

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM515773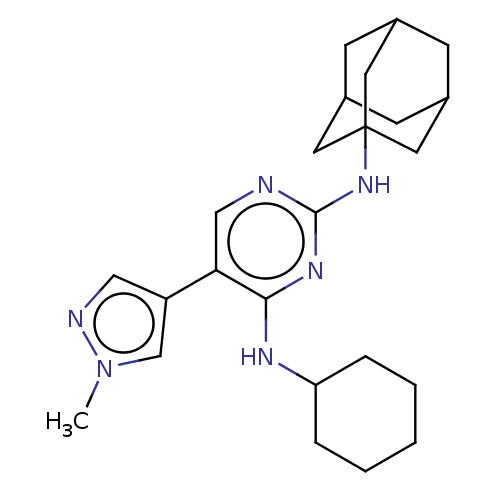 (US11053225, Compound 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515838 (US11053225, Compound 139) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515828 (US11053225, Compound 129 | US11053225, Compound 19...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515870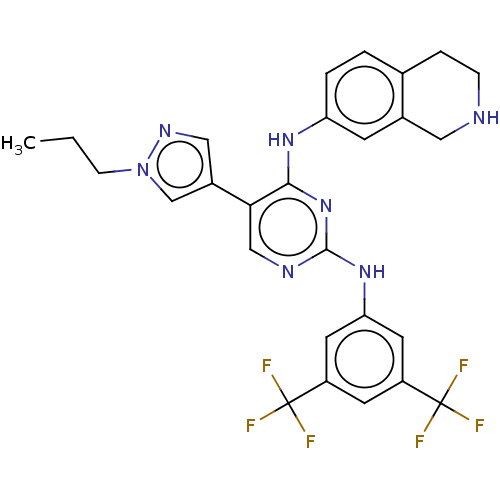 (US11053225, Compound 172) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515820 (US11053225, Compound 101 | US11053225, Compound 19...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515810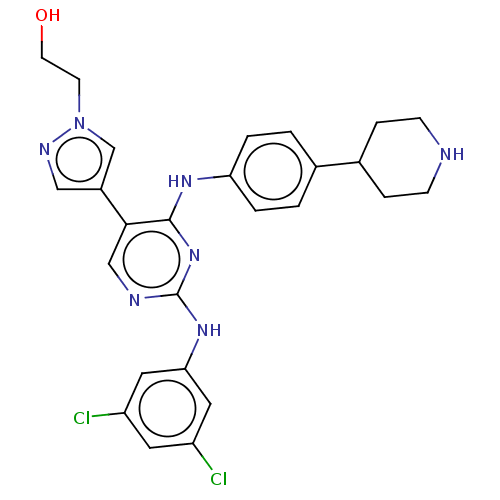 (US11053225, Compound 201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515869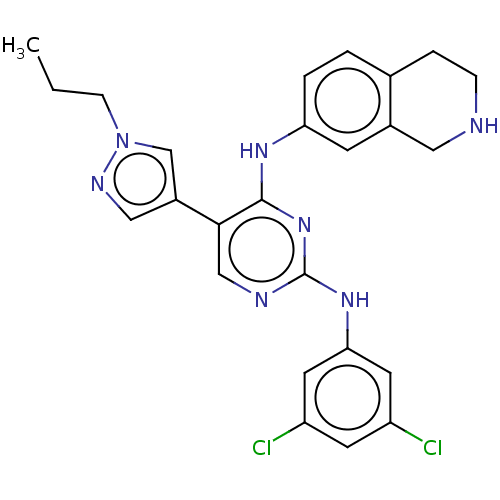 (US11053225, Compound 171) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515814 (US11053225, Compound 84) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515817 (US11053225, Compound 89) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515826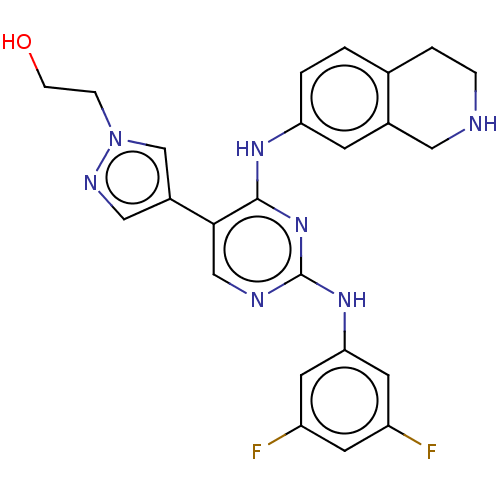 (US11053225, Compound 127) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515794 (US11053225, Compound 75) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515836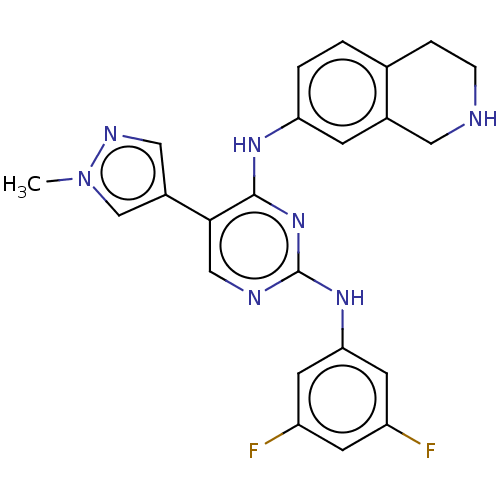 (US11053225, Compound 137) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515865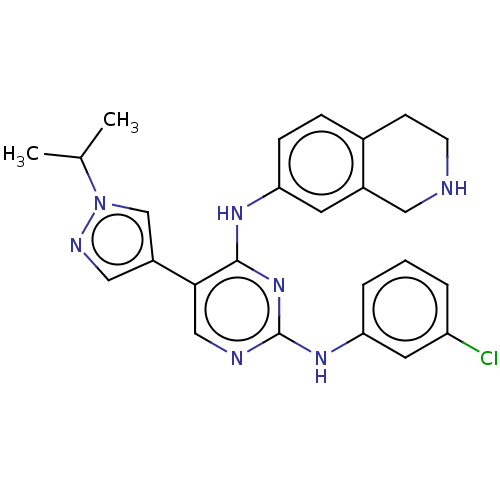 (US11053225, Compound 167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM515786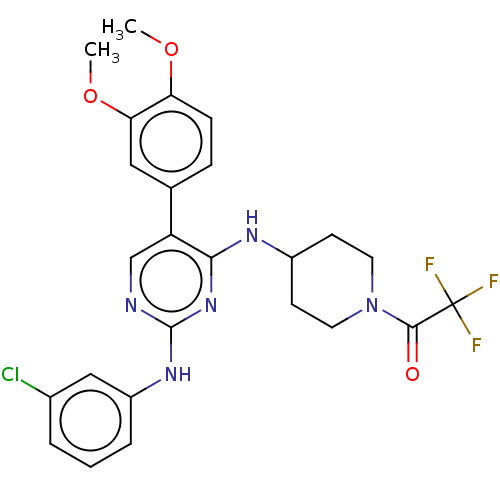 (US11053225, Compound 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515846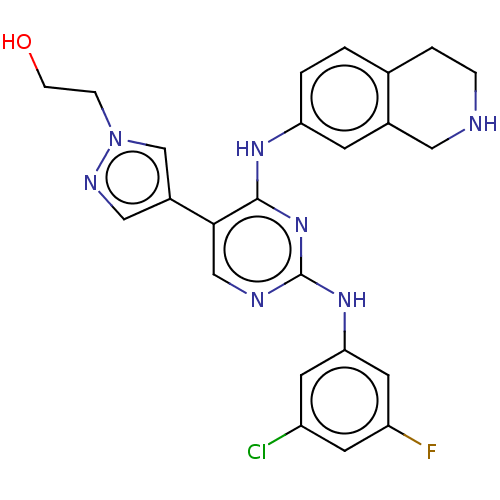 (US11053225, Compound 147) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515871 (US11053225, Compound 173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515847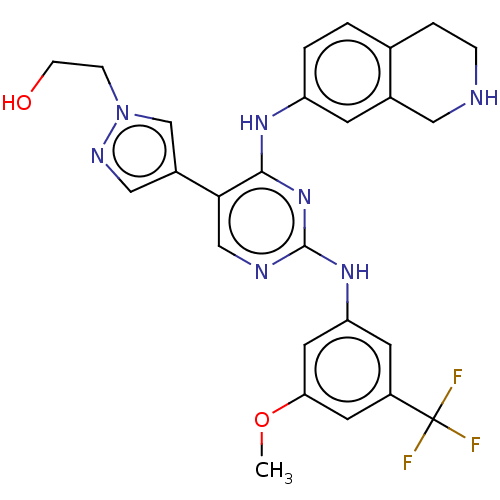 (US11053225, Compound 148) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515860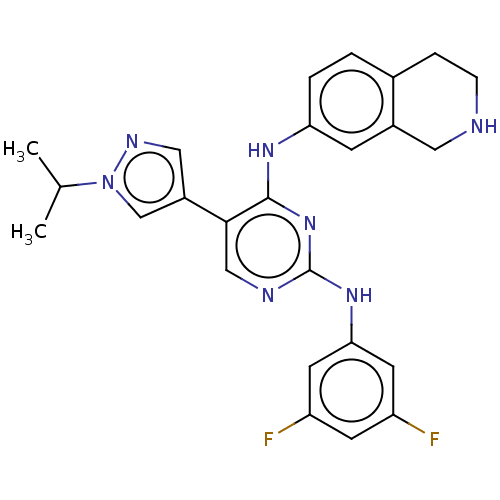 (US11053225, Compound 162) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515812 (US11053225, Compound 205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515879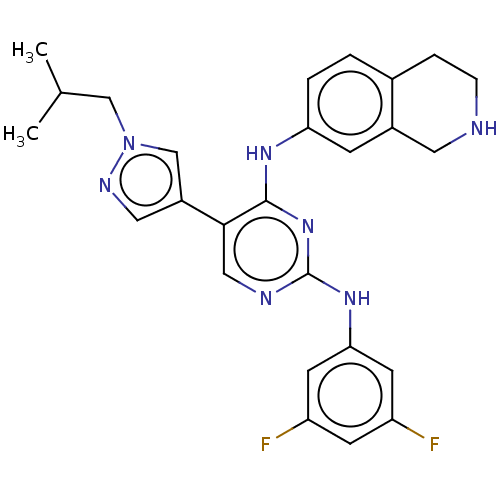 (US11053225, Compound 181) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515830 (US11053225, Compound 131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515858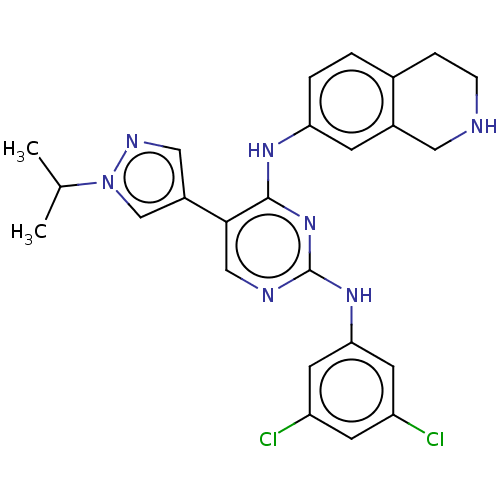 (US11053225, Compound 160) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515867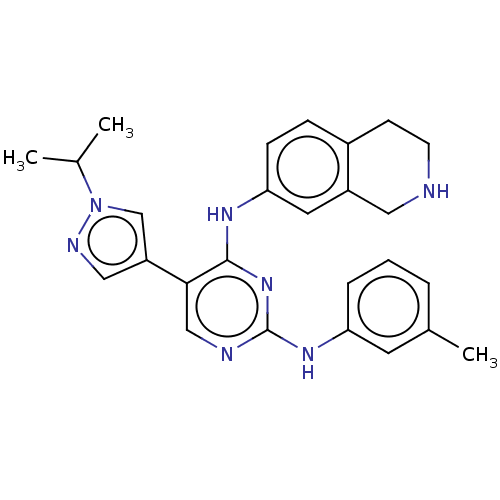 (US11053225, Compound 169) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515877 (US11053225, Compound 179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515857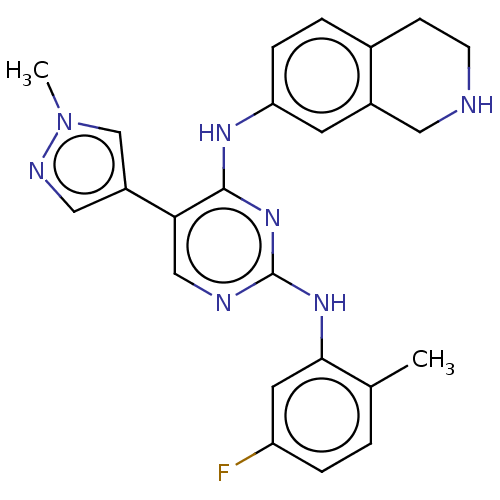 (US11053225, Compound 159) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515799 (US11053225, Compound 103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50384024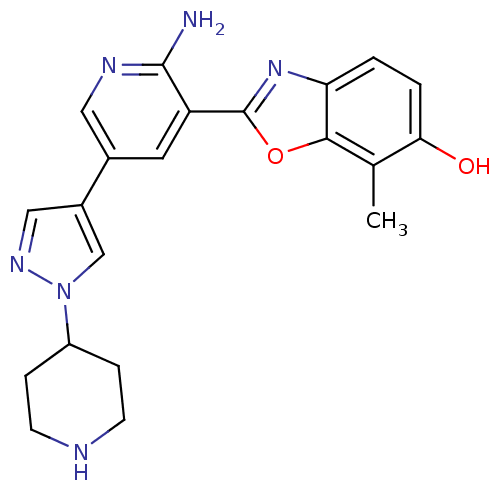 (CHEMBL2032280 | CHEMBL2079349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met