

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554464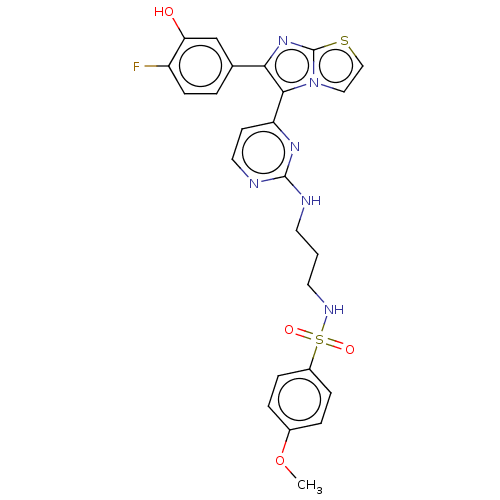 (US11332479, Compound 50III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50585092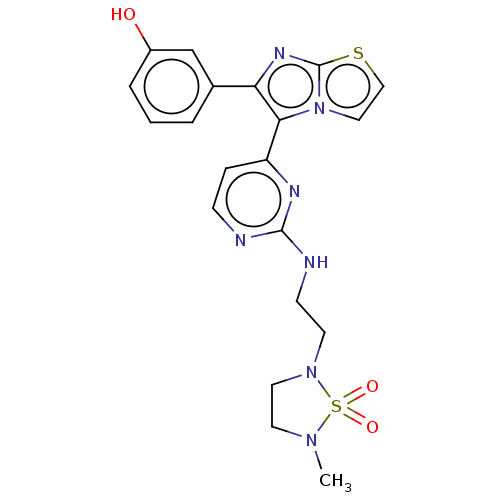 (CHEMBL5087577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554470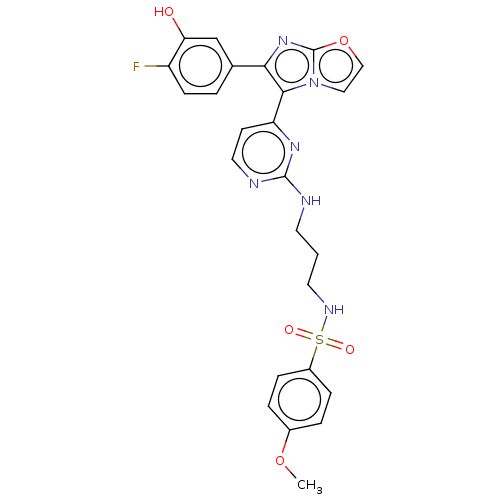 (US11332479, Compound 72IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554469 (US11332479, Compound 70IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554462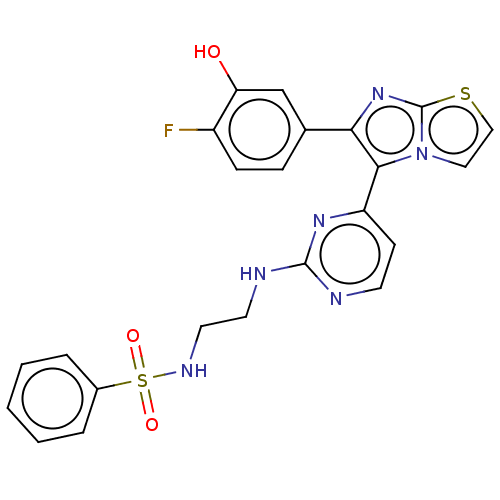 (US11332479, Compound 46III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554471 (US11332479, Compound 73IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554463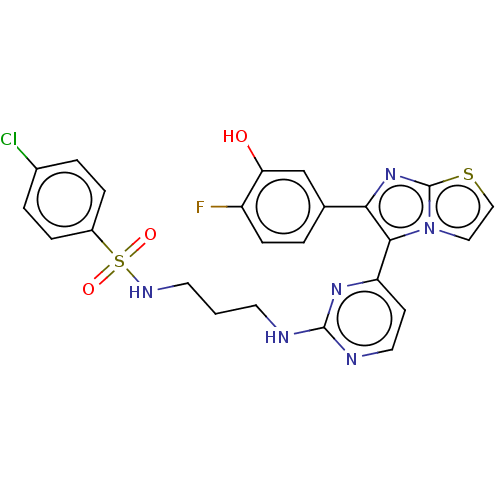 (US11332479, Compound 48III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554467 (US11332479, Compound 64IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554460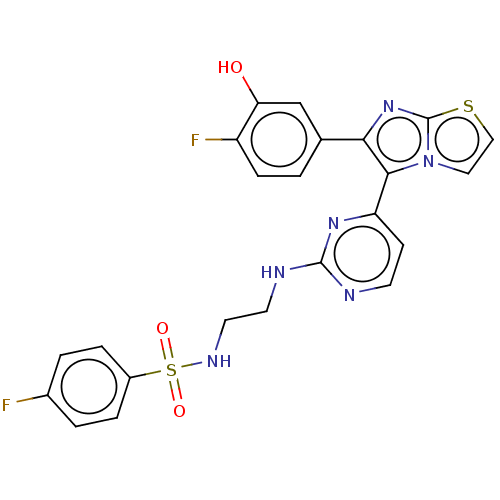 (US11332479, Compound 42III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554468 (US11332479, Compound 65IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50428286 (DABRAFENIB | GSK2118436A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF V600E mutant (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127478 BindingDB Entry DOI: 10.7270/Q2862M5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [696-1022,L858R] (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435722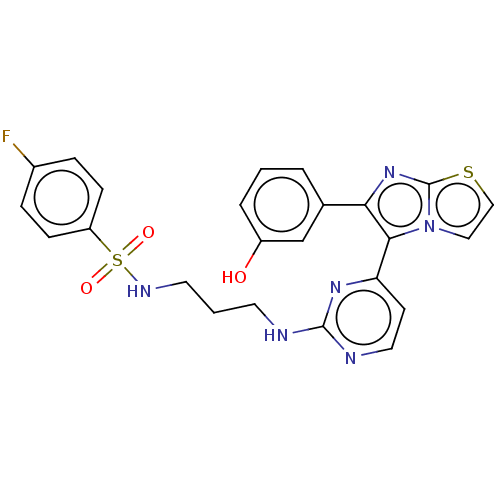 (US10570155, Compound 31III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50585081 (CHEMBL5086749) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554457 (US11332479, Compound 31II) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM435721 (US10570155, Compound 25III | US11332479, Compound ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435721 (US10570155, Compound 25III | US11332479, Compound ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50092079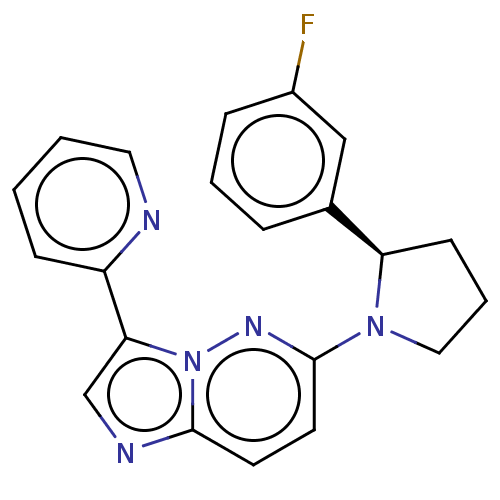 (CHEMBL3582439) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKB (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50092082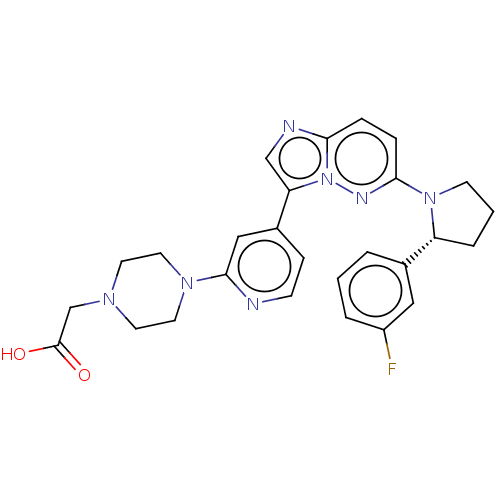 (CHEMBL3582441) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKB (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50092082 (CHEMBL3582441) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKA (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50092082 (CHEMBL3582441) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NGF-activated full length TRKA (unknown origin) expressed in mouse BA/F3 cells assessed as inhibition of cell proliferation | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50092082 (CHEMBL3582441) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of TPM3-fused TRKA (unknown origin) expressed in human KM12 cells assessed as inhibition of cell proliferation after 48 hrs by luciferase ... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50092082 (CHEMBL3582441) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKC (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50092079 (CHEMBL3582439) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKC (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435721 (US10570155, Compound 25III | US11332479, Compound ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554465 (US11332479, Compound 52III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50585092 (CHEMBL5087577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human wild type CRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50560548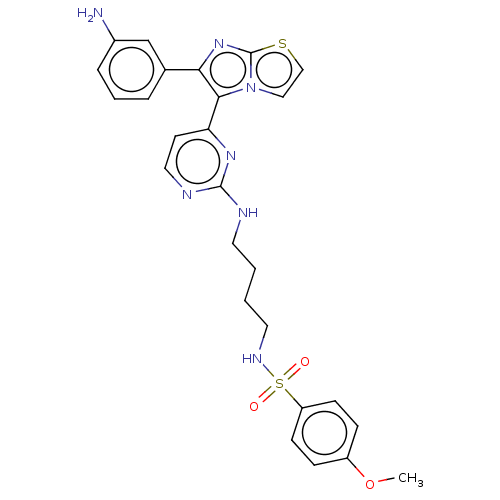 (CHEMBL4795335) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF V600E mutant (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127478 BindingDB Entry DOI: 10.7270/Q2862M5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50585085 (CHEMBL5080553) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554458 (US11332479, Compound 37III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435711 (US10570155, Compound 1II | US11332479, Compound 1I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.85 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435711 (US10570155, Compound 1II | US11332479, Compound 1I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554459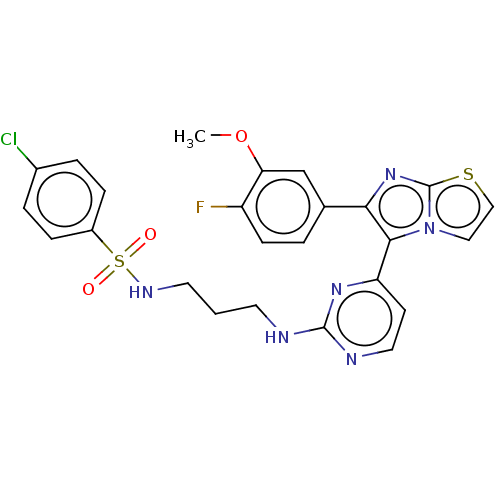 (US11332479, Compound 38III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.89 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50092081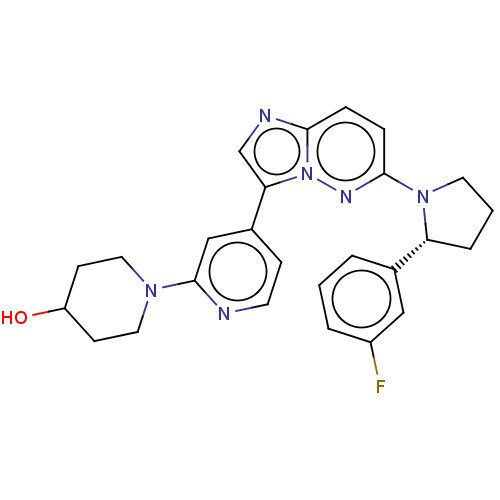 (CHEMBL3582442) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKC (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50092132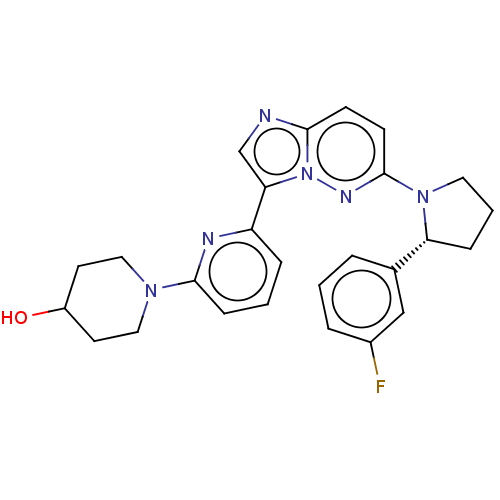 (CHEMBL3582440) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKC (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50585095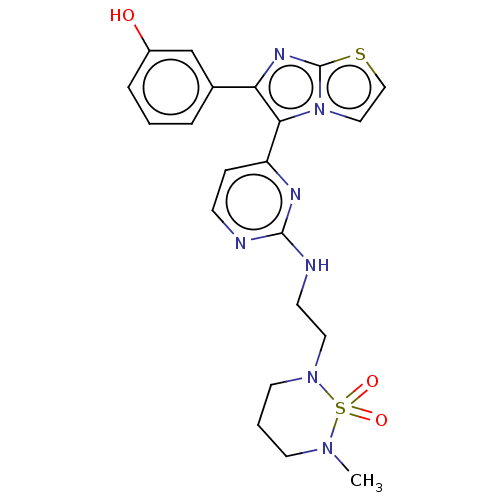 (CHEMBL5079413) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human wild type CRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50585092 (CHEMBL5087577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human wild type BRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50585088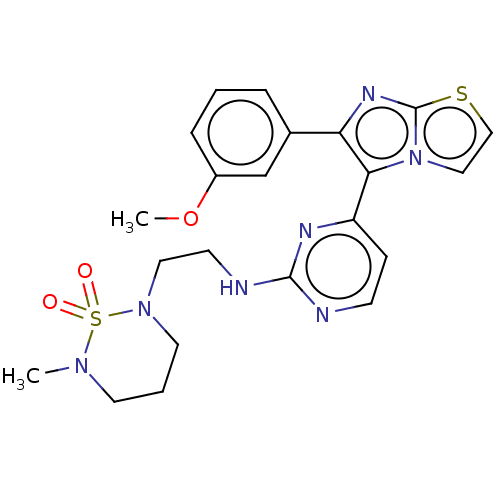 (CHEMBL5073246) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human wild type CRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50585085 (CHEMBL5080553) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human wild type CRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [696-1022,L858R] (Homo sapiens (Human)) | BDBM50289149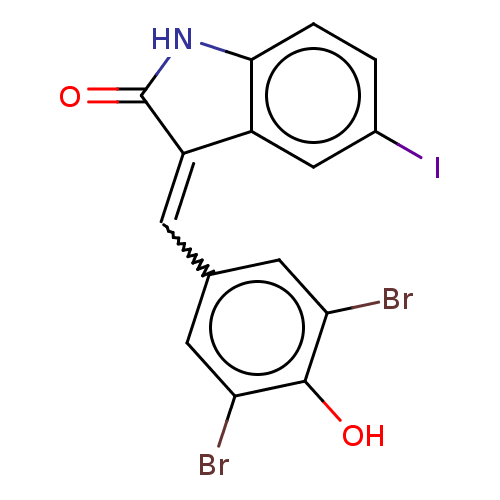 (GW305074X | GW5074 | US10570155, GW5074 | US113324...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50289149 (GW305074X | GW5074 | US10570155, GW5074 | US113324...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.58 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50585088 (CHEMBL5073246) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM554465 (US11332479, Compound 52III) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM435721 (US10570155, Compound 25III | US11332479, Compound ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human wild type BRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [696-1022,L858R] (Homo sapiens (Human)) | BDBM435711 (US10570155, Compound 1II | US11332479, Compound 1I...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.95 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1446 total ) | Next | Last >> |