 Found 115 hits with Last Name = 'choi' and Initial = 'mj'
Found 115 hits with Last Name = 'choi' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50506637

(CHEMBL4476226)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCN(CC2)C(C)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C27H30F3N7O3S/c1-18(38)35-11-13-36(14-12-35)21-9-7-20(8-10-21)32-26-31-15-23(27(28,29)30)25(33-26)37-16-19-5-4-6-24(22(19)17-37)34(2)41(3,39)40/h4-10,15H,11-14,16-17H2,1-3H3,(H,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human AXL using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50506637

(CHEMBL4476226)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCN(CC2)C(C)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C27H30F3N7O3S/c1-18(38)35-11-13-36(14-12-35)21-9-7-20(8-10-21)32-26-31-15-23(27(28,29)30)25(33-26)37-16-19-5-4-6-24(22(19)17-37)34(2)41(3,39)40/h4-10,15H,11-14,16-17H2,1-3H3,(H,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50506648
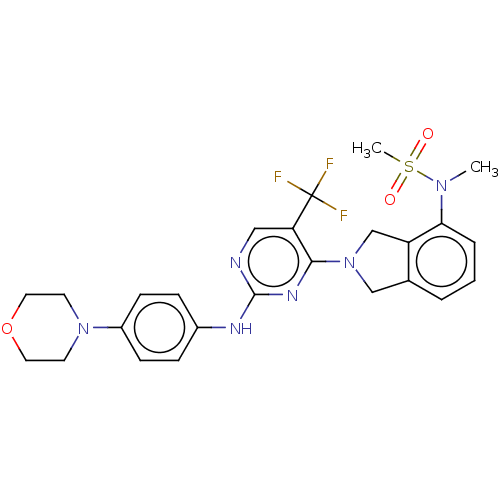
(CHEMBL4445940)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCOCC2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C25H27F3N6O3S/c1-32(38(2,35)36)22-5-3-4-17-15-34(16-20(17)22)23-21(25(26,27)28)14-29-24(31-23)30-18-6-8-19(9-7-18)33-10-12-37-13-11-33/h3-9,14H,10-13,15-16H2,1-2H3,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50506637

(CHEMBL4476226)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCN(CC2)C(C)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C27H30F3N7O3S/c1-18(38)35-11-13-36(14-12-35)21-9-7-20(8-10-21)32-26-31-15-23(27(28,29)30)25(33-26)37-16-19-5-4-6-24(22(19)17-37)34(2)41(3,39)40/h4-10,15H,11-14,16-17H2,1-3H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human AuroraA using [H-LRRASLG] as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50001935

(CHEMBL3233601)Show SMILES COc1ccc(cc1)C1=C(C(=O)N(C)C1=O)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C18H16N2O5S/c1-20-17(21)15(11-3-7-13(25-2)8-4-11)16(18(20)22)12-5-9-14(10-6-12)26(19,23)24/h3-10H,1-2H3,(H2,19,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50506636
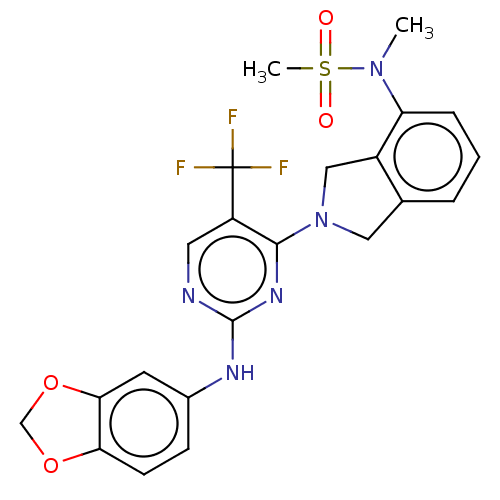
(CHEMBL4445646)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc3OCOc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H20F3N5O4S/c1-29(35(2,31)32)17-5-3-4-13-10-30(11-15(13)17)20-16(22(23,24)25)9-26-21(28-20)27-14-6-7-18-19(8-14)34-12-33-18/h3-9H,10-12H2,1-2H3,(H,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50506637

