 Found 15887 hits with Last Name = 'cho' and Initial = 'a'
Found 15887 hits with Last Name = 'cho' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50407749

(CHEMBL2112110)Show SMILES OC[C@@H]1O[C@@H](C[C@H]1O)n1cnc2[C@H](O)CCC=Nc12 |c:17| Show InChI InChI=1S/C12H17N3O4/c16-5-9-8(18)4-10(19-9)15-6-14-11-7(17)2-1-3-13-12(11)15/h3,6-10,16-18H,1-2,4-5H2/t7-,8-,9+,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Binding affinity towards Adenosine deaminase |
J Med Chem 39: 277-84 (1996)
Article DOI: 10.1021/jm9505674
BindingDB Entry DOI: 10.7270/Q2ZS2X6V |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM50273370
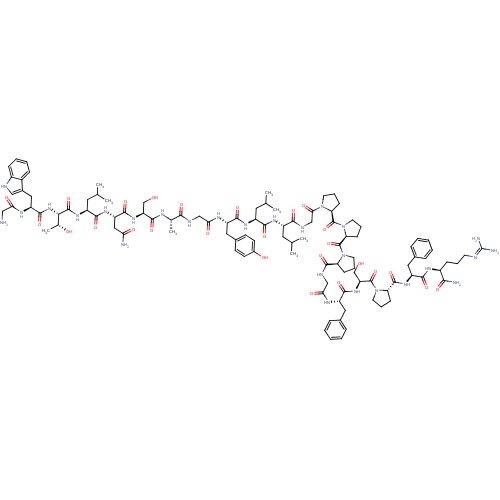
(CHEMBL503473 | GWTLNSAGYLLGPPPGFSPFR-CONH2 | Galan...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(N)=O |r,wU:106.112,4.4,8.12,32.33,37.40,43.48,51.56,63.76,wD:149.157,138.145,134.142,125.131,114.119,99.104,92.96,16.25,59.60,79.83,(16.53,-9.13,;15.19,-9.9,;15.2,-11.44,;13.86,-9.13,;12.52,-9.9,;11.19,-9.13,;11.19,-7.59,;12.53,-6.82,;9.86,-6.82,;9.86,-5.28,;11.2,-4.51,;12.52,-5.28,;11.2,-2.97,;8.13,-7.82,;6.39,-6.82,;6.39,-5.28,;4.66,-7.83,;4.66,-9.36,;5.99,-10.13,;5.99,-11.67,;7.32,-12.44,;8.66,-11.67,;9.99,-12.44,;8.66,-10.13,;7.33,-9.36,;2.92,-6.82,;2.92,-5.28,;4.26,-4.52,;1.19,-4.28,;1.19,-2.75,;2.52,-1.97,;3.86,-2.74,;2.52,-.43,;3.86,.34,;1.2,.34,;1.19,1.88,;2.53,2.65,;-.14,2.65,;-.14,4.19,;1.19,4.96,;-1.48,1.88,;-2.81,2.65,;-2.81,4.19,;-4.14,1.88,;-5.48,2.64,;-5.47,4.19,;-6.81,4.96,;-4.14,4.96,;-4.15,.34,;-5.48,-.43,;-6.81,.34,;-5.48,-1.97,;-6.81,-2.74,;-8.14,-1.97,;-8.14,-.43,;-9.47,-2.74,;-4.14,-2.74,;-4.14,-4.28,;-5.48,-5.05,;-2.81,-5.05,;-2.81,-6.59,;-1.48,-7.36,;-.15,-6.59,;-1.48,-8.9,;-2.85,-9.59,;-4.14,-8.76,;-4.22,-7.22,;-5.71,-6.82,;-6.55,-8.12,;-8.07,-8.35,;-8.62,-9.79,;-7.65,-10.99,;-6.13,-10.75,;-5.58,-9.31,;-.14,-9.67,;1.19,-8.9,;1.19,-7.36,;2.53,-9.67,;2.53,-11.21,;-1.47,-4.28,;-.15,-5.05,;-1.47,-2.74,;12.53,-11.44,;13.86,-12.21,;11.19,-12.21,;11.19,-13.75,;12.53,-14.52,;13.86,-13.75,;12.53,-16.06,;11.28,-16.96,;11.76,-18.43,;13.29,-18.43,;13.77,-16.97,;15.24,-16.49,;15.64,-15,;16.65,-17.91,;16.41,-19.42,;17.78,-20.13,;18.87,-19.04,;18.18,-17.67,;19.08,-15.88,;20.62,-15.8,;18.31,-14.55,;16.78,-14.38,;16.46,-12.88,;17.8,-12.12,;18.94,-13.14,;20.31,-12.44,;21.65,-13.21,;20.31,-10.9,;21.64,-10.13,;22.97,-10.89,;24.3,-10.12,;22.98,-12.43,;24.31,-13.2,;24.32,-14.74,;25.65,-15.5,;25.66,-17.04,;26.99,-17.81,;28.33,-17.04,;28.32,-15.5,;26.99,-14.73,;25.64,-12.43,;25.64,-10.89,;26.98,-13.19,;28.31,-12.42,;29.65,-13.18,;30.98,-12.41,;28.31,-10.88,;26.97,-10.11,;29.64,-10.1,;31.05,-10.72,;32.07,-9.57,;31.3,-8.24,;29.8,-8.57,;28.51,-7.72,;27.17,-8.47,;28.53,-6.17,;29.88,-5.42,;31.2,-6.21,;32.54,-5.45,;32.56,-3.91,;33.91,-3.16,;35.23,-3.95,;35.21,-5.49,;33.87,-6.24,;29.9,-3.88,;31.24,-3.12,;28.57,-3.1,;28.59,-1.55,;29.93,-.8,;29.95,.74,;28.63,1.53,;28.65,3.07,;29.99,3.82,;30.01,5.36,;31.32,3.03,;27.27,-.77,;27.29,.77,;25.92,-1.52,)| Show InChI InChI=1S/C107H153N27O26/c1-57(2)42-70(123-93(147)71(43-58(3)4)124-95(149)74(47-64-33-35-66(138)36-34-64)121-86(141)52-115-91(145)60(7)118-100(154)78(55-135)128-98(152)77(49-84(109)139)125-94(148)72(44-59(5)6)127-103(157)89(61(8)137)130-99(153)76(119-85(140)50-108)48-65-51-114-68-27-16-15-26-67(65)68)92(146)117-54-88(143)131-38-20-31-82(131)105(159)134-41-21-32-83(134)106(160)133-40-18-29-80(133)101(155)116-53-87(142)120-73(45-62-22-11-9-12-23-62)96(150)129-79(56-136)104(158)132-39-19-30-81(132)102(156)126-75(46-63-24-13-10-14-25-63)97(151)122-69(90(110)144)28-17-37-113-107(111)112/h9-16,22-27,33-36,51,57-61,69-83,89,114,135-138H,17-21,28-32,37-50,52-56,108H2,1-8H3,(H2,109,139)(H2,110,144)(H,115,145)(H,116,155)(H,117,146)(H,118,154)(H,119,140)(H,120,142)(H,121,141)(H,122,151)(H,123,147)(H,124,149)(H,125,148)(H,126,156)(H,127,157)(H,128,152)(H,129,150)(H,130,153)(H4,111,112,113)/t60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Binding affinity to human GalR1 |
J Med Chem 53: 1871-5 (2010)
Article DOI: 10.1021/jm9018349
BindingDB Entry DOI: 10.7270/Q26D5TXJ |
More data for this
Ligand-Target Pair | |
Adenosine deaminase
(Homo sapiens (Human)) | BDBM50369126
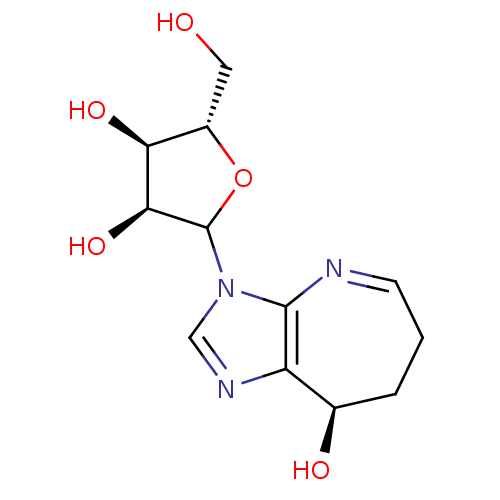
