

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50173647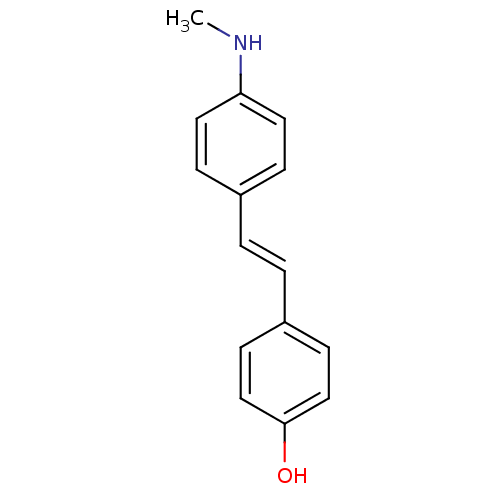 ((E)-4-(4-(methylamino)styryl)phenol | 4-(4-(methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to amyloid beta (1-42) aggregates in human Alzheimer's disease brain sections | J Med Chem 55: 883-92 (2012) Article DOI: 10.1021/jm201400q BindingDB Entry DOI: 10.7270/Q2X92F5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50239956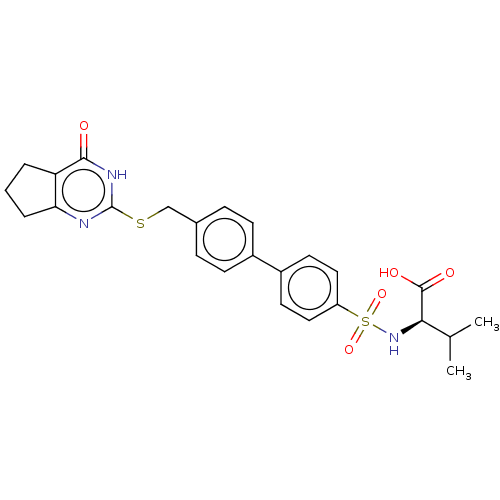 (CHEMBL4061792) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using varying levels of fTHP-15 as substrate preincubated for 30 mins ... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50239954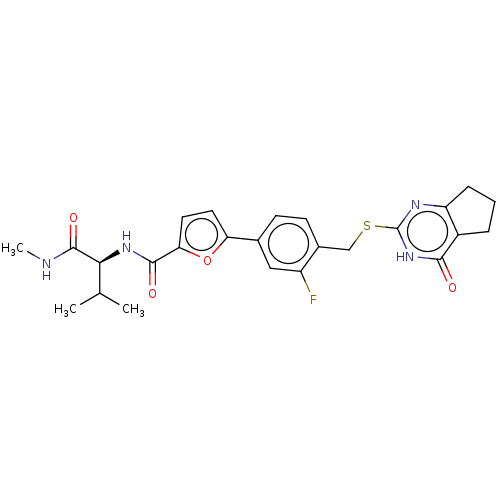 (CHEMBL4080520) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using varying levels of fTHP-15 as substrate preincubated for 30 mins ... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50239957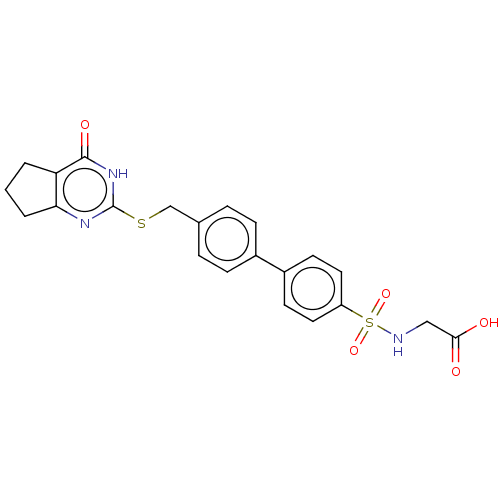 (CHEMBL4089861) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using varying levels of fTHP-15 as substrate preincubated for 30 mins ... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50485236 (BAY-949172 | CHEBI:79033 | FLORBETABEN F18 | Florb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to amyloid beta (1-42) aggregates in human Alzheimer's disease brain sections | J Med Chem 55: 883-92 (2012) Article DOI: 10.1021/jm201400q BindingDB Entry DOI: 