(CHEMBL4476226)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCN(CC2)C(C)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C27H30F3N7O3S/c1-18(38)35-11-13-36(14-12-35)21-9-7-20(8-10-21)32-26-31-15-23(27(28,29)30)25(33-26)37-16-19-5-4-6-24(22(19)17-37)34(2)41(3,39)40/h4-10,15H,11-14,16-17H2,1-3H3,(H,31,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50506637

(CHEMBL4476226)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCN(CC2)C(C)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C27H30F3N7O3S/c1-18(38)35-11-13-36(14-12-35)21-9-7-20(8-10-21)32-26-31-15-23(27(28,29)30)25(33-26)37-16-19-5-4-6-24(22(19)17-37)34(2)41(3,39)40/h4-10,15H,11-14,16-17H2,1-3H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human SYK using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50001936

(CHEMBL3233602)Show SMILES CN1C(=O)C(=C(C1=O)c1ccc(cc1)S(N)(=O)=O)c1ccc(Cl)cc1 |c:4| Show InChI InChI=1S/C17H13ClN2O4S/c1-20-16(21)14(10-2-6-12(18)7-3-10)15(17(20)22)11-4-8-13(9-5-11)25(19,23)24/h2-9H,1H3,(H2,19,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50001934

(CHEMBL3233600)Show SMILES CN1C(=O)C(=C(C1=O)c1ccc(cc1)S(N)(=O)=O)c1ccc(C)cc1 |c:4| Show InChI InChI=1S/C18H16N2O4S/c1-11-3-5-12(6-4-11)15-16(18(22)20(2)17(15)21)13-7-9-14(10-8-13)25(19,23)24/h3-10H,1-2H3,(H2,19,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50001936

(CHEMBL3233602)Show SMILES CN1C(=O)C(=C(C1=O)c1ccc(cc1)S(N)(=O)=O)c1ccc(Cl)cc1 |c:4| Show InChI InChI=1S/C17H13ClN2O4S/c1-20-16(21)14(10-2-6-12(18)7-3-10)15(17(20)22)11-4-8-13(9-5-11)25(19,23)24/h2-9H,1H3,(H2,19,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50506637

(CHEMBL4476226)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCN(CC2)C(C)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C27H30F3N7O3S/c1-18(38)35-11-13-36(14-12-35)21-9-7-20(8-10-21)32-26-31-15-23(27(28,29)30)25(33-26)37-16-19-5-4-6-24(22(19)17-37)34(2)41(3,39)40/h4-10,15H,11-14,16-17H2,1-3H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human PLK1 using casein as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50506637

(CHEMBL4476226)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCN(CC2)C(C)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C27H30F3N7O3S/c1-18(38)35-11-13-36(14-12-35)21-9-7-20(8-10-21)32-26-31-15-23(27(28,29)30)25(33-26)37-16-19-5-4-6-24(22(19)17-37)34(2)41(3,39)40/h4-10,15H,11-14,16-17H2,1-3H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human FAK using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50001933
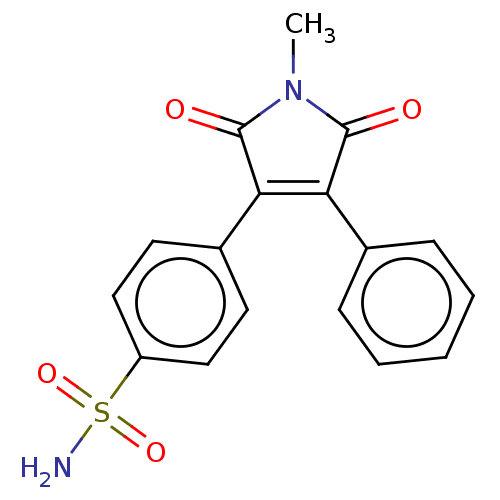
(CHEMBL3233599)Show SMILES CN1C(=O)C(=C(C1=O)c1ccc(cc1)S(N)(=O)=O)c1ccccc1 |c:4| Show InChI InChI=1S/C17H14N2O4S/c1-19-16(20)14(11-5-3-2-4-6-11)15(17(19)21)12-7-9-13(10-8-12)24(18,22)23/h2-10H,1H3,(H2,18,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM50506637