(CONFORMYCIN)Show SMILES OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2[C@H](O)CCC=Nc12 |r,c:18| Show InChI InChI=1S/C12H17N3O5/c16-4-7-9(18)10(19)12(20-7)15-5-14-8-6(17)2-1-3-13-11(8)15/h3,5-7,9-10,12,16-19H,1-2,4H2/t6-,7+,9+,10+,12?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Binding affinity towards Adenosine deaminase |
J Med Chem 39: 277-84 (1996)
Article DOI: 10.1021/jm9505674
BindingDB Entry DOI: 10.7270/Q2ZS2X6V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
J Clin Psychiatry 5-12 (1994)
BindingDB Entry DOI: 10.7270/Q2CR5RW8 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50443007
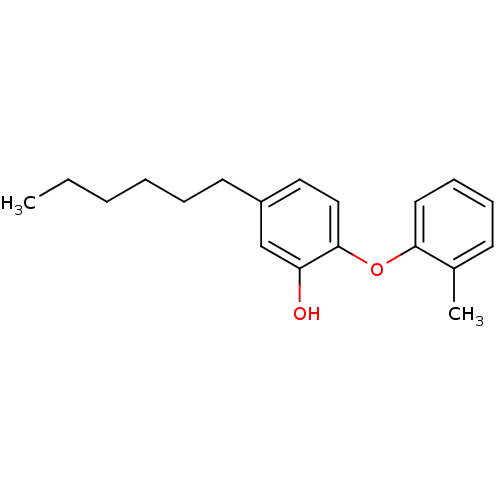
(CHEMBL1236225 | PT70)Show InChI InChI=1S/C19H24O2/c1-3-4-5-6-10-16-12-13-19(17(20)14-16)21-18-11-8-7-9-15(18)2/h7-9,11-14,20H,3-6,10H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis InhA using trans-2-dodecenoyl-CoA as substrate |
Eur J Med Chem 146: 318-343 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.047
BindingDB Entry DOI: 10.7270/Q2GF0X12 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM50378616
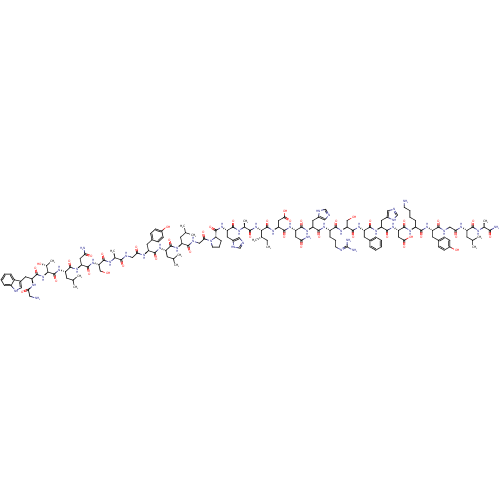
(GALANIN)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(N)=O |r,wU:119.123,135.139,162.167,173.179,200.207,224.233,13.20,42.48,50.61,113.119,93.96,wD:2.2,127.131,145.150,156.161,183.190,191.198,216.224,4.4,8.8,23.23,34.40,66.69,71.76,77.84,85.92,97.112,(19.04,-36.25,;18.27,-34.92,;16.73,-34.92,;15.96,-36.25,;15.96,-33.59,;14.42,-33.59,;13.65,-34.92,;14.42,-36.25,;12.11,-34.92,;11.34,-36.25,;11.34,-33.59,;9.8,-33.59,;9.03,-34.92,;9.03,-32.26,;9.8,-30.92,;9.03,-29.59,;9.66,-28.19,;8.52,-27.16,;7.18,-27.92,;7.5,-29.43,;7.49,-32.26,;6.72,-33.59,;7.49,-34.92,;5.19,-33.67,;4.35,-34.96,;2.86,-34.56,;2.78,-33.03,;4.21,-32.48,;4.62,-30.99,;6.1,-30.59,;3.53,-29.9,;2.04,-30.3,;.95,-29.21,;-.53,-29.61,;1.35,-27.72,;.26,-26.63,;-1.23,-27.03,;-2.31,-25.94,;-1.62,-28.52,;2.83,-27.32,;3.24,-25.83,;2.15,-24.75,;4.72,-25.44,;5.81,-26.53,;5.41,-28.01,;3.93,-28.41,;6.5,-29.11,;5.24,-23.51,;3.83,-22.09,;2.34,-22.49,;4.34,-20.16,;5.83,-19.76,;6.92,-20.85,;8.4,-20.45,;9.5,-21.54,;9.09,-23.02,;10.19,-24.11,;7.61,-23.42,;6.52,-22.33,;2.93,-18.75,;1.44,-19.14,;1.04,-20.63,;.03,-17.72,;-1.46,-18.12,;-1.86,-19.61,;-.77,-20.7,;-3.35,-20.01,;-3.75,-21.5,;-4.43,-18.92,;-5.93,-19.32,;-6.32,-20.81,;-7.01,-18.23,;-8.5,-18.63,;-8.9,-20.12,;-6.61,-16.74,;-7.7,-15.65,;-9.19,-16.05,;-7.3,-14.17,;-8.39,-13.08,;-9.88,-13.47,;-10.97,-12.39,;-10.28,-14.97,;-5.82,-13.77,;-5.42,-12.28,;-6.51,-11.19,;-3.93,-11.88,;-3.53,-10.4,;-4.62,-9.31,;-6.11,-9.71,;-4.22,-7.82,;-2.84,-12.97,;-1.35,-12.57,;-.95,-11.09,;-.27,-13.66,;1.22,-13.27,;2.31,-14.35,;1.91,-15.84,;3.79,-13.96,;4.12,-12.45,;5.58,-11.97,;6.06,-10.51,;7.59,-10.51,;8.07,-11.97,;9.48,-12.6,;9.64,-14.13,;8.39,-15.04,;6.99,-14.41,;6.82,-12.88,;4.89,-15.04,;4.49,-16.53,;3,-16.93,;5.58,-17.62,;7.06,-17.22,;-.66,-15.15,;.43,-16.24,;-2.15,-15.55,;16.73,-32.26,;18.27,-32.26,;15.96,-30.92,;16.73,-29.59,;15.96,-28.26,;14.42,-28.26,;13.65,-29.59,;13.65,-26.93,;18.27,-29.59,;19.04,-28.26,;19.04,-30.92,;20.57,-30.92,;21.35,-32.26,;22.88,-32.26,;23.66,-33.59,;23.66,-30.92,;21.35,-29.59,;22.88,-29.59,;20.57,-28.26,;21.35,-26.93,;20.57,-25.59,;21.35,-24.26,;20.72,-22.86,;21.87,-21.82,;23.2,-22.59,;22.88,-24.09,;22.88,-26.93,;23.66,-28.26,;23.66,-25.59,;25.19,-25.59,;25.97,-26.93,;27.5,-26.93,;28.27,-25.59,;29.81,-25.59,;30.58,-24.26,;32.12,-24.26,;29.81,-22.92,;25.97,-24.26,;27.5,-24.26,;25.19,-22.92,;25.97,-21.59,;27.5,-21.59,;28.27,-20.26,;25.19,-20.26,;25.97,-18.92,;23.66,-20.26,;22.88,-18.92,;21.35,-18.92,;20.57,-17.59,;19.04,-17.59,;18.27,-16.26,;19.04,-14.93,;20.57,-14.93,;21.35,-16.26,;23.66,-17.59,;25.19,-17.59,;22.88,-16.26,;23.66,-14.93,;22.88,-13.59,;23.66,-12.26,;23.03,-10.86,;24.18,-9.82,;25.51,-10.59,;25.19,-12.1,;25.19,-14.93,;25.97,-16.26,;25.97,-13.59,;27.5,-13.59,;28.27,-14.93,;29.81,-14.93,;30.58,-16.26,;30.58,-13.59,;28.27,-12.26,;29.81,-12.26,;27.5,-10.93,;28.27,-9.6,;29.81,-9.6,;30.58,-10.93,;32.12,-10.93,;32.89,-12.26,;34.43,-12.26,;27.5,-8.26,;28.27,-6.92,;25.97,-8.26,;25.19,-6.92,;23.66,-6.92,;22.88,-5.59,;21.35,-5.59,;20.57,-4.26,;21.35,-2.93,;20.57,-1.59,;22.88,-2.93,;23.66,-4.26,;25.97,-5.59,;27.5,-5.59,;25.19,-4.26,;25.97,-2.93,;27.5,-2.93,;28.27,-4.26,;28.27,-1.59,;29.81,-1.59,;30.58,-2.93,;32.12,-2.93,;32.89,-4.26,;32.89,-1.59,;30.58,-.26,;32.12,-.26,;29.81,1.07,;30.58,2.4,;29.81,3.74,;32.12,2.4,;32.89,3.74,;32.89,1.07,)| Show