

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM92596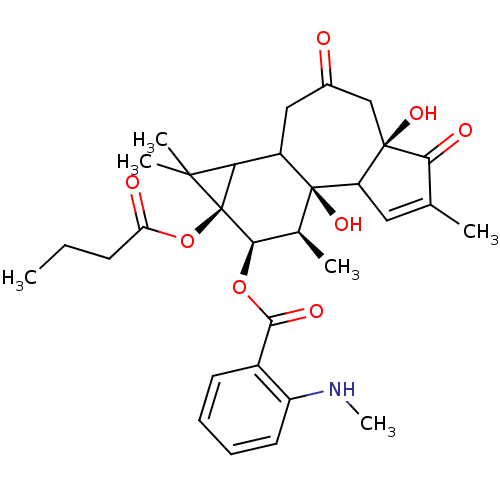 (Sapintoxin D) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50107112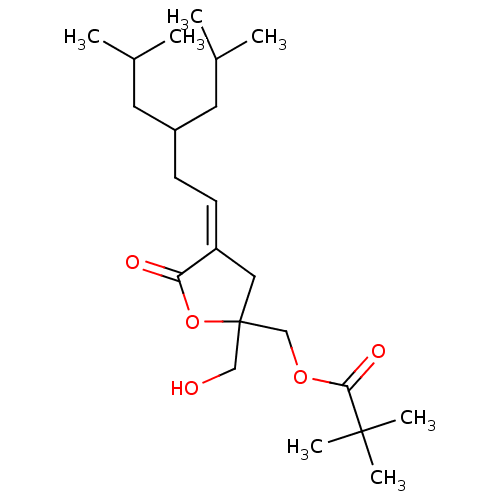 ((Z) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16 | -49.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM92595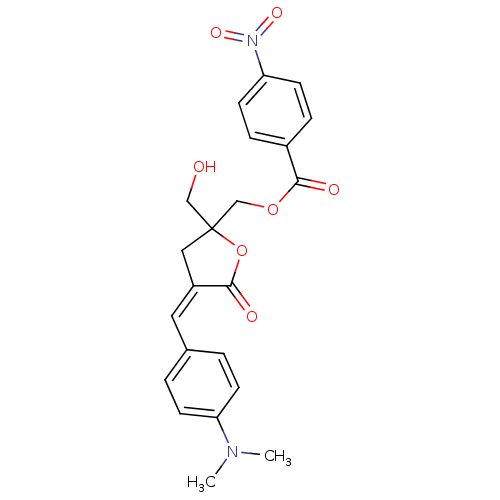 (DAG-lactone (130C045)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.54 | -49.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK alpha (Homo sapiens (Human)) | BDBM92596 (Sapintoxin D) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50015677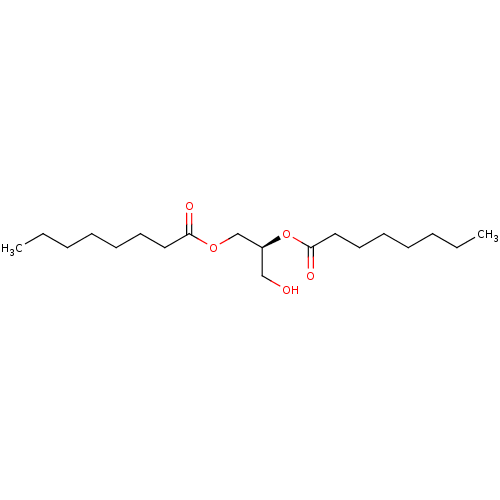 ((S)-1-hydroxymethyl-2-octanoyloxy-ethyl ester | 1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK beta (Homo sapiens (Human)) | BDBM92596 (Sapintoxin D) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12.6 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM92596 (Sapintoxin D) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18.2 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50107112 ((Z) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 402 | -35.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK alpha (Homo sapiens (Human)) | BDBM50107112 ((Z) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.03E+3 | -33.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK beta (Homo sapiens (Human)) | BDBM50107112 ((Z) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.07E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK alpha (Homo sapiens (Human)) | BDBM50015677 ((S)-1-hydroxymethyl-2-octanoyloxy-ethyl ester | 1,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.82E+3 | -30.