(CHEMBL4476226)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCN(CC2)C(C)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C27H30F3N7O3S/c1-18(38)35-11-13-36(14-12-35)21-9-7-20(8-10-21)32-26-31-15-23(27(28,29)30)25(33-26)37-16-19-5-4-6-24(22(19)17-37)34(2)41(3,39)40/h4-10,15H,11-14,16-17H2,1-3H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human MER using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50001933

(CHEMBL3233599)Show SMILES CN1C(=O)C(=C(C1=O)c1ccc(cc1)S(N)(=O)=O)c1ccccc1 |c:4| Show InChI InChI=1S/C17H14N2O4S/c1-19-16(20)14(11-5-3-2-4-6-11)15(17(19)21)12-7-9-13(10-8-12)24(18,22)23/h2-10H,1H3,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50506637

(CHEMBL4476226)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCN(CC2)C(C)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C27H30F3N7O3S/c1-18(38)35-11-13-36(14-12-35)21-9-7-20(8-10-21)32-26-31-15-23(27(28,29)30)25(33-26)37-16-19-5-4-6-24(22(19)17-37)34(2)41(3,39)40/h4-10,15H,11-14,16-17H2,1-3H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human TYRO3 using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50001935

(CHEMBL3233601)Show SMILES COc1ccc(cc1)C1=C(C(=O)N(C)C1=O)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C18H16N2O5S/c1-20-17(21)15(11-3-7-13(25-2)8-4-11)16(18(20)22)12-5-9-14(10-6-12)26(19,23)24/h3-10H,1-2H3,(H2,19,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50506657

(CHEMBL4457670)Show SMILES COc1cc(Nc2ncc(c(n2)N2Cc3cccc(N(C)S(C)(=O)=O)c3C2)C(F)(F)F)cc(OC)c1 Show InChI InChI=1S/C23H24F3N5O4S/c1-30(36(4,32)33)20-7-5-6-14-12-31(13-18(14)20)21-19(23(24,25)26)11-27-22(29-21)28-15-8-16(34-2)10-17(9-15)35-3/h5-11H,12-13H2,1-4H3,(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50001934

(CHEMBL3233600)Show SMILES CN1C(=O)C(=C(C1=O)c1ccc(cc1)S(N)(=O)=O)c1ccc(C)cc1 |c:4| Show InChI InChI=1S/C18H16N2O4S/c1-11-3-5-12(6-4-11)15-16(18(22)20(2)17(15)21)13-7-9-14(10-8-13)25(19,23)24/h3-10H,1-2H3,(H2,19,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50001931