InChI InChI=1S/C146H213N43O40/c1-15-75(10)119(187-123(207)78(13)167-129(213)101(51-84-60-154-68-162-84)182-143(227)110-31-24-42-189(110)116(200)65-161-124(208)93(43-71(2)3)173-130(214)95(45-73(6)7)174-132(216)98(49-82-34-38-88(194)39-35-82)170-114(198)63-159-122(206)77(12)166-141(225)108(66-190)186-137(221)105(55-112(150)196)179-131(215)96(46-74(8)9)183-145(229)120(79(14)192)188-140(224)100(168-113(197)58-148)50-83-59-158-90-28-20-19-27-89(83)90)144(228)184-107(57-118(203)204)139(223)180-104(54-111(149)195)136(220)178-102(52-85-61-155-69-163-85)134(218)172-92(30-23-41-157-146(152)153)127(211)185-109(67-191)142(226)176-99(47-80-25-17-16-18-26-80)133(217)177-103(53-86-62-156-70-164-86)135(219)181-106(56-117(201)202)138(222)171-91(29-21-22-40-147)126(210)175-97(48-81-32-36-87(193)37-33-81)125(209)160-64-115(199)169-94(44-72(4)5)128(212)165-76(11)121(151)205/h16-20,25-28,32-39,59-62,68-79,91-110,119-120,158,190-194H,15,21-24,29-31,40-58,63-67,147-148H2,1-14H3,(H2,149,195)(H2,150,196)(H2,151,205)(H,154,162)(H,155,163)(H,156,164)(H,159,206)(H,160,209)(H,161,208)(H,165,212)(H,166,225)(H,167,213)(H,168,197)(H,169,199)(H,170,198)(H,171,222)(H,172,218)(H,173,214)(H,174,216)(H,175,210)(H,176,226)(H,177,217)(H,178,220)(H,179,215)(H,180,223)(H,181,219)(H,182,227)(H,183,229)(H,184,228)(H,185,211)(H,186,221)(H,187,207)(H,188,224)(H,201,202)(H,203,204)(H4,152,153,157)/t75-,76-,77-,78-,79+,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,119-,120-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Binding affinity to human GalR1 |
J Med Chem 53: 1871-5 (2010)
Article DOI: 10.1021/jm9018349
BindingDB Entry DOI: 10.7270/Q26D5TXJ |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM50273369
(CHEMBL526003 | GWTLNSAGYLLGPQQFFGLM-CONH2 | Galani...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C104H151N25O26S/c1-54(2)39-70(91(142)113-52-87(139)129-37-20-27-80(129)103(154)120-69(33-35-82(107)134)93(144)119-68(32-34-81(106)133)94(145)124-76(44-61-23-16-13-17-24-61)99(150)123-74(43-60-21-14-12-15-22-60)92(143)112-51-86(138)116-71(40-55(3)4)95(146)118-67(89(109)140)36-38-156-11)121-96(147)72(41-56(5)6)122-98(149)75(45-62-28-30-64(132)31-29-62)117-85(137)50-111-90(141)58(9)114-102(153)79(53-130)127-100(151)78(47-83(108)135)125-97(148)73(42-57(7)8)126-104(155)88(59(10)131)128-101(152)77(115-84(136)48-105)46-63-49-110-66-26-19-18-25-65(63)66/h12-19,21-26,28-31,49,54-59,67-80,88,110,130-132H,20,27,32-48,50-53,105H2,1-11H3,(H2,106,133)(H2,107,134)(H2,108,135)(H2,109,140)(H,111,141)(H,112,143)(H,113,142)(H,114,153)(H,115,136)(H,116,138)(H,117,137)(H,118,146)(H,119,144)(H,120,154)(H,121,147)(H,122,149)(H,123,150)(H,124,145)(H,125,148)(H,126,155)(H,127,151)(H,128,152)/t58-,59+,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Binding affinity to human GalR1 |
J Med Chem 53: 1871-5 (2010)
Article DOI: 10.1021/jm9018349
BindingDB Entry DOI: 10.7270/Q26D5TXJ |
More data for this
Ligand-Target Pair | |
Aspartate--tRNA ligase
(Escherichia coli) | BDBM50339907
(((S)-2-amino-3-carboxypropanoyl)(((2R,3S,4R,5R)-5-...)Show SMILES N[C@@H](CC(O)=O)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H19N7O9S/c15-5(1-7(22)23)13(26)20-31(27,28)29-2-6-9(24)10(25)14(30-6)21-4-19-8-11(16)17-3-18-12(8)21/h3-6,9-10,14,24-25H,1-2,15H2,(H,20,26)(H,22,23)(H2,16,17,18)/t5-,6+,9+,10+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Aspartyl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escheric... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075807
(1-(3-{1-[2-(4-Iodo-phenyl)-ethyl]-piperidin-4-yl}-...)Show SMILES Ic1ccc(CCN2CCC(CCCn3c(COc4ccccc4)nc4c(OCCCN5CCCCC5)cccc34)CC2)cc1 Show InChI InChI=1S/C38H49IN4O2/c39-33-17-15-32(16-18-33)21-28-42-26-19-31(20-27-42)10-8-25-43-35-13-7-14-36(44-29-9-24-41-22-5-2-6-23-41)38(35)40-37(43)30-45-34-11-3-1-4-12-34/h1,3-4,7,11-18,31H,2,5-6,8-10,19-30H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85034

