

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate after 5 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate after 4 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 10 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Rattus norvegicus (rat)) | BDBM50018011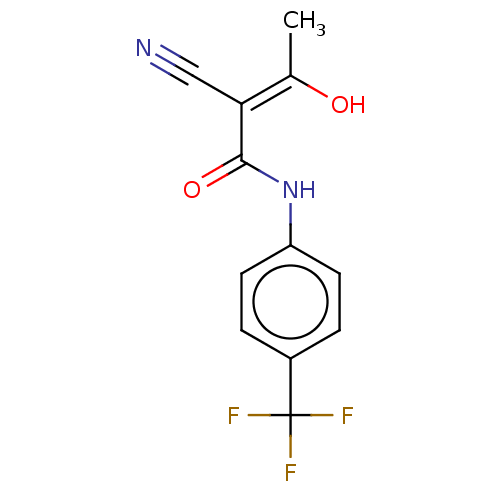 (Aubagio | CHEBI:68540 | HMR-1726 | TERIFLUNOMIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged rat DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay | J Med Chem 63: 4929-4956 (2020) Article DOI: 10.1021/acs.jmedchem.0c00311 BindingDB Entry DOI: 10.7270/Q2M90D6Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50305505 (CHEMBL227855 | OZ-209 | dispiro[adamantane-2,2'-[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | Bioorg Med Chem Lett 20: 563-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.088 BindingDB Entry DOI: 10.7270/Q2RJ4JKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Mus musculus) | BDBM50018011 (Aubagio | CHEBI:68540 | HMR-1726 | TERIFLUNOMIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged mouse DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay | J Med Chem 63: 4929-4956 (2020) Article DOI: 10.1021/acs.jmedchem.0c00311 BindingDB Entry DOI: 10.7270/Q2M90D6Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550378 (CHEMBL4799175) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550361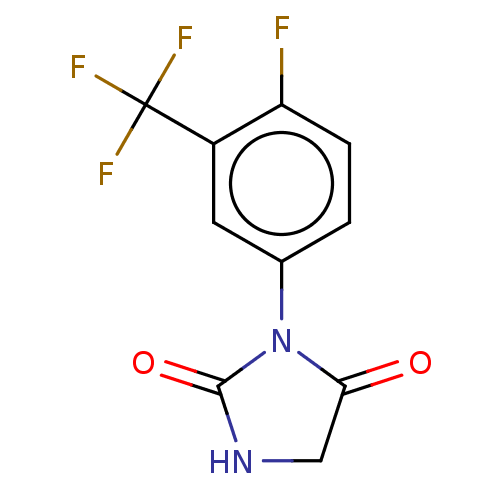 (CHEMBL4793070) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550377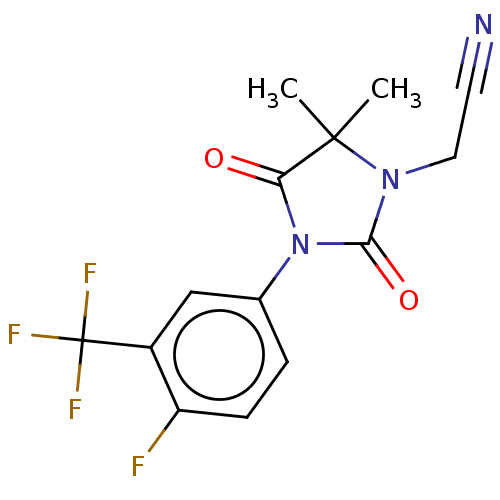 (CHEMBL4758277) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Homo sapiens (Human)) | BDBM50018011 (Aubagio | CHEBI:68540 | HMR-1726 | TERIFLUNOMIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged human DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay | J Med Chem 63: 4929-4956 (2020) Article DOI: 10.1021/acs.jmedchem.0c00311 BindingDB Entry DOI: 10.7270/Q2M90D6Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50135912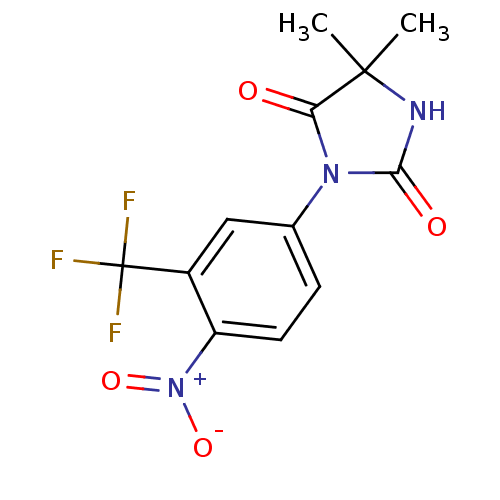 (5,5-Dimethyl-3-(alpha,alpha,alpha-trifluoro-4-nitr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50090677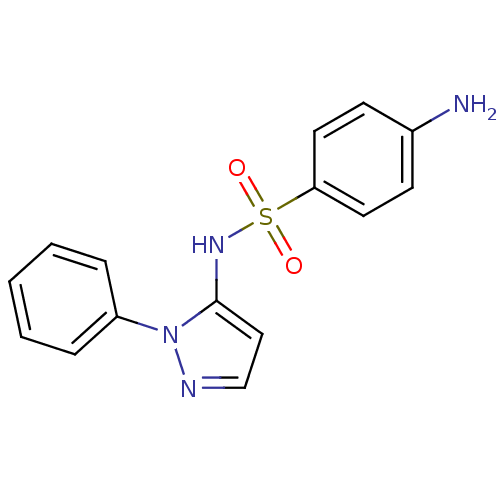 (4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 20 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550354 (CHEMBL4787997) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550357 (CHEMBL4755665) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50180847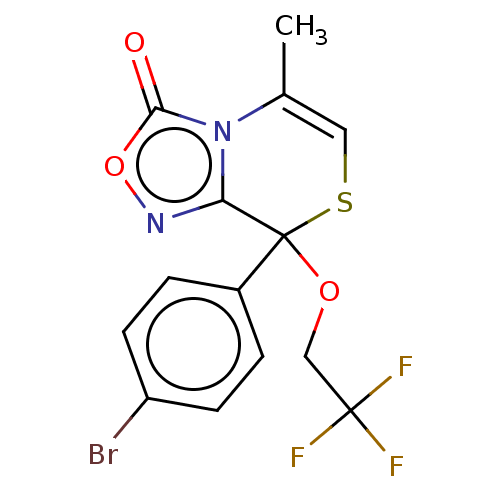 (CHEMBL3408523) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate after 40 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM85509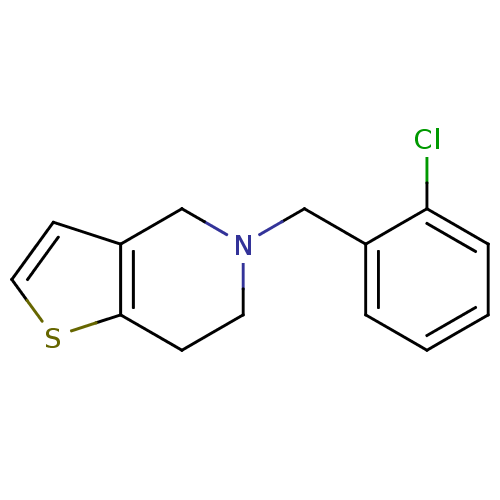 (CAS_55142-85-3 | NSC_5472 | Ticlopidine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate after 40 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50236897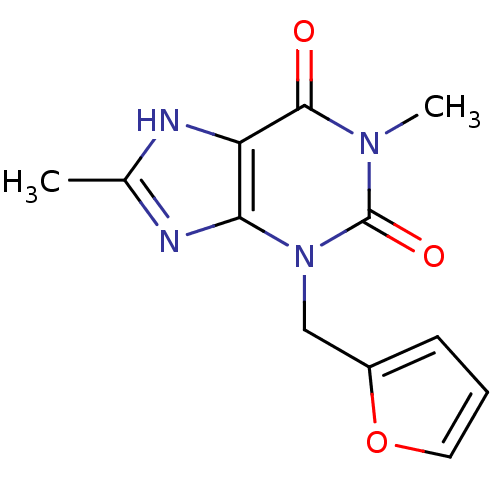 (3-(furan-2-ylmethyl)-1,8-dimethyl-1H-purine-2,6(3H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 30 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550379 (CHEMBL4778974) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550372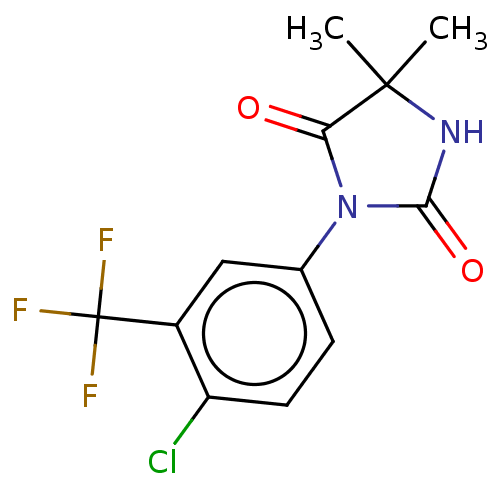 (CHEMBL4750103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550366 (CHEMBL4778298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50180846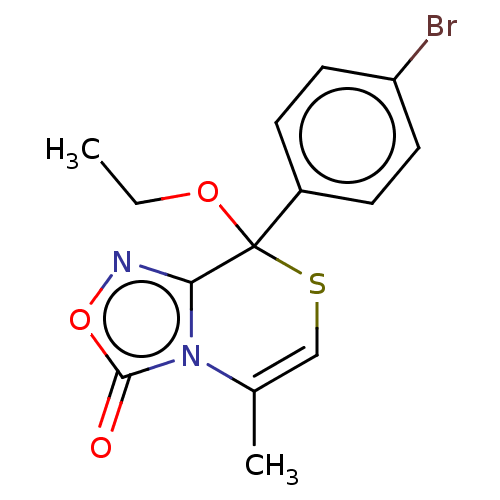 (CHEMBL211204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate after 40 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50201426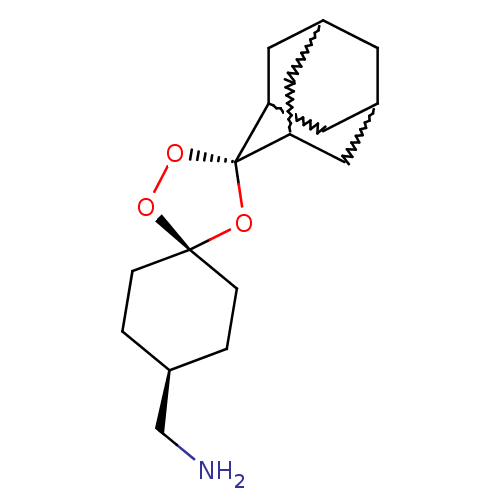 (dispiro[adamantane-2,2'-[1,3,5]trioxolane-4',1''-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of hERG by patch clamp assay | Bioorg Med Chem Lett 17: 1260-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.007 BindingDB Entry DOI: 10.7270/Q2N017B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550353 (CHEMBL4790269) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Mus musculus) | BDBM50365230 (CHEMBL1956285 | US9238653, Table 5, Compound 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged mouse DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay | J Med Chem 63: 4929-4956 (2020) Article DOI: 10.1021/acs.jmedchem.0c00311 BindingDB Entry DOI: 10.7270/Q2M90D6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550376 (CHEMBL4786505) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50180841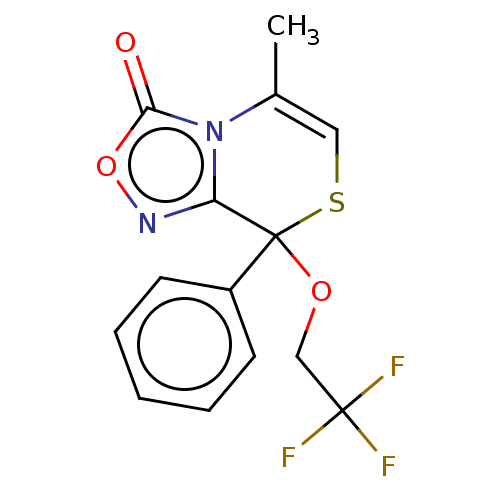 (CHEMBL3819606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate after 40 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50180842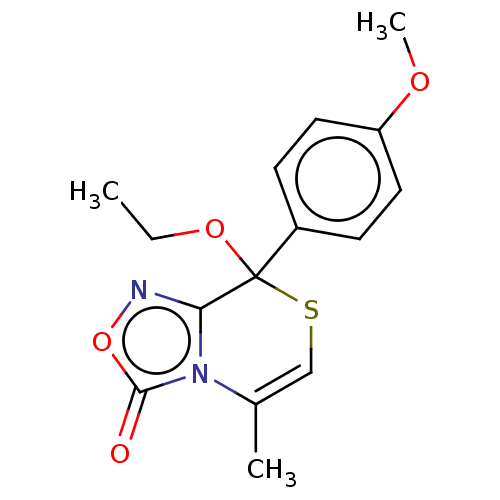 (CHEMBL3818643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate after 40 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50201425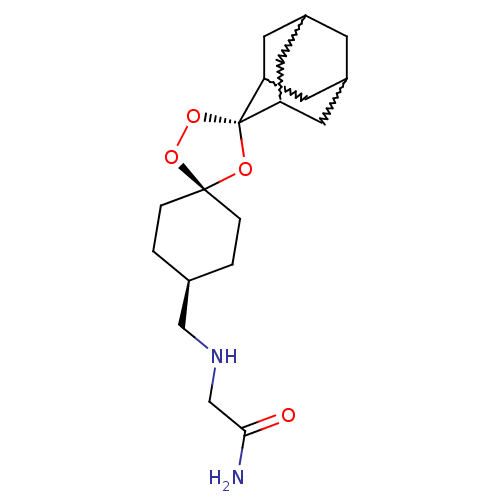 (2-({dispiro[adamantane-2,2'-[1,3,5]trioxolane-4',1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center Curated by ChEMBL | Assay Description Inhibition of hERG by patch clamp assay | Bioorg Med Chem Lett 17: 1260-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.007 BindingDB Entry DOI: 10.7270/Q2N017B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550350 (CHEMBL4786811) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50180846 (CHEMBL211204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 30 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550347 (CHEMBL4572306) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550355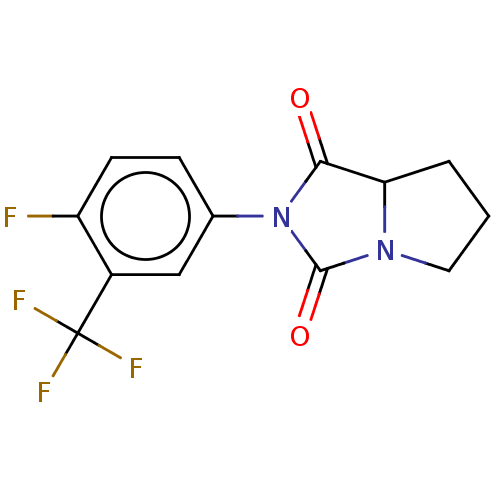 (CHEMBL4778185) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50180844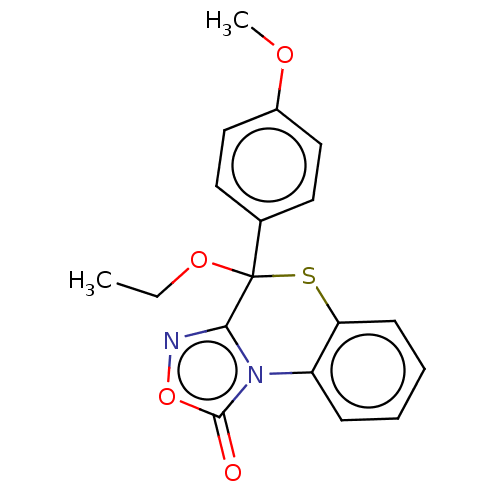 (CHEMBL3818560) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate after 40 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550370 (CHEMBL4799429) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550368 (CHEMBL4782426) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495154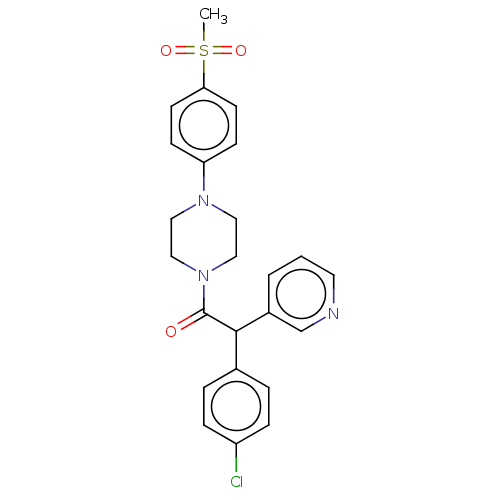 (CHEMBL3104490) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50180846 (CHEMBL211204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate after 10 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550356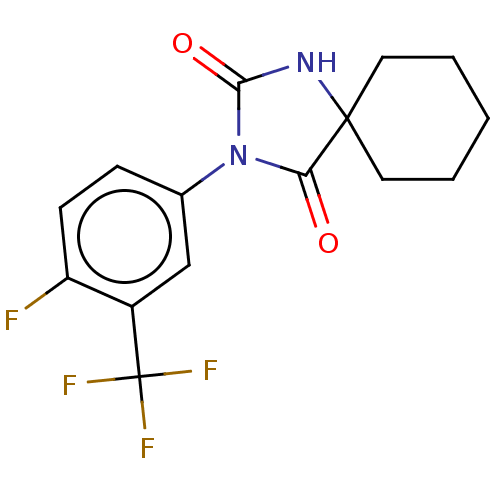 (CHEMBL4747499) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550362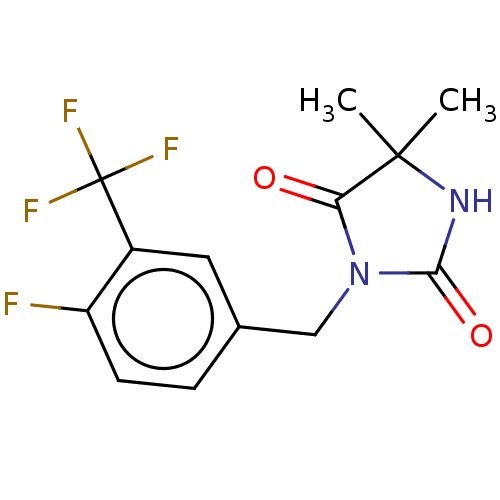 (CHEMBL4743529) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550374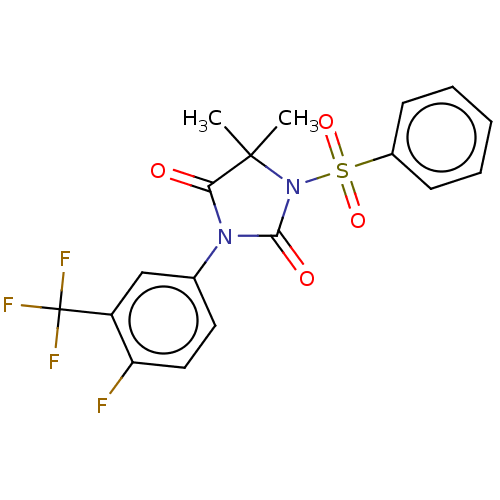 (CHEMBL4748557) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Rattus norvegicus (rat)) | BDBM50365230 (CHEMBL1956285 | US9238653, Table 5, Compound 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of C-terminal His6-tagged rat DHODH expressed in Escherichia coli BL21(DE3) cells using L-DHO as substrate by DCIP dye based assay | J Med Chem 63: 4929-4956 (2020) Article DOI: 10.1021/acs.jmedchem.0c00311 BindingDB Entry DOI: 10.7270/Q2M90D6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550380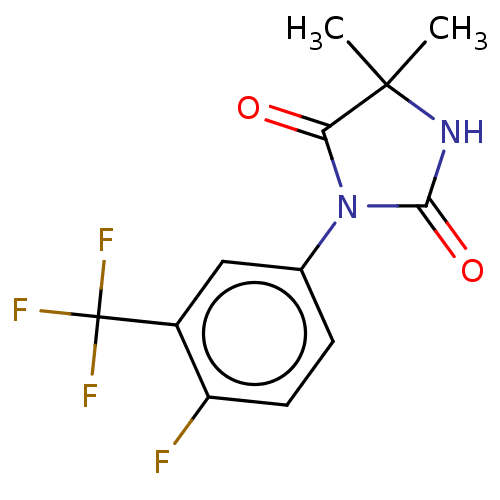 (CHEMBL4465426) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550367 (CHEMBL4748422) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50180840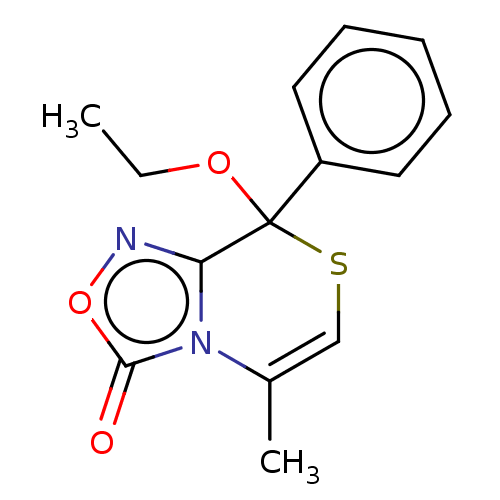 (CHEMBL3819185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate after 40 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550358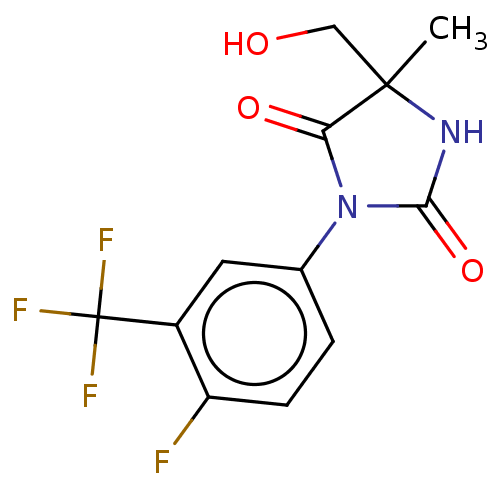 (CHEMBL4793158) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50180847 (CHEMBL3408523) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 30 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50495150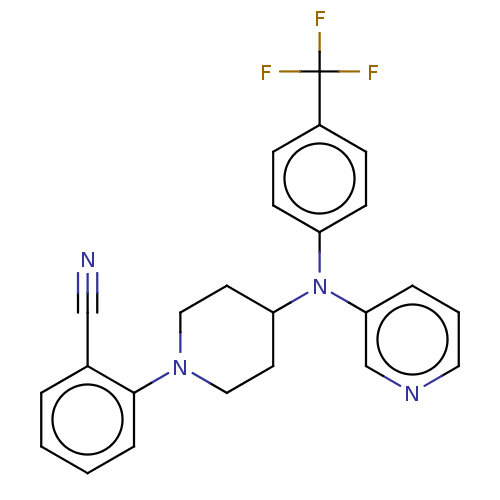 (CHEMBL3104537) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epichem Pty Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as 6beta-hydroxylation of testosterone | J Med Chem 56: 10158-70 (2013) Article DOI: 10.1021/jm401610c BindingDB Entry DOI: 10.7270/Q2VH5RT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50180842 (CHEMBL3818643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 30 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50550359 (CHEMBL4763136) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type androgen receptor in human LAPC4 cells assessed as inhibition of DHT-induced luciferase activity measured after 24 hrs by luc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M0492B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A5 (Homo sapiens (Human)) | BDBM50538344 (CHEMBL4633246) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of CYP3A4/5 in human liver microsomes using midazolam as substrate preincubated for 30 mins followed by substrate addition and measured af... | J Med Chem 63: 4929-4956 (2020) Article DOI: 10.1021/acs.jmedchem.0c00311 BindingDB Entry DOI: 10.7270/Q2M90D6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 265 total ) | Next | Last >> |