(CHEMBL3233597)Show SMILES COc1ccc(cc1)C1=C(C(=O)NC1=O)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C17H14N2O5S/c1-24-12-6-2-10(3-7-12)14-15(17(21)19-16(14)20)11-4-8-13(9-5-11)25(18,22)23/h2-9H,1H3,(H2,18,22,23)(H,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50305810
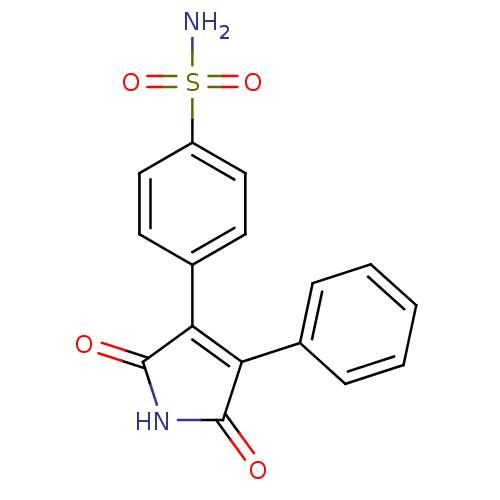
(1H-3-(4-sulfamoylphenyl)-4-phenyl-pyrrole-2,5-dion...)Show SMILES NS(=O)(=O)c1ccc(cc1)C1=C(C(=O)NC1=O)c1ccccc1 |t:11| Show InChI InChI=1S/C16H12N2O4S/c17-23(21,22)12-8-6-11(7-9-12)14-13(15(19)18-16(14)20)10-4-2-1-3-5-10/h1-9H,(H2,17,21,22)(H,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM82561
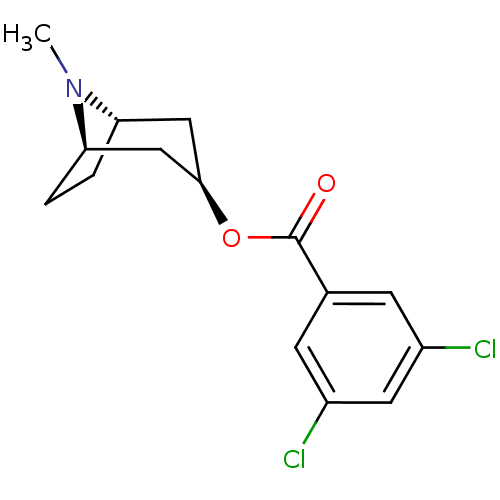
(CAS_40796-97-2 | TROPANYL 3,5-DICHLOROBENZOATE | T...)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C15H17Cl2NO2/c1-18-12-2-3-13(18)8-14(7-12)20-15(19)9-4-10(16)6-11(17)5-9/h4-6,12-14H,2-3,7-8H2,1H3/t12-,13+,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Antagonist activity at 5HT3A receptor expressed in Xenopus oocyte |
Bioorg Med Chem 17: 4793-6 (2009)
Article DOI: 10.1016/j.bmc.2009.04.029
BindingDB Entry DOI: 10.7270/Q2668F4R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50506649

(CHEMBL4546231)Show SMILES COc1cc(ccc1Nc1ncc(c(n1)N1Cc2cccc(N(C)S(C)(=O)=O)c2C1)C(F)(F)F)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C28H32F3N7O4S/c1-18(39)36-10-12-37(13-11-36)20-8-9-23(25(14-20)42-3)33-27-32-15-22(28(29,30)31)26(34-27)38-16-19-6-5-7-24(21(19)17-38)35(2)43(4,40)41/h5-9,14-15H,10-13,16-17H2,1-4H3,(H,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50295828
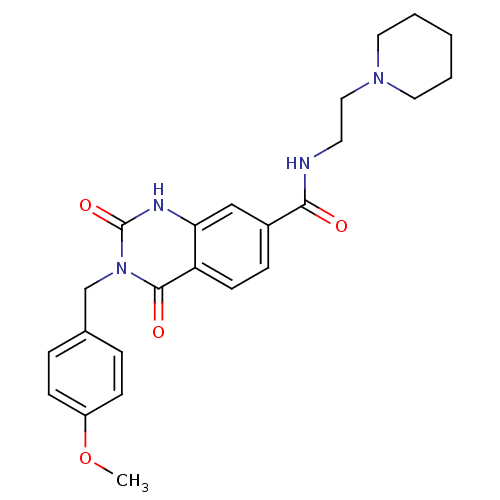
(3-(4-methoxybenzyl)-2,4-dioxo-N-(2-(piperidin-1-yl...)Show SMILES COc1ccc(Cn2c(=O)[nH]c3cc(ccc3c2=O)C(=O)NCCN2CCCCC2)cc1 Show InChI InChI=1S/C24H28N4O4/c1-32-19-8-5-17(6-9-19)16-28-23(30)20-10-7-18(15-21(20)26-24(28)31)22(29)25-11-14-27-12-3-2-4-13-27/h5-10,15H,2-4,11-14,16H2,1H3,(H,25,29)(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Antagonist activity at 5HT3A receptor expressed in Xenopus oocyte |
Bioorg Med Chem 17: 4793-6 (2009)
Article DOI: 10.1016/j.bmc.2009.04.029
BindingDB Entry DOI: 10.7270/Q2668F4R |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50009859
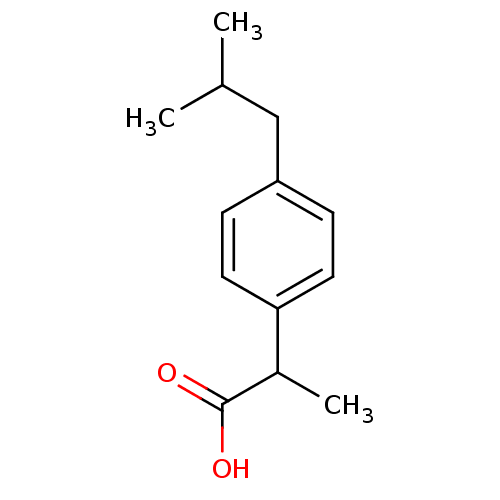
((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...)Show InChI InChI=1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50001930

