,2-7H2,1H3,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of human alcohol dehydrogenase beta 1 activity |
J Med Chem 41: 1696-701 (1998)
Article DOI: 10.1021/jm9707380
BindingDB Entry DOI: 10.7270/Q2VM4BC5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alcohol dehydrogenase 1A
(Homo sapiens (Human)) | BDBM50064286

(CHEMBL45844 | CYCLOBUTYL(CYCLOPENTYL)FORMAMIDE | N...)Show InChI InChI=1S/C10H17NO/c12-8-11(10-6-3-7-10)9-4-1-2-5-9/h8-10H,1-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of human alcohol dehydrogenase alpha activity |
J Med Chem 41: 1696-701 (1998)
Article DOI: 10.1021/jm9707380
BindingDB Entry DOI: 10.7270/Q2VM4BC5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552441

(CHEMBL4747747) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Alcohol dehydrogenase 1C
(Homo sapiens (Human)) | BDBM50064274
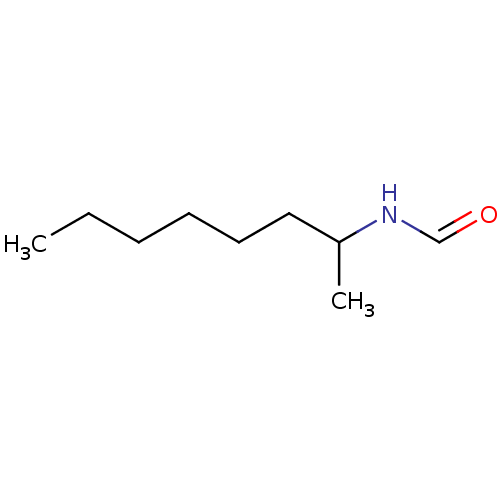
(CHEMBL48122 | N-(1-Methyl-heptyl)-formamide)Show InChI InChI=1S/C9H19NO/c1-3-4-5-6-7-9(2)10-8-11/h8-9H,3-7H2,1-2H3,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of human alcohol dehydrogenase gamma2 activity |
J Med Chem 41: 1696-701 (1998)
Article DOI: 10.1021/jm9707380
BindingDB Entry DOI: 10.7270/Q2VM4BC5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM642633
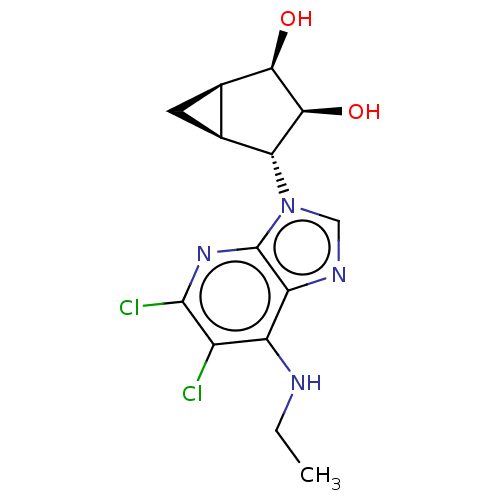
(US20230416245, Compound 71)Show SMILES CCNc1c(Cl)c(Cl)nc2n(cnc12)[C@@H]1[C@H]2C[C@H]2[C@@H](O)[C@H]1O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552429

(CHEMBL4752345) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
All-trans-retinol dehydrogenase [NAD(+)] ADH7
(Homo sapiens (Human)) | BDBM50064266

(CHEMBL43719 | HEPTYLFORMAMIDE | N-Heptyl-formamide)Show InChI InChI=1S/C8H17NO/c1-2-3-4-5-6-7-9-8-10/h8H,2-7H2,1H3,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of human alcohol dehydrogenase sigma activity |
J Med Chem 41: 1696-701 (1998)
Article DOI: 10.1021/jm9707380
BindingDB Entry DOI: 10.7270/Q2VM4BC5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552444
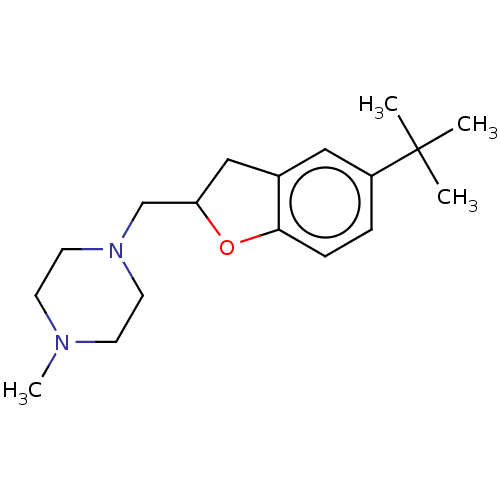
(CHEMBL4764535) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552442
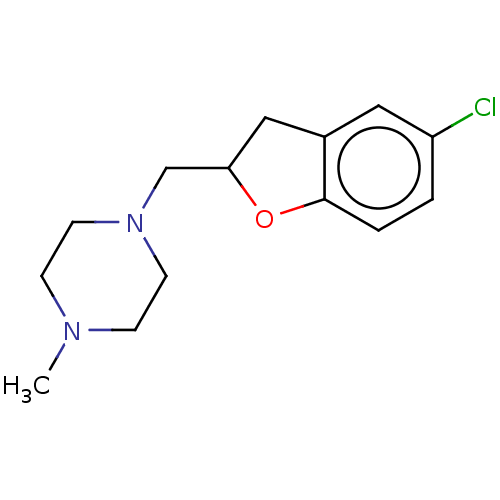
(CHEMBL4792753) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50552443
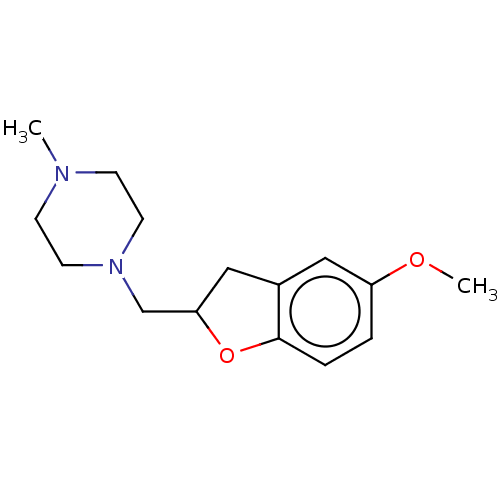
(CHEMBL4758983) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 3 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50552442