(BW-501 | BW-501C | BW501)Show InChI InChI=1S/C17H20ClN3O/c18-15-9-4-5-10-16(15)22-12-6-11-20-17(19)13-21-14-7-2-1-3-8-14/h1-5,7-10,21H,6,11-13H2,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50075724
(4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...)Show SMILES CN(C)c1ccc2c(Oc3cc(ccc3C22OC(=O)c3cc(ccc23)C(=O)N([C@@H](CCCCN)C(N)=O)C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)N(C)C(=O)CCc2ccc(O)cc2)N(C)C)c1 Show InChI InChI=1S/C79H96N16O16/c1-91(2)49-24-31-55-64(42-49)110-65-43-50(92(3)4)25-32-56(65)79(55)54-30-23-48(41-53(54)77(109)111-79)74(106)95(61(69(83)101)16-9-10-36-80)76(108)62-17-12-38-94(62)75(107)58(15-11-37-86-78(84)85)88-72(104)60(44-67(82)99)89-70(102)57(33-34-66(81)98)87-71(103)59(39-46-13-7-6-8-14-46)90-73(105)63(40-47-20-28-52(97)29-21-47)93(5)68(100)35-22-45-18-26-51(96)27-19-45/h6-8,13-14,18-21,23-32,41-43,57-63,96-97H,9-12,15-17,22,33-40,44,80H2,1-5H3,(H2,81,98)(H2,82,99)(H2,83,101)(H,87,103)(H,88,104)(H,89,102)(H,90,105)(H4,84,85,86)/t57-,58-,59-,60-,61-,62-,63+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM50307256
(CHEMBL604373 | GWTLNSAGYLLGPrPKPQQwFwLL-CONH2 | Ga...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(N)=O |r,wU:181.202,169.182,155.166,150.159,134.151,118.130,107.113,96.110,80.91,64.74,4.4,wD:177.186,197.209,161.174,126.138,89.94,73.77,55.65,41.56,30.40,16.28,8.12,(-10.91,-15.15,;-10.14,-13.82,;-10.9,-12.49,;-8.59,-13.83,;-7.82,-12.49,;-6.29,-12.5,;-5.52,-13.84,;-6.29,-15.17,;-3.97,-13.84,;-3.2,-12.51,;-3.97,-11.17,;-3.19,-9.85,;-5.5,-11.18,;-3.21,-15.18,;-1.67,-15.19,;-.9,-13.86,;-.9,-16.52,;-1.68,-17.86,;-1.62,-19.4,;-.32,-20.25,;-.73,-21.73,;-2.27,-21.8,;-3.25,-22.99,;-4.76,-22.74,;-5.3,-21.3,;-4.34,-20.11,;-2.81,-20.36,;.63,-16.54,;1.4,-17.87,;.62,-19.21,;2.94,-17.88,;3.72,-16.55,;2.94,-15.21,;1.41,-15.2,;.65,-13.86,;1.42,-12.54,;2.96,-12.53,;3.73,-13.87,;3.7,-19.21,;5.25,-19.16,;5.97,-17.8,;6.06,-20.47,;5.33,-21.83,;3.79,-21.88,;2.85,-20.67,;1.4,-21.19,;1.46,-22.73,;.34,-23.8,;.71,-25.29,;2.19,-25.72,;3.3,-24.65,;2.93,-23.16,;7.6,-20.42,;8.41,-21.74,;7.69,-23.08,;9.94,-21.67,;10.68,-20.31,;9.86,-19.01,;10.59,-17.64,;12.12,-17.6,;9.77,-16.33,;10.76,-22.98,;12.31,-22.93,;13.03,-21.57,;13.11,-24.23,;12.39,-25.6,;10.86,-25.65,;10.13,-27.01,;10.94,-28.32,;8.58,-27.06,;14.66,-24.17,;15.38,-22.81,;14.55,-21.5,;16.91,-22.75,;17.56,-21.34,;19.18,-21.81,;19.22,-23.48,;17.65,-24.07,;17.55,-25.6,;16.18,-26.3,;18.85,-26.45,;18.77,-27.98,;17.39,-28.68,;17.29,-30.22,;15.92,-30.91,;15.83,-32.45,;20.22,-25.75,;21.51,-26.6,;21.42,-28.13,;22.89,-25.91,;22.89,-24.35,;24.56,-24.11,;25.29,-25.62,;24.1,-26.8,;24.2,-28.34,;22.93,-29.19,;25.62,-28.92,;26.9,-28.07,;28.28,-28.74,;29.56,-27.88,;30.94,-28.56,;32.23,-27.69,;32.11,-26.16,;33.6,-28.38,;25.82,-30.45,;27.24,-31.05,;28.52,-30.19,;27.44,-32.58,;26.15,-33.45,;26.89,-34.98,;28.55,-34.74,;28.85,-33.08,;30.19,-32.31,;30.19,-30.77,;31.52,-33.08,;31.52,-34.62,;32.86,-35.39,;34.2,-34.61,;32.86,-36.92,;31.52,-37.7,;30.19,-36.92,;28.86,-37.7,;30.19,-35.39,;34.2,-37.7,;34.2,-39.24,;32.86,-40.01,;35.54,-40.01,;36.87,-39.23,;36.87,-37.69,;38.2,-36.92,;35.53,-36.92,;35.54,-41.54,;36.87,-42.31,;36.87,-43.85,;38.2,-41.54,;39.53,-42.32,;39.53,-43.84,;38.22,-44.61,;38.21,-46.16,;39.56,-46.92,;39.56,-48.46,;40.89,-46.14,;40.87,-44.61,;38.2,-39.99,;39.52,-39.23,;40.86,-40,;39.52,-37.69,;40.86,-36.91,;40.86,-35.37,;39.52,-34.61,;42.19,-34.61,;43.53,-35.37,;42.19,-33.07,;43.53,-32.29,;43.53,-30.76,;44.86,-33.06,;44.86,-34.6,;46.19,-35.37,;46.19,-32.28,;47.52,-33.05,;47.52,-34.6,;48.86,-32.28,;48.86,-30.74,;47.52,-29.97,;46.19,-30.74,;47.52,-28.44,;50.19,-33.05,;51.54,-32.29,;51.54,-30.74,;52.86,-33.05,;52.86,-34.59,;51.54,-35.37,;51.54,-36.91,;50.19,-34.59,;54.2,-32.28,;55.53,-33.05,;55.53,-34.59,;56.86,-32.28,;58.19,-33.04,;59.53,-32.27,;59.53,-30.73,;60.86,-33.04,;60.86,-34.58,;59.53,-35.35,;58.13,-34.73,;57.11,-35.88,;57.88,-37.21,;57.4,-38.68,;58.43,-39.82,;59.95,-39.49,;60.41,-38.03,;59.37,-36.89,;62.19,-32.28,;63.53,-33.05,;63.53,-34.58,;64.86,-32.28,;66.19,-33.04,;56.86,-30.74,;58.19,-29.97,;55.53,-29.97,;-8.59,-11.16,;-10.13,-11.15,;-7.81,-9.83,)| Show InChI InChI=1S/C138H199N35O30/c1-72(2)54-95(117(144)183)160-122(188)97(56-74(5)6)163-127(193)104(63-83-68-150-90-35-21-18-32-87(83)90)166-126(192)101(59-79-28-14-13-15-29-79)164-128(194)103(62-82-67-149-89-34-20-17-31-86(82)89)165-121(187)91(45-47-110(141)177)156-120(186)92(46-48-111(142)178)157-133(199)108-39-26-52-172(108)136(202)93(36-22-23-49-139)158-134(200)109-40-27-53-173(109)137(203)94(37-24-50-147-138(145)146)159-132(198)107-38-25-51-171(107)115(182)70-152-119(185)96(55-73(3)4)161-123(189)98(57-75(7)8)162-125(191)100(60-80-41-43-84(176)44-42-80)155-114(181)69-151-118(184)77(11)153-131(197)106(71-174)169-129(195)105(64-112(143)179)167-124(190)99(58-76(9)10)168-135(201)116(78(12)175)170-130(196)102(154-113(180)65-140)61-81-66-148-88-33-19-16-30-85(81)88/h13-21,28-35,41-44,66-68,72-78,91-109,116,148-150,174-176H,22-27,36-40,45-65,69-71,139-140H2,1-12H3,(H2,141,177)(H2,142,178)(H2,143,179)(H2,144,183)(H,151,184)(H,152,185)(H,153,197)(H,154,180)(H,155,181)(H,156,186)(H,157,199)(H,158,200)(H,159,198)(H,160,188)(H,161,189)(H,162,191)(H,163,193)(H,164,194)(H,165,187)(H,166,192)(H,167,190)(H,168,201)(H,169,195)(H,170,196)(H4,145,146,147)/t77-,78+,91-,92-,93-,94+,95-,96-,97-,98-,99-,100-,101-,102-,103+,104+,105-,106-,107-,108-,109-,116-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Binding affinity to human GalR1 |
J Med Chem 53: 1871-5 (2010)
Article DOI: 10.1021/jm9018349
BindingDB Entry DOI: 10.7270/Q26D5TXJ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50075720
((HO-LVA23)2-{2-[3-(4-Hydroxy-phenyl)-2-(methyl-4-H...)Show SMILES CN([C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)C(=O)Cc1ccc(O)cc1 Show InChI InChI=1S/C53H74N16O12/c1-68(44(74)28-32-15-19-34(71)20-16-32)41(27-31-13-17-33(70)18-14-31)50(80)67-38(26-30-8-3-2-4-9-30)47(77)64-36(21-22-42(54)72)46(76)66-39(29-43(55)73)48(78)65-37(11-6-24-62-53(59)60)51(81)69-25-7-12-40(69)49(79)63-35(45(56)75)10-5-23-61-52(57)58/h2-4,8-9,13-20,35-41,70-71H,5-7,10-12,21-29H2,1H3,(H2,54,72)(H2,55,73)(H2,56,75)(H,63,79)(H,64,77)(H,65,78)(H,66,76)(H,67,80)(H4,57,58,61)(H4,59,60,62)/t35-,36-,37-,38-,39-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Protachykinin-1
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM50307254
(CHEMBL604990 | GWTLNSAGYLLGPPPALALA-CONH2 | M40)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C94H145N23O24/c1-46(2)33-61(82(129)100-44-76(124)115-30-19-24-71(115)93(140)117-32-20-25-72(117)94(141)116-31-18-23-70(116)91(138)104-54(14)81(128)108-63(35-48(5)6)84(131)103-53(13)80(127)107-62(34-47(3)4)83(130)101-51(11)78(97)125)109-85(132)64(36-49(7)8)110-87(134)66(38-56-26-28-58(120)29-27-56)106-75(123)43-99-79(126)52(12)102-90(137)69(45-118)113-88(135)68(40-73(96)121)111-86(133)65(37-50(9)10)112-92(139)77(55(15)119)114-89(136)67(105-74(122)41-95)39-57-42-98-60-22-17-16-21-59(57)60/h16-17,21-22,26-29,42,46-55,61-72,77,98,118-120H,18-20,23-25,30-41,43-45,95H2,1-15H3,(H2,96,121)(H2,97,125)(H,99,126)(H,100,129)(H,101,130)(H,102,137)(H,103,131)(H,104,138)(H,105,122)(H,106,123)(H,107,127)(H,108,128)(H,109,132)(H,110,134)(H,111,133)(H,112,139)(H,113,135)(H,114,136)/t51-,52-,53-,54-,55+,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,77-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Binding affinity to human GalR1 |
J Med Chem 53: 1871-5 (2010)
Article DOI: 10.1021/jm9018349
BindingDB Entry DOI: 10.7270/Q26D5TXJ |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075796
(2-Phenoxymethyl-4-(3-piperidin-1-yl-propoxy)-1-{3-...)Show SMILES C(COc1cccc2n(CCCC3CCN(CCCN4CCCCC4)CC3)c(COc3ccccc3)nc12)CN1CCCCC1 Show InChI InChI=1S/C38H57N5O2/c1-4-15-34(16-5-1)45-32-37-39-38-35(17-10-18-36(38)44-31-13-27-41-23-8-3-9-24-41)43(37)28-11-14-33-19-29-42(30-20-33)26-12-25-40-21-6-2-7-22-40/h1,4-5,10,15-18,33H,2-3,6-9,11-14,19-32H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075811