(CHEMBL3233596)Show SMILES Cc1ccc(cc1)C1=C(C(=O)NC1=O)c1ccc(cc1)S(N)(=O)=O |t:8| Show InChI InChI=1S/C17H14N2O4S/c1-10-2-4-11(5-3-10)14-15(17(21)19-16(14)20)12-6-8-13(9-7-12)24(18,22)23/h2-9H,1H3,(H2,18,22,23)(H,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in mouse RAW264.7 cells assessed as decrease in LPS-induced PGE2 production treated prior to LPS challenge by enzyme immunoassay |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50506634

(CHEMBL4441802)Show SMILES COc1cc(Nc2ncc(c(n2)N2Cc3cccc(N(C)S(C)(=O)=O)c3C2)C(F)(F)F)cc(c1)C(F)(F)F Show InChI InChI=1S/C23H21F6N5O3S/c1-33(38(3,35)36)19-6-4-5-13-11-34(12-17(13)19)20-18(23(27,28)29)10-30-21(32-20)31-15-7-14(22(24,25)26)8-16(9-15)37-2/h4-10H,11-12H2,1-3H3,(H,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50001933

(CHEMBL3233599)Show SMILES CN1C(=O)C(=C(C1=O)c1ccc(cc1)S(N)(=O)=O)c1ccccc1 |c:4| Show InChI InChI=1S/C17H14N2O4S/c1-19-16(20)14(11-5-3-2-4-6-11)15(17(19)21)12-7-9-13(10-8-12)24(18,22)23/h2-10H,1H3,(H2,18,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50001934

(CHEMBL3233600)Show SMILES CN1C(=O)C(=C(C1=O)c1ccc(cc1)S(N)(=O)=O)c1ccc(C)cc1 |c:4| Show InChI InChI=1S/C18H16N2O4S/c1-11-3-5-12(6-4-11)15-16(18(22)20(2)17(15)21)13-7-9-14(10-8-13)25(19,23)24/h3-10H,1-2H3,(H2,19,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50001935

(CHEMBL3233601)Show SMILES COc1ccc(cc1)C1=C(C(=O)N(C)C1=O)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C18H16N2O5S/c1-20-17(21)15(11-3-7-13(25-2)8-4-11)16(18(20)22)12-5-9-14(10-6-12)26(19,23)24/h3-10H,1-2H3,(H2,19,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50001936

(CHEMBL3233602)Show SMILES CN1C(=O)C(=C(C1=O)c1ccc(cc1)S(N)(=O)=O)c1ccc(Cl)cc1 |c:4| Show InChI InChI=1S/C17H13ClN2O4S/c1-20-16(21)14(10-2-6-12(18)7-3-10)15(17(20)22)11-4-8-13(9-5-11)25(19,23)24/h2-9H,1H3,(H2,19,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins prior to substr... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50424306