(CHEMBL4792753) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-Histamine from human histamine 4 receptor transfected in HEK293T cells incubated for 16 hrs by liquid scintillation counter anal... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115924
BindingDB Entry DOI: 10.7270/Q2833WNJ |
More data for this
Ligand-Target Pair | |
Alcohol dehydrogenase 1A
(Homo sapiens (Human)) | BDBM50064271

(CHEMBL45704 | N-Cyclopentyl-N-cyclopropyl-formamid...)Show InChI InChI=1S/C9H15NO/c11-7-10(9-5-6-9)8-3-1-2-4-8/h7-9H,1-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of human alcohol dehydrogenase alpha activity |
J Med Chem 41: 1696-701 (1998)
Article DOI: 10.1021/jm9707380
BindingDB Entry DOI: 10.7270/Q2VM4BC5 |
More data for this
Ligand-Target Pair | |
All-trans-retinol dehydrogenase [NAD(+)] ADH1B
(Homo sapiens (Human)) | BDBM50064270

(CHEMBL291214 | N-Cyclohexylmethyl-formamide)Show InChI InChI=1S/C8H15NO/c10-7-9-6-8-4-2-1-3-5-8/h7-8H,1-6H2,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of human alcohol dehydrogenase beta 1 activity |
J Med Chem 41: 1696-701 (1998)
Article DOI: 10.1021/jm9707380
BindingDB Entry DOI: 10.7270/Q2VM4BC5 |
More data for this
Ligand-Target Pair | |
All-trans-retinol dehydrogenase [NAD(+)] ADH1B
(Homo sapiens (Human)) | BDBM50064274

(CHEMBL48122 | N-(1-Methyl-heptyl)-formamide)Show InChI InChI=1S/C9H19NO/c1-3-4-5-6-7-9(2)10-8-11/h8-9H,3-7H2,1-2H3,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of human alcohol dehydrogenase beta 1 activity |
J Med Chem 41: 1696-701 (1998)
Article DOI: 10.1021/jm9707380
BindingDB Entry DOI: 10.7270/Q2VM4BC5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alcohol dehydrogenase 1A
(Homo sapiens (Human)) | BDBM50064267
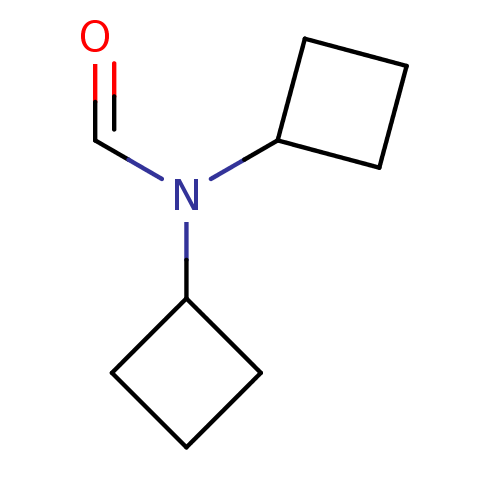
(CHEMBL297125 | N,N-Dicyclobutyl-formamide)Show InChI InChI=1S/C9H15NO/c11-7-10(8-3-1-4-8)9-5-2-6-9/h7-9H,1-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of human alcohol dehydrogenase alpha activity |
J Med Chem 41: 1696-701 (1998)
Article DOI: 10.1021/jm9707380
BindingDB Entry DOI: 10.7270/Q2VM4BC5 |
More data for this
Ligand-Target Pair | |
Alcohol dehydrogenase 1A
(Homo sapiens (Human)) | BDBM50064287
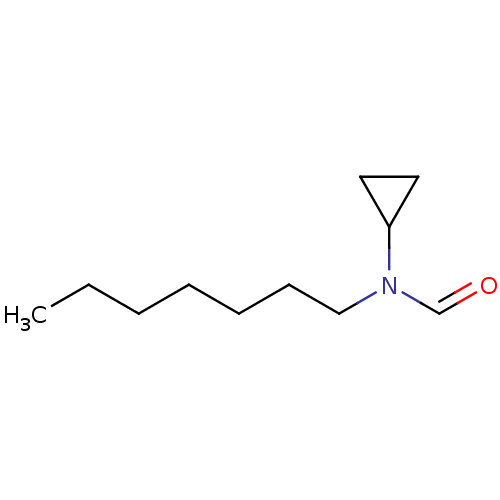
(CHEMBL46655 | N-Cyclopropyl-N-heptyl-formamide)Show InChI InChI=1S/C11H21NO/c1-2-3-4-5-6-9-12(10-13)11-7-8-11/h10-11H,2-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Iowa
Curated by ChEMBL
| Assay Description
Inhibition of human alcohol dehydrogenase alpha activity |
J Med Chem 41: 1696-701 (1998)
Article DOI: 10.1021/jm9707380
BindingDB Entry DOI: 10.7270/Q2VM4BC5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data