10.7270/Q2X92F5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50239961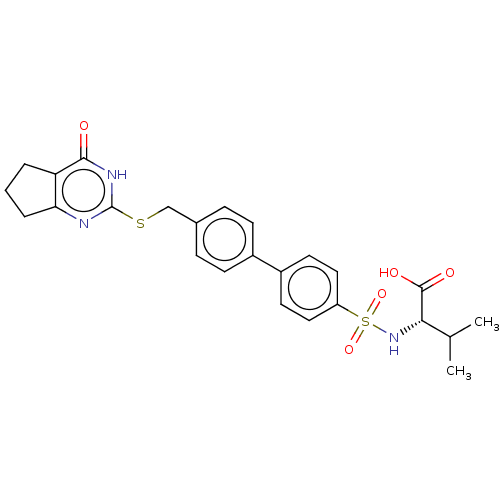 (CHEMBL4062836) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using varying levels of fTHP-15 as substrate preincubated for 30 mins ... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50484946 ((18F)AV-45 | 18F-AV-45 | AV-45 F-18 | Amyvid | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University School of Medicine Curated by ChEMBL | Assay Description Binding affinity to amyloid beta (1-42) aggregates in human Alzheimer's disease brain sections | J Med Chem 55: 883-92 (2012) Article DOI: 10.1021/jm201400q BindingDB Entry DOI: 10.7270/Q2X92F5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50239951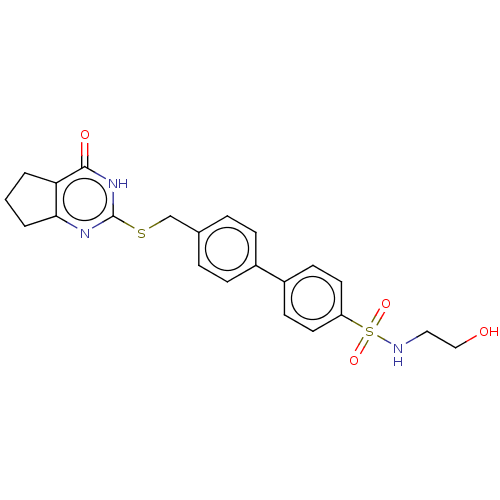 (CHEMBL4100008) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using varying levels of fTHP-15 as substrate preincubated for 30 mins ... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50239952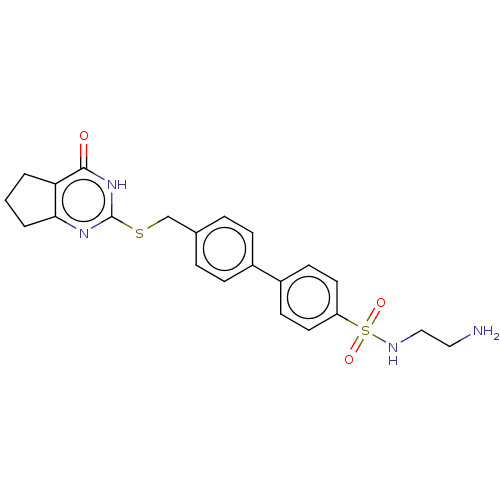 (CHEMBL4081997) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using varying levels of fTHP-15 as substrate preincubated for 30 mins ... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50361639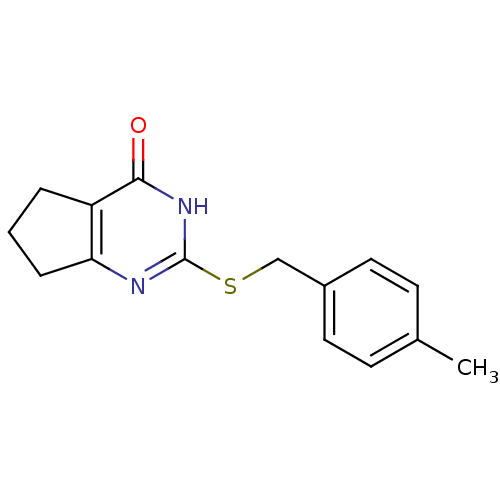 (CHEMBL1371684) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using varying levels of fTHP-15 as substrate preincubated for 30 mins ... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate kinase (Mycobacterium tuberculosis) | BDBM50223787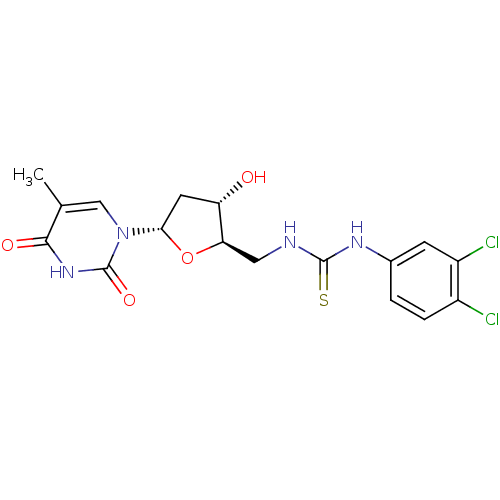 (CHEMBL235088 | N-(5'-deoxy-alpha-D-thymidin-5'-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Florida Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis thymidylate kinase | J Med Chem 55: 852-70 (2012) Article DOI: 10.1021/jm201349f BindingDB Entry DOI: 10.7270/Q2BZ66HV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222640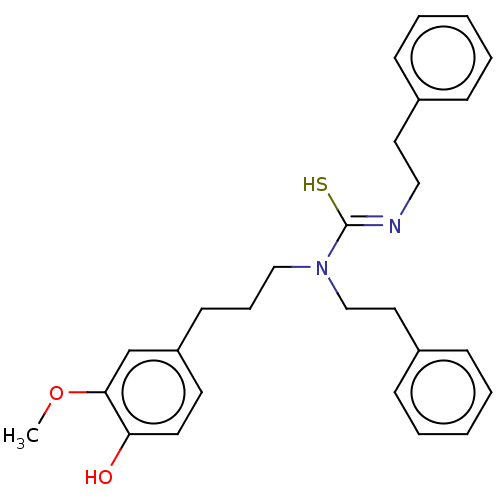 (CHEMBL415543) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222640 (CHEMBL415543) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM546731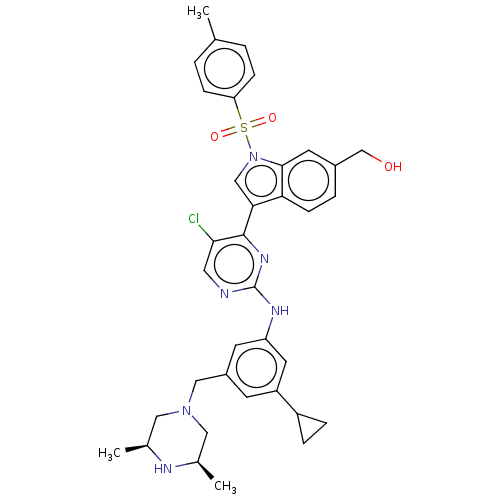 ((3-(5-chloro-2-((3-cyclopropyl-5-(((3R, 5S)-3,5-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476709 (2-((5-chloro-4-(6-methyl-1H-indole-3-yl)pyrimidine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476704 ( (3-(5-chloro-2-((3-cyclopropyl-5-(((3R,5S)-3,5-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476709 (2-((5-chloro-4-(6-methyl-1H-indole-3-yl)pyrimidine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222695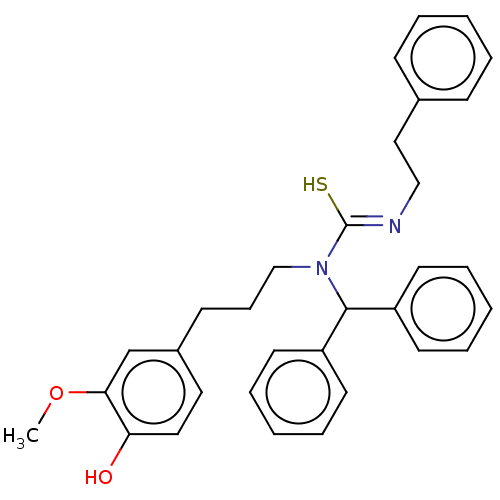 (CHEMBL155433) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222637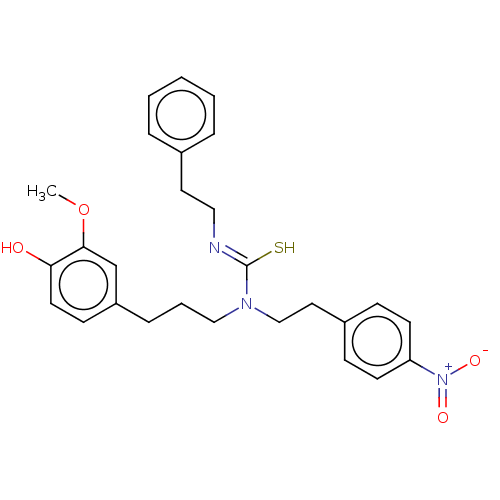 (CHEMBL154743) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476704 ( (3-(5-chloro-2-((3-cyclopropyl-5-(((3R,5S)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM546731 ((3-(5-chloro-2-((3-cyclopropyl-5-(((3R, 5S)-3,5-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222696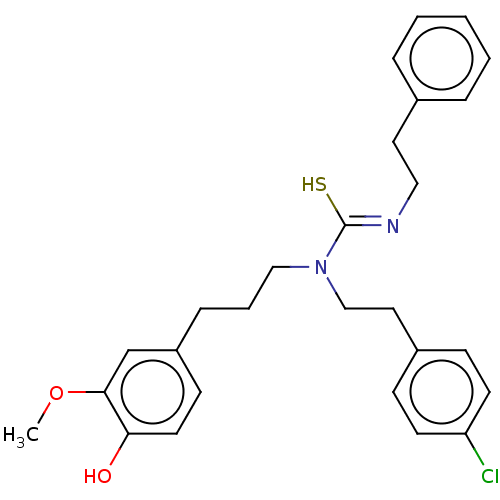 (CHEMBL156041) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222635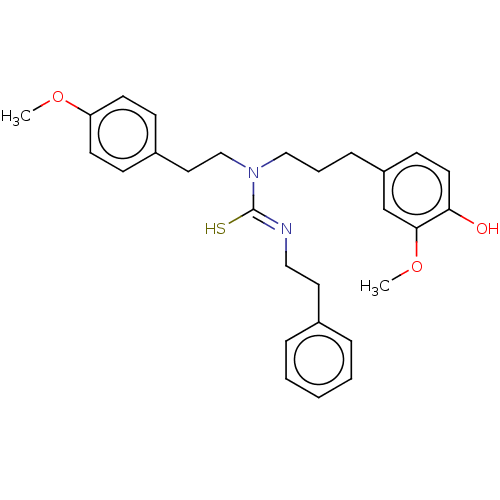 (CHEMBL267205) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222647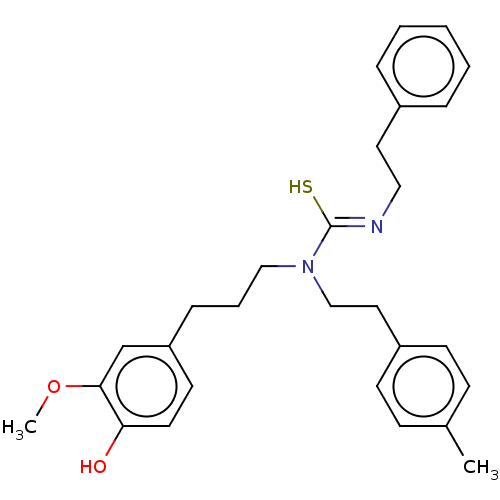 (CHEMBL154990) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222649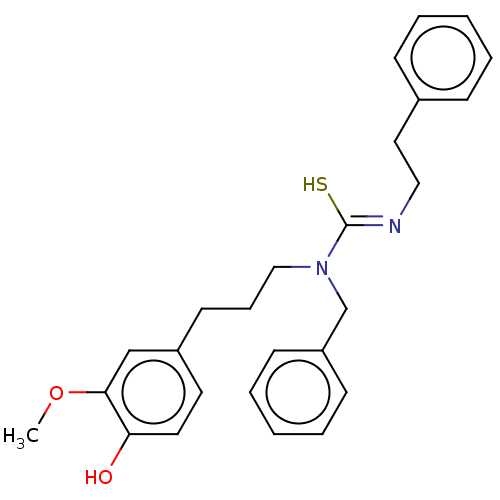 (CHEMBL155730) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476700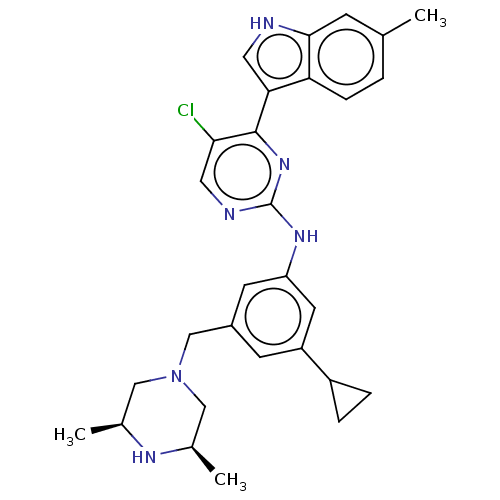 (5-chloro-N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476700 (5-chloro-N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476702 (N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethylpiperazin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476702 (N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethylpiperazin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222641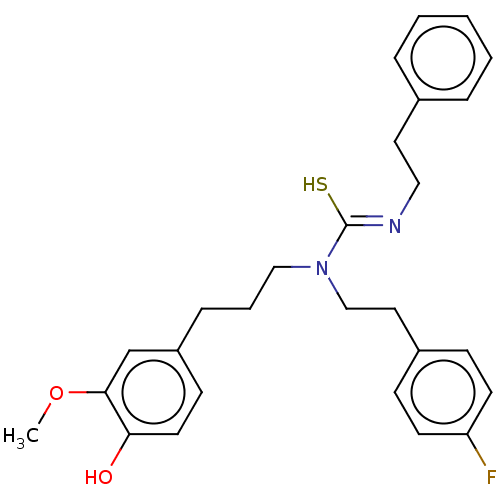 (CHEMBL157962) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476695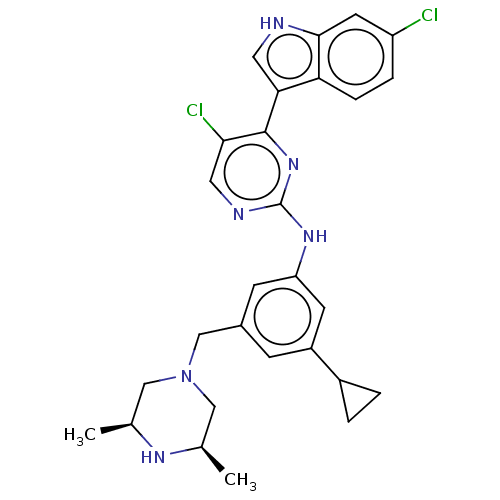 (5-chloro-4-(6-chloro-1H-indole-3-yl)-N-(3-cyclopro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476695 (5-chloro-4-(6-chloro-1H-indole-3-yl)-N-(3-cyclopro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476711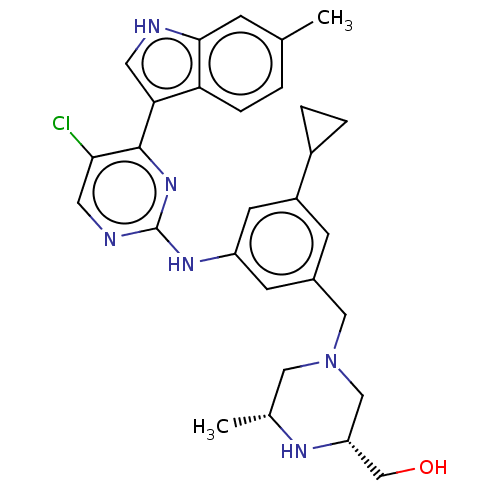 ( ((2R,6R)-4-(3-((5-chloro-4-(6-methyl-1H-indole-3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476711 ( ((2R,6R)-4-(3-((5-chloro-4-(6-methyl-1H-indole-3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50239957 (CHEMBL4089861) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using fTHP-15 as substrate preincubated for 30 mins followed by substr... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476699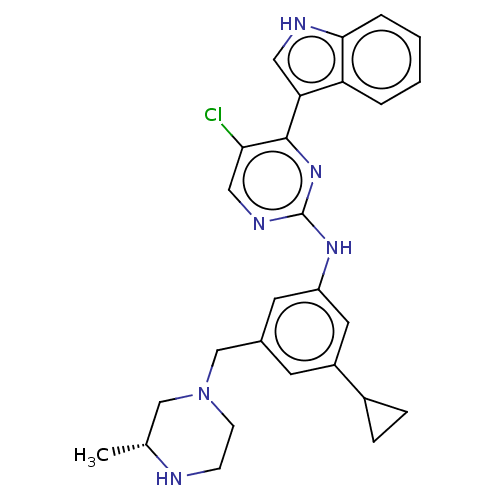 ((R)-5-chloro-N-(3-cyclopropyl-5-((3-methylpiperazi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476700 (5-chloro-N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476700 (5-chloro-N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476699 ((R)-5-chloro-N-(3-cyclopropyl-5-((3-methylpiperazi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476695 (5-chloro-4-(6-chloro-1H-indole-3-yl)-N-(3-cyclopro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476695 (5-chloro-4-(6-chloro-1H-indole-3-yl)-N-(3-cyclopro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50239961 (CHEMBL4062836) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemistry, Scripps Florida , 130 Scripps Way, Jupiter, Florida 33458, United States. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human MMP13 expressed in mouse NSO cells using fTHP-15 as substrate preincubated for 30 mins followed by substr... | J Med Chem 60: 5816-5825 (2017) Article DOI: 10.1021/acs.jmedchem.7b00514 BindingDB Entry DOI: 