(CHEMBL2314485)Show SMILES OC(=O)c1nnn(CCN(c2ccccc2)S(=O)(=O)c2ccccc2)c1C(O)=O Show InChI InChI=1S/C18H16N4O6S/c23-17(24)15-16(18(25)26)21(20-19-15)11-12-22(13-7-3-1-4-8-13)29(27,28)14-9-5-2-6-10-14/h1-10H,11-12H2,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 (unknown origin) using PGH2 as substrate incubated for 30 mins prior to substrate addition by PGH2-coupled spectrophotometric an... |
Bioorg Med Chem Lett 23: 75-80 (2012)
Article DOI: 10.1016/j.bmcl.2012.11.019
BindingDB Entry DOI: 10.7270/Q2DB8359 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50001931

(CHEMBL3233597)Show SMILES COc1ccc(cc1)C1=C(C(=O)NC1=O)c1ccc(cc1)S(N)(=O)=O |t:9| Show InChI InChI=1S/C17H14N2O5S/c1-24-12-6-2-10(3-7-12)14-15(17(21)19-16(14)20)11-4-8-13(9-5-11)25(18,22)23/h2-9H,1H3,(H2,18,22,23)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50295834

(3-(4-chlorobenzyl)-N-(3-morpholinopropyl)-2,4-diox...)Show SMILES Clc1ccc(Cn2c(=O)[nH]c3cc(ccc3c2=O)C(=O)NCCCN2CCOCC2)cc1 Show InChI InChI=1S/C23H25ClN4O4/c24-18-5-2-16(3-6-18)15-28-22(30)19-7-4-17(14-20(19)26-23(28)31)21(29)25-8-1-9-27-10-12-32-13-11-27/h2-7,14H,1,8-13,15H2,(H,25,29)(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Antagonist activity at 5HT3A receptor expressed in Xenopus oocyte |
Bioorg Med Chem 17: 4793-6 (2009)
Article DOI: 10.1016/j.bmc.2009.04.029
BindingDB Entry DOI: 10.7270/Q2668F4R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50295826
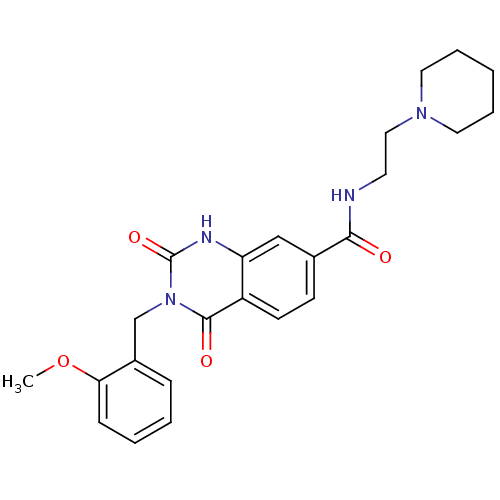
(3-(2-methoxybenzyl)-2,4-dioxo-N-(2-(piperidin-1-yl...)Show SMILES COc1ccccc1Cn1c(=O)[nH]c2cc(ccc2c1=O)C(=O)NCCN1CCCCC1 Show InChI InChI=1S/C24H28N4O4/c1-32-21-8-4-3-7-18(21)16-28-23(30)19-10-9-17(15-20(19)26-24(28)31)22(29)25-11-14-27-12-5-2-6-13-27/h3-4,7-10,15H,2,5-6,11-14,16H2,1H3,(H,25,29)(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Antagonist activity at 5HT3A receptor expressed in Xenopus oocyte |
Bioorg Med Chem 17: 4793-6 (2009)
Article DOI: 10.1016/j.bmc.2009.04.029
BindingDB Entry DOI: 10.7270/Q2668F4R |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50001930

(CHEMBL3233596)Show SMILES Cc1ccc(cc1)C1=C(C(=O)NC1=O)c1ccc(cc1)S(N)(=O)=O |t:8| Show InChI InChI=1S/C17H14N2O4S/c1-10-2-4-11(5-3-10)14-15(17(21)19-16(14)20)12-6-8-13(9-7-12)24(18,22)23/h2-9H,1H3,(H2,18,22,23)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50506644