by time resolved-fluorescence resonance energy transfer analysis | Bioorg Med Chem Lett 22: 4044-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.083 BindingDB Entry DOI: 10.7270/Q2FF3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512366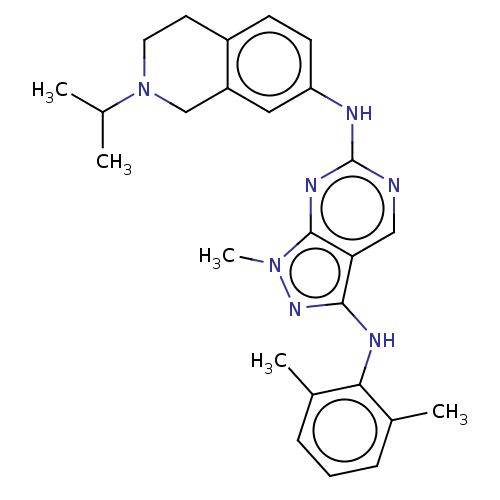 (US11084824, Example 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515831 (US11053225, Compound 132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243386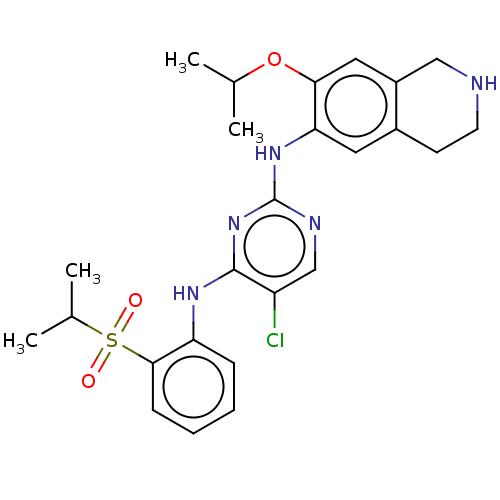 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(7-is...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM515783 (US11053225, Compound 65) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515861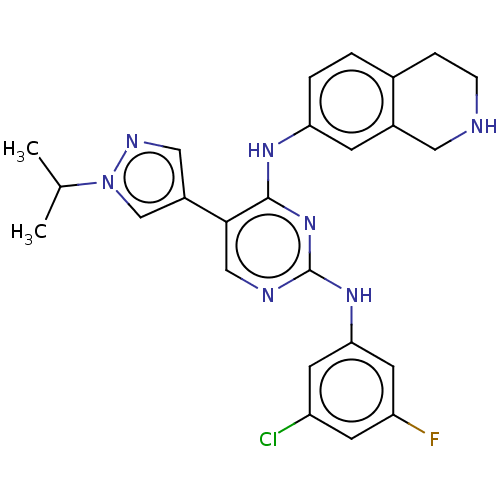 (US11053225, Compound 163) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243402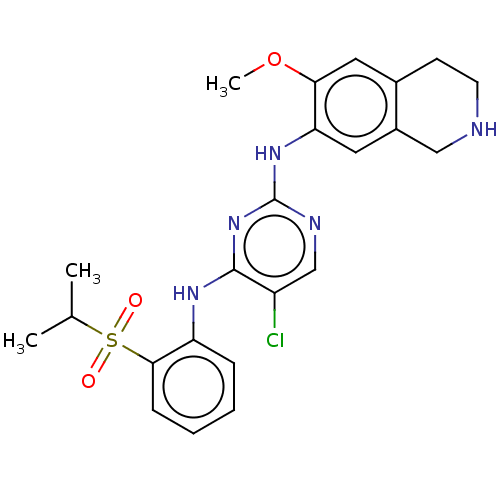 (5-chloro-N4-(2-(isopropylsulfonyl)phenyl)-N2-(6-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of crizotinib-resistant ALK G1269A mutant (unknown