(3-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...)Show SMILES O=C(CCN1CCC(CCCn2c(COc3ccccc3)nc3c(OCCCN4CCCCC4)cccc23)CC1)c1ccccc1 Show InChI InChI=1S/C39H50N4O3/c44-36(33-14-4-1-5-15-33)22-29-42-27-20-32(21-28-42)13-11-26-43-35-18-10-19-37(45-30-12-25-41-23-8-3-9-24-41)39(35)40-38(43)31-46-34-16-6-2-7-17-34/h1-2,4-7,10,14-19,32H,3,8-9,11-13,20-31H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM194780

(7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...)Show SMILES O=c1ccc2ccc(OCCCCN3CCN(CC3)c3cccc4sccc34)cc2[nH]1 Show InChI InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111736
BindingDB Entry DOI: 10.7270/Q2W0996H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM85033
(CAS_357-56-2 | Dextromoramide | NSC_9648)Show SMILES CC(CN1CCOCC1)C(C(=O)N1CCCC1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H32N2O2/c1-21(20-26-16-18-29-19-17-26)25(22-10-4-2-5-11-22,23-12-6-3-7-13-23)24(28)27-14-8-9-15-27/h2-7,10-13,21H,8-9,14-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM84994
(CAS_163091 | NSC_163091 | ORG-5222)Show InChI InChI=1S/C17H16ClNO/c1-19-9-14-12-4-2-3-5-16(12)20-17-7-6-11(18)8-13(17)15(14)10-19/h2-8,14-15H,9-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075803
(4-(4-{3-[2-Phenoxymethyl-4-(3-piperidin-1-yl-propo...)Show SMILES O=C(CCCN1CCC(CCCn2c(COc3ccccc3)nc3c(OCCCN4CCCCC4)cccc23)CC1)c1ccccc1 Show InChI InChI=1S/C40H52N4O3/c45-37(34-15-4-1-5-16-34)20-12-26-43-29-22-33(23-30-43)14-11-28-44-36-19-10-21-38(46-31-13-27-42-24-8-3-9-25-42)40(36)41-39(44)32-47-35-17-6-2-7-18-35/h1-2,4-7,10,15-19,21,33H,3,8-9,11-14,20,22-32H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50458119
(Leusa)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C16H25N7O7S/c1-7(2)3-8(17)15(26)22-31(27,28)29-4-9-11(24)12(25)16(30-9)23-6-21-10-13(18)19-5-20-14(10)23/h5-9,11-12,16,24-25H,3-4,17H2,1-2H3,(H,22,26)(H2,18,19,20)/t8-,9+,11+,12+,16+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Leucyl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escherichi... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50010859
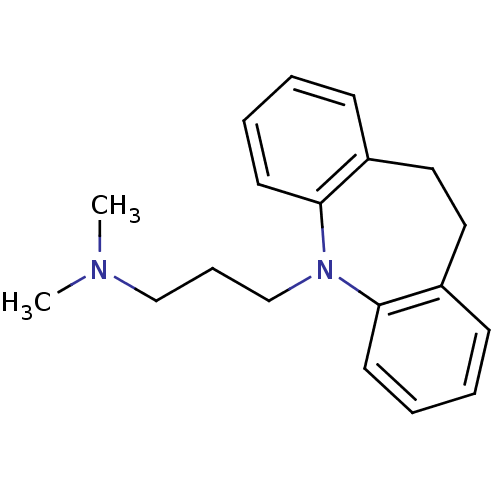
(CHEMBL11 | IMIPRAMINE HYDROCHLORIDE | IMIPRAMINE P...)Show InChI InChI=1S/C19H24N2/c1-20(2)14-7-15-21-18-10-5-3-8-16(18)12-13-17-9-4-6-11-19(17)21/h3-6,8-11H,7,12-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50469874

(CHEMBL59780)Show SMILES CCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)c1ccccc1)C(N)=O Show InChI InChI=1S/C49H61N9O8/c1-4-5-21-37(42(50)59)54-47(64)40-23-14-25-57(40)49(66)41-24-15-26-58(41)48(65)39(27-32-16-8-6-9-17-32)56-46(63)38(28-34-29-51-36-22-13-12-20-35(34)36)55-44(61)31(3)52-43(60)30(2)53-45(62)33-18-10-7-11-19-33/h6-13,16-20,22,29-31,37-41,51H,4-5,14-15,21,23-28H2,1-3H3,(H2,50,59)(H,52,60)(H,53,62)(H,54,64)(H,55,61)(H,56,63)/t30-,31-,37-,38-,39-,40-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Institute for Molecular Biology
Curated by ChEMBL
| Assay Description
Binding affinity against human NK2 receptors expressed in CHO cells using [3H]GR-100679 as radioligand |
J Med Chem 37: 1991-5 (1994)
Article DOI: 10.1021/jm00039a012
BindingDB Entry DOI: 10.7270/Q2DZ0C10 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM84994