(CHEMBL4558560)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2cccc(c2)C(F)(F)F)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H19F6N5O2S/c1-32(36(2,34)35)18-8-3-5-13-11-33(12-16(13)18)19-17(22(26,27)28)10-29-20(31-19)30-15-7-4-6-14(9-15)21(23,24)25/h3-10H,11-12H2,1-2H3,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50295824
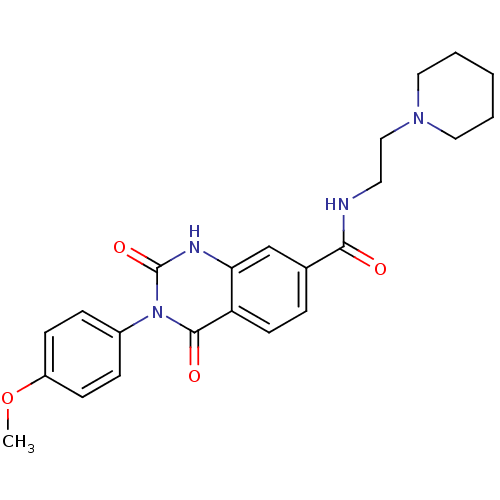
(3-(4-methoxyphenyl)-2,4-dioxo-N-(2-(piperidin-1-yl...)Show SMILES COc1ccc(cc1)-n1c(=O)[nH]c2cc(ccc2c1=O)C(=O)NCCN1CCCCC1 Show InChI InChI=1S/C23H26N4O4/c1-31-18-8-6-17(7-9-18)27-22(29)19-10-5-16(15-20(19)25-23(27)30)21(28)24-11-14-26-12-3-2-4-13-26/h5-10,15H,2-4,11-14H2,1H3,(H,24,28)(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Antagonist activity at 5HT3A receptor expressed in Xenopus oocyte |
Bioorg Med Chem 17: 4793-6 (2009)
Article DOI: 10.1016/j.bmc.2009.04.029
BindingDB Entry DOI: 10.7270/Q2668F4R |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as decrease in PGH2 production using arachidonic acid as substrate treated with enzyme for 10 mins pri... |
Bioorg Med Chem Lett 24: 1958-62 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.074
BindingDB Entry DOI: 10.7270/Q2SN0BGQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50295823