origin) using peptide substrate incubated for 30 mins in presence of ATP by fluorescenc... | Citation and Details Article DOI: 10.1016/j.bmc.2015.12.004 BindingDB Entry DOI: 10.7270/Q269778Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243393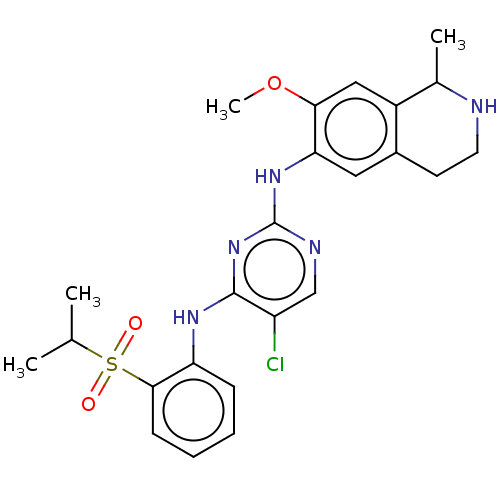 (5-chloro-N4-2-(isopropylsulfonyl)phenyl)-N2-(7-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515876 (US11053225, Compound 178) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515880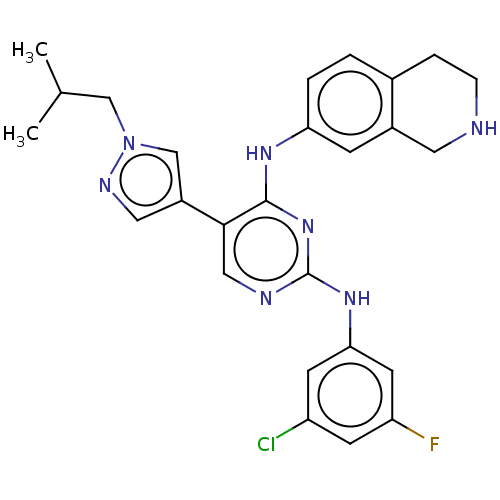 (US11053225, Compound 182) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515803 (US11053225, Compound 110) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512368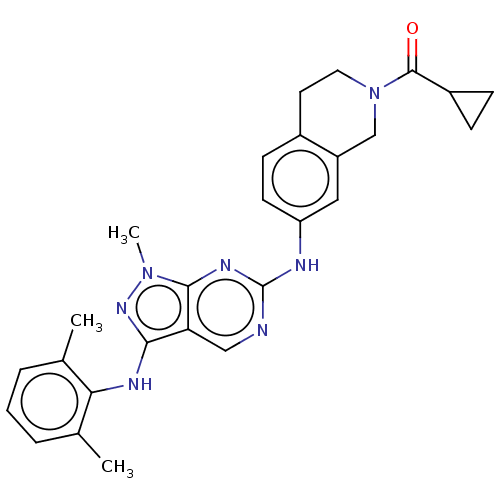 (US11084824, Example 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512369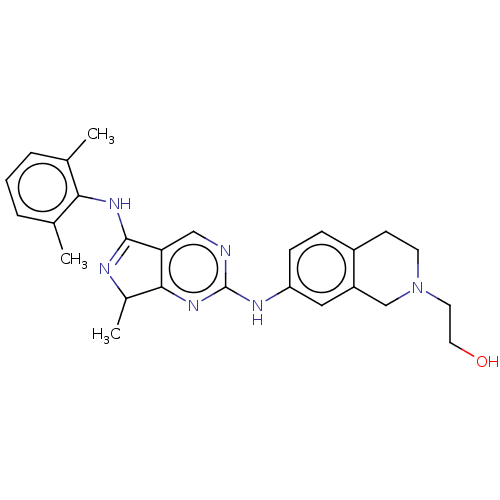 (US11084824, Example 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515844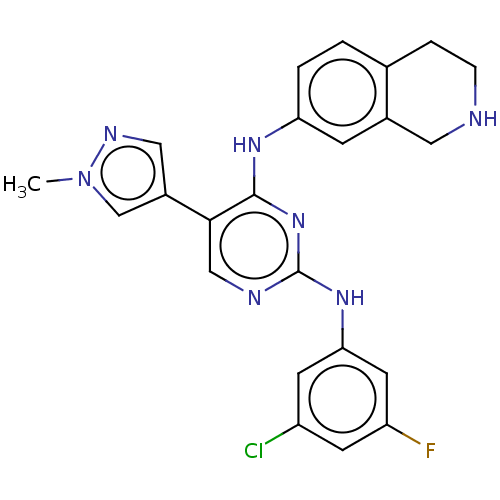 (US11053225, Compound 145) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50380974 (CHEMBL2016903) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant VEGFR-2 kinase domain by homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 2837-42 (2012) Article DOI: 10.1016/j.bmcl.2012.02.073 BindingDB Entry DOI: 10.7270/Q2RN38W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM243393 (5-chloro-N4-2-(isopropylsulfonyl)phenyl)-N2-(7-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM243395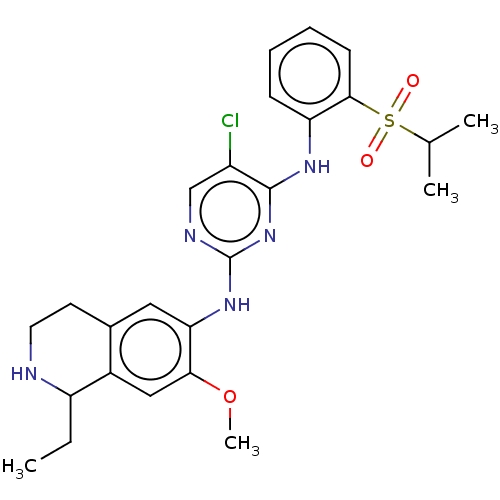 (5-chloro-N4-2-(isopropylsulfonyl)phenyl)-N2-(7-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description A proliferation inhibitory activity against the ALK of the compound represented by Chemical Formula 1 according to the present invention at an enzyme... | US Patent US10053458 (2018) BindingDB Entry DOI: 10.7270/Q2ZS2ZH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512364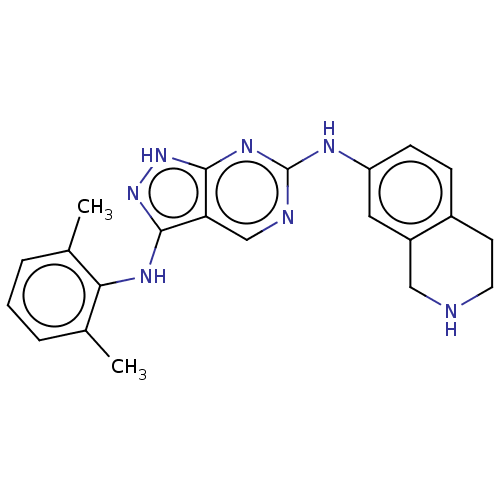 (Preparation of N3-(2,6-dimethylphenyl)-N6-(1,2,3,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM512370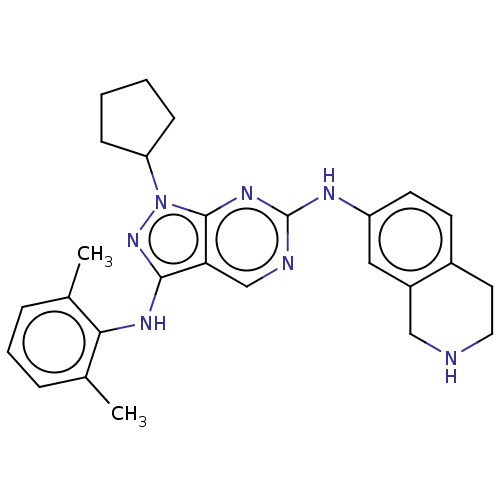 (US11084824, Example 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description BTK enzyme evaluation with each compound of examples of the invention was performed by using BTK enzyme inhibition diagnosis kit (Cisbio, Codolet, Fr... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP27KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515873 (US11053225, Compound 175) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515864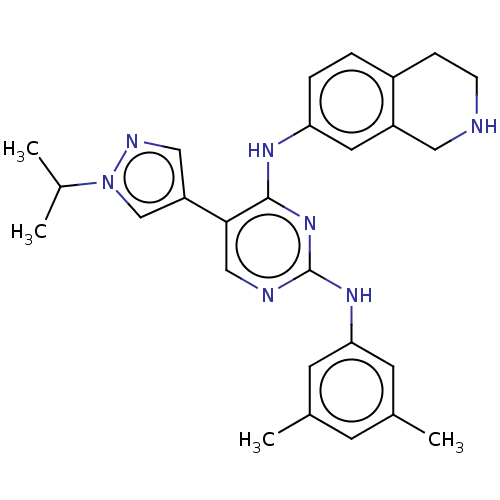 (US11053225, Compound 166) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515854 (US11053225, Compound 156) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM515821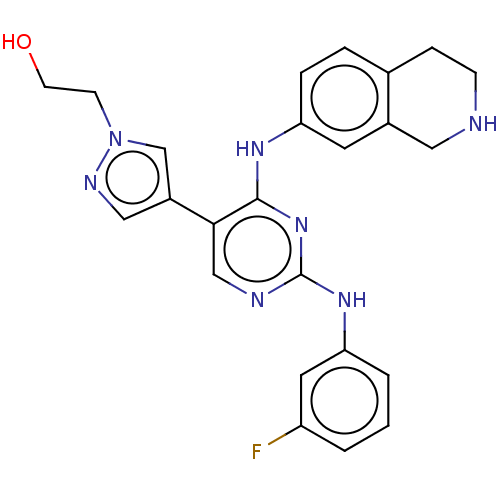 (US11053225, Compound 102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor TYRO3 (Homo sapiens (Human)) | BDBM515805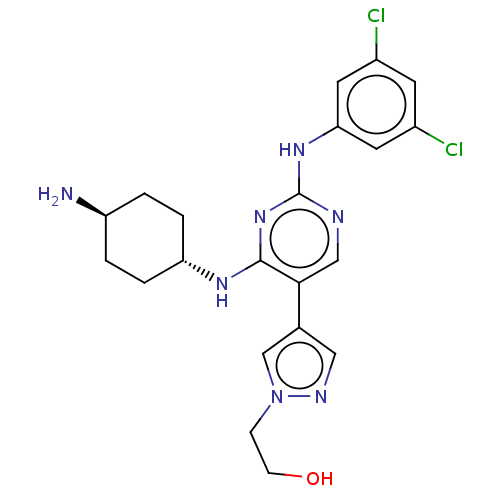 (US11053225, Compound 113) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1866 total ) | Next | Last >> |