(CAS_163091 | NSC_163091 | ORG-5222)Show InChI InChI=1S/C17H16ClNO/c1-19-9-14-12-4-2-3-5-16(12)20-17-7-6-11(18)8-13(17)15(14)10-19/h2-8,14-15H,9-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305851
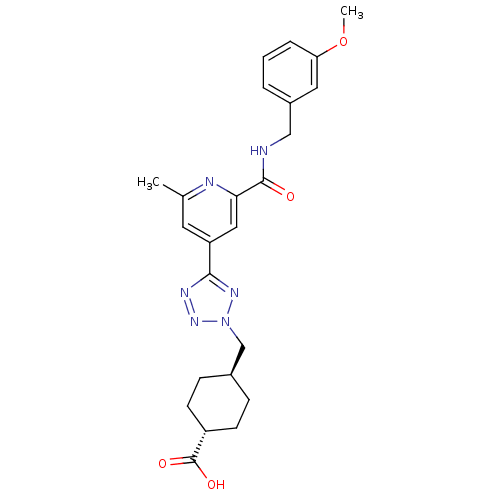
(CHEMBL596273 | trans-4-((5-(2-(3-methoxybenzylcarb...)Show SMILES COc1cccc(CNC(=O)c2cc(cc(C)n2)-c2nnn(C[C@H]3CC[C@@H](CC3)C(O)=O)n2)c1 |r,wU:26.30,wD:23.23,(7.62,-43.42,;8.41,-44.75,;9.95,-44.72,;10.71,-43.37,;12.25,-43.35,;13.03,-44.67,;12.28,-46.01,;13.07,-47.34,;14.61,-47.32,;15.4,-48.64,;14.64,-49.98,;16.94,-48.62,;17.69,-47.28,;19.23,-47.26,;20.02,-48.58,;19.27,-49.92,;20.07,-51.25,;17.72,-49.95,;19.99,-45.92,;19.34,-44.51,;20.47,-43.47,;21.82,-44.22,;23.22,-43.58,;24.56,-44.32,;24.59,-45.86,;25.93,-46.61,;27.25,-45.82,;27.22,-44.27,;25.88,-43.53,;28.6,-46.57,;28.63,-48.1,;29.92,-45.77,;21.52,-45.73,;10.74,-46.04,)| Show InChI InChI=1S/C24H28N6O4/c1-15-10-19(12-21(26-15)23(31)25-13-17-4-3-5-20(11-17)34-2)22-27-29-30(28-22)14-16-6-8-18(9-7-16)24(32)33/h3-5,10-12,16,18H,6-9,13-14H2,1-2H3,(H,25,31)(H,32,33)/t16-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant MMP13 using MCA-Arg-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-Glu-Arg-NH2 as substrate preincubated for 60 mins followe... |
J Med Chem 59: 313-27 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01434
BindingDB Entry DOI: 10.7270/Q20G3N0F |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305851

(CHEMBL596273 | trans-4-((5-(2-(3-methoxybenzylcarb...)Show SMILES COc1cccc(CNC(=O)c2cc(cc(C)n2)-c2nnn(C[C@H]3CC[C@@H](CC3)C(O)=O)n2)c1 |r,wU:26.30,wD:23.23,(7.62,-43.42,;8.41,-44.75,;9.95,-44.72,;10.71,-43.37,;12.25,-43.35,;13.03,-44.67,;12.28,-46.01,;13.07,-47.34,;14.61,-47.32,;15.4,-48.64,;14.64,-49.98,;16.94,-48.62,;17.69,-47.28,;19.23,-47.26,;20.02,-48.58,;19.27,-49.92,;20.07,-51.25,;17.72,-49.95,;19.99,-45.92,;19.34,-44.51,;20.47,-43.47,;21.82,-44.22,;23.22,-43.58,;24.56,-44.32,;24.59,-45.86,;25.93,-46.61,;27.25,-45.82,;27.22,-44.27,;25.88,-43.53,;28.6,-46.57,;28.63,-48.1,;29.92,-45.77,;21.52,-45.73,;10.74,-46.04,)| Show InChI InChI=1S/C24H28N6O4/c1-15-10-19(12-21(26-15)23(31)25-13-17-4-3-5-20(11-17)34-2)22-27-29-30(28-22)14-16-6-8-18(9-7-16)24(32)33/h3-5,10-12,16,18H,6-9,13-14H2,1-2H3,(H,25,31)(H,32,33)/t16-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(PIG) | BDBM84994

(CAS_163091 | NSC_163091 | ORG-5222)Show InChI InChI=1S/C17H16ClNO/c1-19-9-14-12-4-2-3-5-16(12)20-17-7-6-11(18)8-13(17)15(14)10-19/h2-8,14-15H,9-10H2,1H3 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50133938
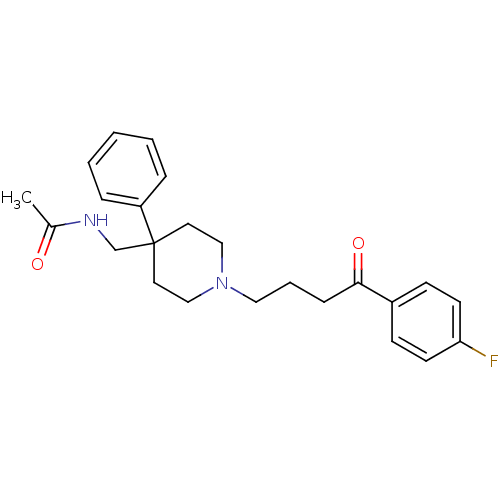
(Aceperone | CHEMBL136298 | N-{1-[4-(4-Fluoro-pheny...)Show SMILES CC(=O)NCC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccccc1 Show InChI InChI=1S/C24H29FN2O2/c1-19(28)26-18-24(21-6-3-2-4-7-21)13-16-27(17-14-24)15-5-8-23(29)20-9-11-22(25)12-10-20/h2-4,6-7,9-12H,5,8,13-18H2,1H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50075725
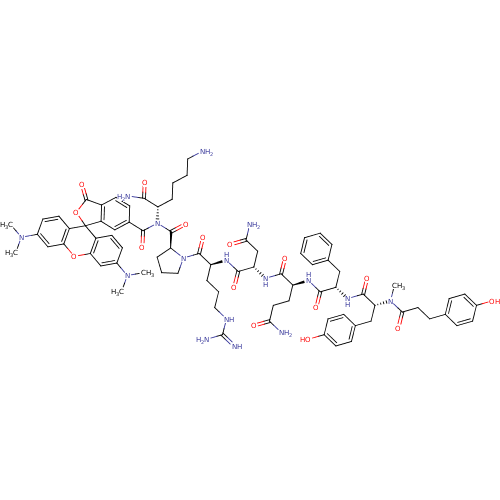
(4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(6C-...)Show SMILES CN(C)c1ccc2c(Oc3cc(ccc3C22OC(=O)c3ccc(cc23)C(=O)N([C@@H](CCCCN)C(N)=O)C(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)N(C)C(=O)CCc2ccc(O)cc2)N(C)C)c1 Show InChI InChI=1S/C79H96N16O16/c1-91(2)49-24-31-54-64(42-49)110-65-43-50(92(3)4)25-32-55(65)79(54)56-41-48(23-30-53(56)77(109)111-79)74(106)95(61(69(83)101)16-9-10-36-80)76(108)62-17-12-38-94(62)75(107)58(15-11-37-86-78(84)85)88-72(104)60(44-67(82)99)89-70(102)57(33-34-66(81)98)87-71(103)59(39-46-13-7-6-8-14-46)90-73(105)63(40-47-20-28-52(97)29-21-47)93(5)68(100)35-22-45-18-26-51(96)27-19-45/h6-8,13-14,18-21,23-32,41-43,57-63,96-97H,9-12,15-17,22,33-40,44,80H2,1-5H3,(H2,81,98)(H2,82,99)(H2,83,101)(H,87,103)(H,88,104)(H,89,102)(H,90,105)(H4,84,85,86)/t57-,58-,59-,60-,61-,62-,63+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50075722