(3-(4-fluorophenyl)-2,4-dioxo-N-(2-(piperidin-1-yl)...)Show SMILES Fc1ccc(cc1)-n1c(=O)[nH]c2cc(ccc2c1=O)C(=O)NCCN1CCCCC1 Show InChI InChI=1S/C22H23FN4O3/c23-16-5-7-17(8-6-16)27-21(29)18-9-4-15(14-19(18)25-22(27)30)20(28)24-10-13-26-11-2-1-3-12-26/h4-9,14H,1-3,10-13H2,(H,24,28)(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Antagonist activity at 5HT3A receptor expressed in Xenopus oocyte |
Bioorg Med Chem 17: 4793-6 (2009)
Article DOI: 10.1016/j.bmc.2009.04.029
BindingDB Entry DOI: 10.7270/Q2668F4R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50196473
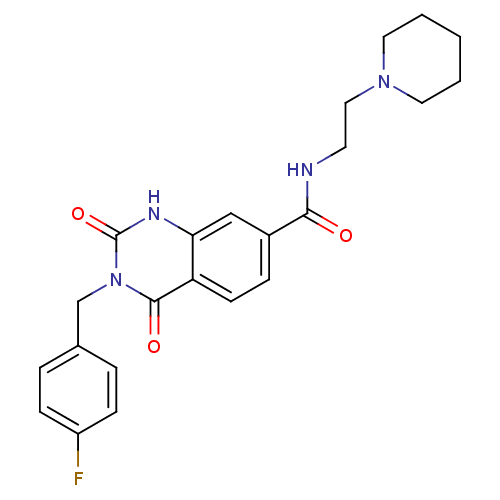
(3-(4-fluorobenzyl)-2,4-dioxo-N-(2-(piperidin-1-yl)...)Show SMILES Fc1ccc(Cn2c(=O)[nH]c3cc(ccc3c2=O)C(=O)NCCN2CCCCC2)cc1 Show InChI InChI=1S/C23H25FN4O3/c24-18-7-4-16(5-8-18)15-28-22(30)19-9-6-17(14-20(19)26-23(28)31)21(29)25-10-13-27-11-2-1-3-12-27/h4-9,14H,1-3,10-13,15H2,(H,25,29)(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Antagonist activity at 5HT3A receptor expressed in Xenopus oocyte |
Bioorg Med Chem 17: 4793-6 (2009)
Article DOI: 10.1016/j.bmc.2009.04.029
BindingDB Entry DOI: 10.7270/Q2668F4R |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50196475
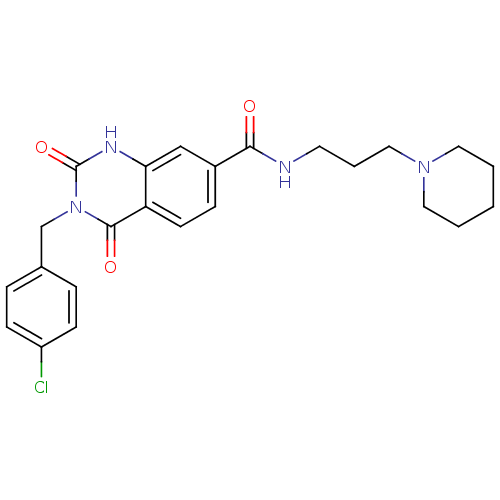
(3-(4-chlorobenzyl)-2,4-dioxo-N-(3-(piperidin-1-yl)...)Show SMILES Clc1ccc(Cn2c(=O)[nH]c3cc(ccc3c2=O)C(=O)NCCCN2CCCCC2)cc1 Show InChI InChI=1S/C24H27ClN4O3/c25-19-8-5-17(6-9-19)16-29-23(31)20-10-7-18(15-21(20)27-24(29)32)22(30)26-11-4-14-28-12-2-1-3-13-28/h5-10,15H,1-4,11-14,16H2,(H,26,30)(H,27,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Antagonist activity against T-type calcium channel alpha1G |
Bioorg Med Chem 17: 4793-6 (2009)
Article DOI: 10.1016/j.bmc.2009.04.029
BindingDB Entry DOI: 10.7270/Q2668F4R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50295829

(3-cyclohexyl-2,4-dioxo-N-(2-(piperidin-1-yl)ethyl)...)Show SMILES O=C(NCCN1CCCCC1)c1ccc2c(c1)[nH]c(=O)n(C1CCCCC1)c2=O Show InChI InChI=1S/C22H30N4O3/c27-20(23-11-14-25-12-5-2-6-13-25)16-9-10-18-19(15-16)24-22(29)26(21(18)28)17-7-3-1-4-8-17/h9-10,15,17H,1-8,11-14H2,(H,23,27)(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Antagonist activity at 5HT3A receptor expressed in Xenopus oocyte |
Bioorg Med Chem 17: 4793-6 (2009)
Article DOI: 10.1016/j.bmc.2009.04.029
BindingDB Entry DOI: 10.7270/Q2668F4R |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1G
(Homo sapiens (Human)) | BDBM50196465

(3-(4-chlorobenzyl)-2,4-dioxo-N-(2-(piperidin-1-yl)...)Show SMILES Clc1ccc(Cn2c(=O)[nH]c3cc(ccc3c2=O)C(=O)NCCN2CCCCC2)cc1 Show InChI InChI=1S/C23H25ClN4O3/c24-18-7-4-16(5-8-18)15-28-22(30)19-9-6-17(14-20(19)26-23(28)31)21(29)25-10-13-27-11-2-1-3-12-27/h4-9,14H,1-3,10-13,15H2,(H,25,29)(H,26,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University
Curated by ChEMBL
| Assay Description
Antagonist activity against T-type calcium channel alpha1G |
Bioorg Med Chem 17: 4793-6 (2009)
Article DOI: 10.1016/j.bmc.2009.04.029
BindingDB Entry DOI: 10.7270/Q2668F4R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data