10.7270/Q23F4RTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476710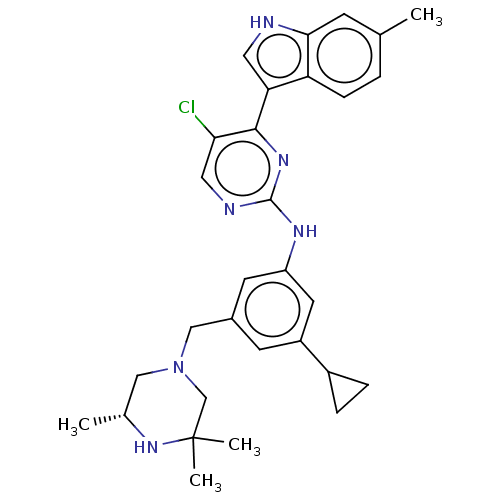 ((R)-5-chloro-N-(3-cyclopropyl-5-((3,3,5-trimethylp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476710 ((R)-5-chloro-N-(3-cyclopropyl-5-((3,3,5-trimethylp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476711 ( ((2R,6R)-4-(3-((5-chloro-4-(6-methyl-1H-indole-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476711 ( ((2R,6R)-4-(3-((5-chloro-4-(6-methyl-1H-indole-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476708 (3-(5-chloro-2-((3-cyclopropyl-5-(((3R,5S)-3,5-dime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222638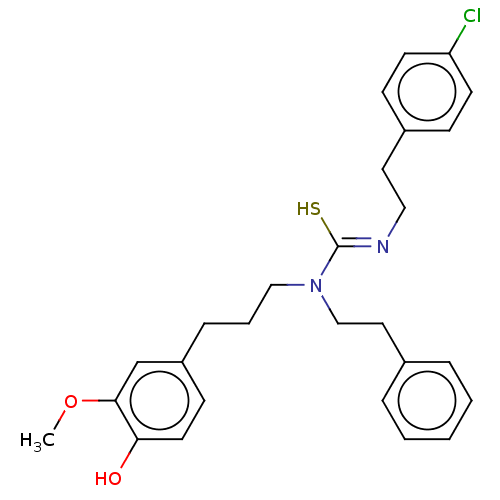 (CHEMBL422702) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476708 (3-(5-chloro-2-((3-cyclopropyl-5-(((3R,5S)-3,5-dime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
HANMI PHARM. CO.. LTD. US Patent | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured.The... | US Patent US10870639 (2020) BindingDB Entry DOI: 10.7270/Q20G3P70 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM476702 (N-(3-cyclopropyl-5-(((3R,5S)-3,5-dimethylpiperazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of some of the compounds described above against FLT3-ITD, FLT3 wild type (WT), VEGFR2 (KDR), and SYK kinase was measured. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VX0KQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 522 total ) | Next | Last >> |