(4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(5C-...)Show SMILES CN([C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N([C@@H](CCCCN)C(N)=O)C(=O)c1ccc2c(c1)C(=O)OC21c2ccc(O)cc2Oc2cc(O)ccc12)C(=O)CCc1ccc(O)cc1 Show InChI InChI=1S/C75H86N14O18/c1-87(64(96)31-18-41-14-20-45(90)21-15-41)59(36-43-16-22-46(91)23-17-43)69(101)86-55(35-42-9-3-2-4-10-42)67(99)83-53(29-30-62(77)94)66(98)85-56(40-63(78)95)68(100)84-54(11-7-33-82-74(80)81)71(103)88-34-8-13-58(88)72(104)89(57(65(79)97)12-5-6-32-76)70(102)44-19-26-50-49(37-44)73(105)107-75(50)51-27-24-47(92)38-60(51)106-61-39-48(93)25-28-52(61)75/h2-4,9-10,14-17,19-28,37-39,53-59,90-93H,5-8,11-13,18,29-36,40,76H2,1H3,(H2,77,94)(H2,78,95)(H2,79,97)(H,83,99)(H,84,100)(H,85,98)(H,86,101)(H4,80,81,82)/t53-,54-,55-,56-,57-,58-,59+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Serine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50339906

(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...)Show SMILES N[C@@H](CO)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C13H19N7O8S/c14-5(1-21)12(24)19-29(25,26)27-2-6-8(22)9(23)13(28-6)20-4-18-7-10(15)16-3-17-11(7)20/h3-6,8-9,13,21-23H,1-2,14H2,(H,19,24)(H2,15,16,17)/t5-,6+,8+,9+,13+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Seryl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escherichia... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305844
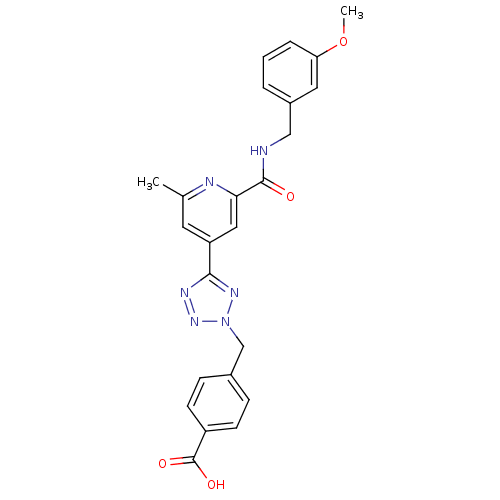
(4-((5-(2-(3-methoxybenzylcarbamoyl)-6-methylpyridi...)Show SMILES COc1cccc(CNC(=O)c2cc(cc(C)n2)-c2nnn(Cc3ccc(cc3)C(O)=O)n2)c1 Show InChI InChI=1S/C24H22N6O4/c1-15-10-19(12-21(26-15)23(31)25-13-17-4-3-5-20(11-17)34-2)22-27-29-30(28-22)14-16-6-8-18(9-7-16)24(32)33/h3-12H,13-14H2,1-2H3,(H,25,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075812
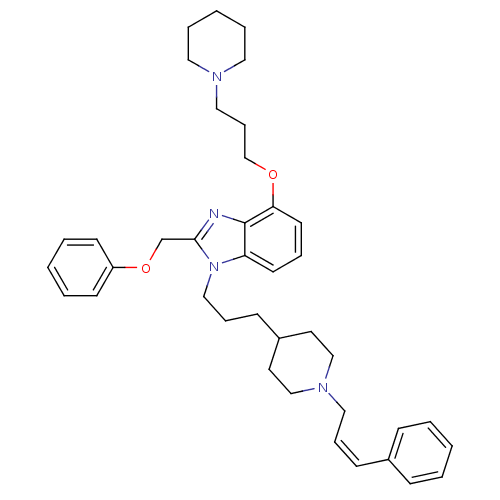
(2-Phenoxymethyl-1-{3-[1-((Z)-3-phenyl-allyl)-piper...)Show SMILES C(COc1cccc2n(CCCC3CCN(C\C=C/c4ccccc4)CC3)c(COc3ccccc3)nc12)CN1CCCCC1 Show InChI InChI=1S/C39H50N4O2/c1-4-14-33(15-5-1)16-11-26-42-29-22-34(23-30-42)17-12-28-43-36-20-10-21-37(44-31-13-27-41-24-8-3-9-25-41)39(36)40-38(43)32-45-35-18-6-2-7-19-35/h1-2,4-7,10-11,14-16,18-21,34H,3,8-9,12-13,17,22-32H2/b16-11- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50075809

(1-[3-(1-Phenethyl-piperidin-4-yl)-propyl]-2-phenox...)Show SMILES C(COc1cccc2n(CCCC3CCN(CCc4ccccc4)CC3)c(COc3ccccc3)nc12)CN1CCCCC1 Show InChI InChI=1S/C38H50N4O2/c1-4-13-32(14-5-1)20-27-41-28-21-33(22-29-41)15-11-26-42-35-18-10-19-36(43-30-12-25-40-23-8-3-9-24-40)38(35)39-37(42)31-44-34-16-6-2-7-17-34/h1-2,4-7,10,13-14,16-19,33H,3,8-9,11-12,15,20-31H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Ability to displace [125I]-peptide YY binding to cloned human Neuropeptide Y receptor type 1 expressed in AV-12 cells |
Bioorg Med Chem Lett 9: 647-52 (1999)
BindingDB Entry DOI: 10.7270/Q2QN65ZK |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50296318

(CHEMBL4177124)Show InChI InChI=1S/C18H21BrO2/c1-2-3-4-5-8-14-11-12-18(16(20)13-14)21-17-10-7-6-9-15(17)19/h6-7,9-13,20H,2-5,8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis InhA |
Eur J Med Chem 146: 318-343 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.047
BindingDB Entry DOI: 10.7270/Q2GF0X12 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316184

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50142473
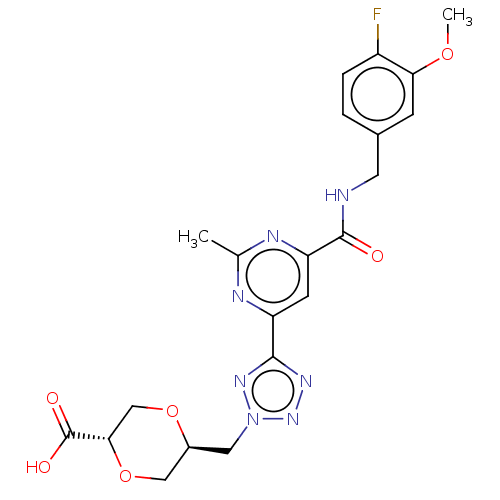
(CHEMBL3758194)Show SMILES COc1cc(CNC(=O)c2cc(nc(C)n2)-c2nnn(C[C@H]3CO[C@@H](CO3)C(O)=O)n2)ccc1F |r| Show InChI InChI=1S/C21H22FN7O6/c1-11-24-15(19-26-28-29(27-19)8-13-9-35-18(10-34-13)21(31)32)6-16(25-11)20(30)23-7-12-3-4-14(22)17(5-12)33-2/h3-6,13,18H,7-10H2,1-2H3,(H,23,30)(H,31,32)/t13-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant MMP13 using MCA-Arg-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-Glu-Arg-NH2 as substrate preincubated for 60 mins followe... |
J Med Chem 59: 313-27 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01434
BindingDB Entry DOI: 10.7270/Q20G3N0F |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19423

(HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...)Show InChI InChI=1S/C19H17N3O2S/c1-12(23)21-15-7-4-13(5-8-15)19(24)22-17-11-14(6-9-16(17)20)18-3-2-10-25-18/h2-11H,20H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged and C-terminal FLAG-tagged full length human recombinant HDAC1 expressed in baculovirus coexpressed in fall armyw... |
Bioorg Med Chem 24: 4008-4015 (2016)
Article DOI: 10.1016/j.bmc.2016.06.040
BindingDB Entry DOI: 10.7270/Q23B6220 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316183
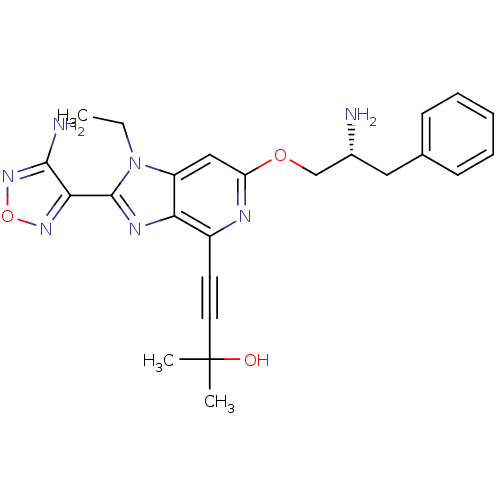
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(OC[C@H](N)Cc3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-18-13-19(33-14-16(25)12-15-8-6-5-7-9-15)27-17(10-11-24(2,3)32)20(18)28-23(31)21-22(26)30-34-29-21/h5-9,13,16,32H,4,12,14,25H2,1-3H3,(H2,26,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50305858
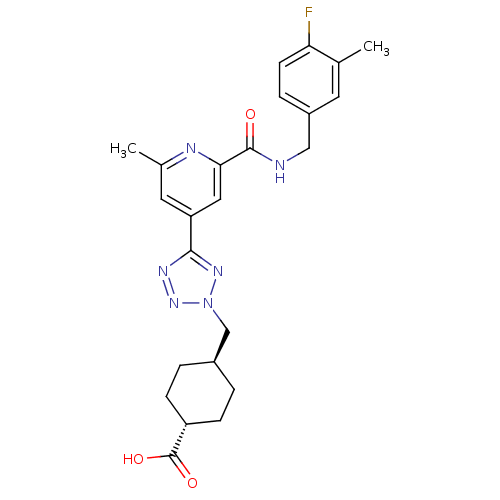
(CHEMBL605928 | trans-4-((5-(2-(4-fluoro-3-methylbe...)Show SMILES Cc1cc(cc(n1)C(=O)NCc1ccc(F)c(C)c1)-c1nnn(C[C@H]2CC[C@@H](CC2)C(O)=O)n1 |r,wU:27.32,wD:24.25,(4.82,-51.39,;4.03,-50.06,;4.78,-48.72,;3.99,-47.4,;2.44,-47.42,;1.69,-48.76,;2.48,-50.09,;.15,-48.78,;-.6,-50.12,;-.64,-47.46,;-2.18,-47.48,;-2.96,-46.15,;-2.21,-44.81,;-3,-43.49,;-4.54,-43.51,;-5.33,-42.19,;-5.29,-44.86,;-6.83,-44.89,;-4.5,-46.18,;4.74,-46.06,;4.1,-44.65,;5.23,-43.61,;6.57,-44.36,;7.97,-43.72,;9.32,-44.46,;9.34,-46,;10.69,-46.75,;12.01,-45.96,;11.98,-44.41,;10.63,-43.67,;13.36,-46.71,;13.39,-48.24,;14.68,-45.91,;6.27,-45.87,)| Show InChI InChI=1S/C24H27FN6O3/c1-14-9-17(5-8-20(14)25)12-26-23(32)21-11-19(10-15(2)27-21)22-28-30-31(29-22)13-16-3-6-18(7-4-16)24(33)34/h5,8-11,16,18H,3-4,6-7,12-13H2,1-2H3,(H,26,32)(H,33,34)/t16-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs |
Bioorg Med Chem Lett 20: 576-80 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.081
BindingDB Entry DOI: 10.7270/Q2JS9QHB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50252513
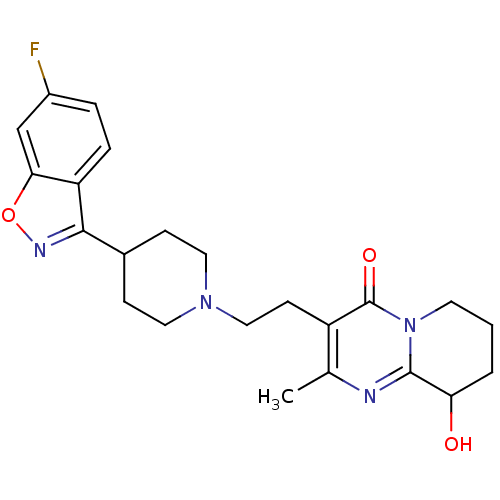
(3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1...)Show SMILES Cc1nc2C(O)CCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O3/c1-14-17(23(30)28-9-2-3-19(29)22(28)25-14)8-12-27-10-6-15(7-11-27)21-18-5-4-16(24)13-20(18)31-26-21/h4-5,13,15,19,29H,2-3,6-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
J Clin Psychiatry 5-12 (1994)
BindingDB Entry DOI: 10.7270/Q2CR5RW8 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50075728
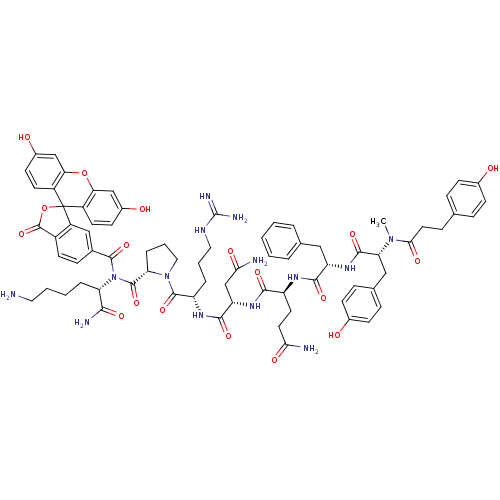
(4HOPh(CH2)2CO-DTyr(Me)-Phe-Gln-Asn-Arg-Pro-Lys(6C-...)Show SMILES CN([C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N([C@@H](CCCCN)C(N)=O)C(=O)c1ccc2C(=O)OC3(c2c1)c1ccc(O)cc1Oc1cc(O)ccc31)C(=O)CCc1ccc(O)cc1 Show InChI InChI=1S/C75H86N14O18/c1-87(64(96)31-18-41-14-20-45(90)21-15-41)59(36-43-16-22-46(91)23-17-43)69(101)86-55(35-42-9-3-2-4-10-42)67(99)83-53(29-30-62(77)94)66(98)85-56(40-63(78)95)68(100)84-54(11-7-33-82-74(80)81)71(103)88-34-8-13-58(88)72(104)89(57(65(79)97)12-5-6-32-76)70(102)44-19-26-49-52(37-44)75(107-73(49)105)50-27-24-47(92)38-60(50)106-61-39-48(93)25-28-51(61)75/h2-4,9-10,14-17,19-28,37-39,53-59,90-93H,5-8,11-13,18,29-36,40,76H2,1H3,(H2,77,94)(H2,78,95)(H2,79,97)(H,83,99)(H,84,100)(H,85,98)(H,86,101)(H4,80,81,82)/t53-,54-,55-,56-,57-,58-,59+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I]- HO-LVA from human Vasopressin V1a receptor in membranes of CHO cells |
J Med Chem 42: 1312-9 (1999)
Article DOI: 10.1021/jm9804782
BindingDB Entry DOI: 10.7270/Q2057F3Q |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(RAT) | BDBM50336640

((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...)Show SMILES COC(=O)C1C(C(C(=O)OC)=C(C)N=C1C)c1ccccc1[N+]([O-])=O |c:13,t:10| Show InChI InChI=1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,13,15H,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data