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM92595 (DAG-lactone (130C045)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.20E+3 | -30.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50015677 ((S)-1-hydroxymethyl-2-octanoyloxy-ethyl ester | 1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70E+3 | -28.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK beta (Homo sapiens (Human)) | BDBM50015677 ((S)-1-hydroxymethyl-2-octanoyloxy-ethyl ester | 1,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6.96E+3 | -28.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK beta (Homo sapiens (Human)) | BDBM92595 (DAG-lactone (130C045)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.26E+4 | -25.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK alpha (Homo sapiens (Human)) | BDBM92595 (DAG-lactone (130C045)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.26E+4 | -25.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 18 |
National Institutes of Health | Assay Description [3H]PDBu binding to the C1 domains of MRCK alpha/beta and PKC alpha/delta was measured using the polyethylene glycol precipitation assay. | J Biol Chem 283: 10543-9 (2008) Article DOI: 10.1074/jbc.M707463200 BindingDB Entry DOI: 10.7270/Q2CF9NQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222640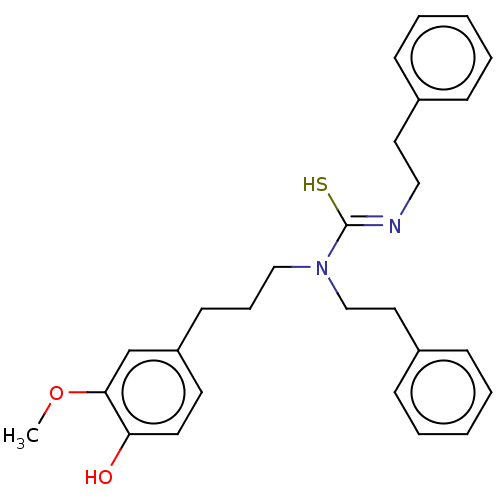 (CHEMBL415543) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222640 (CHEMBL415543) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222695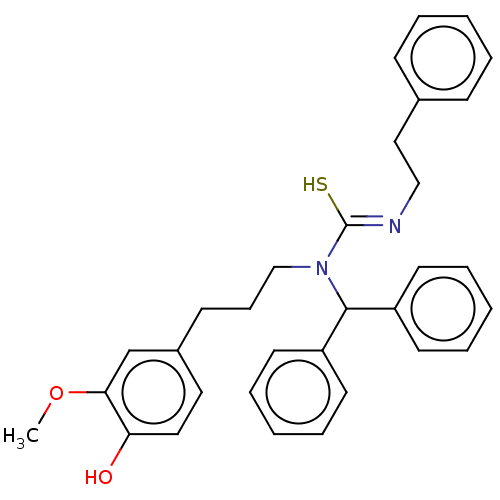 (CHEMBL155433) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222637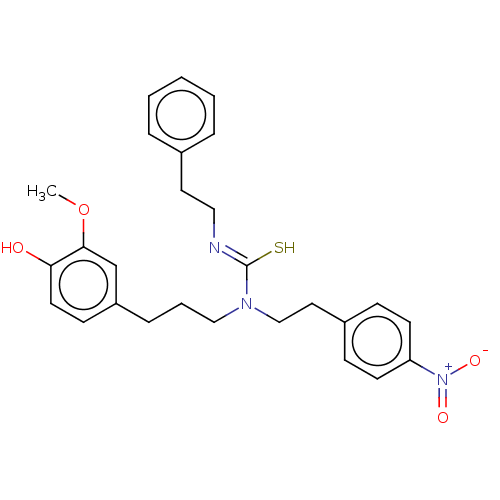 (CHEMBL154743) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222696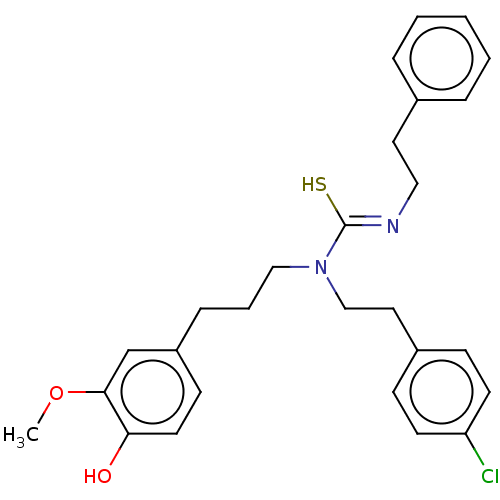 (CHEMBL156041) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222635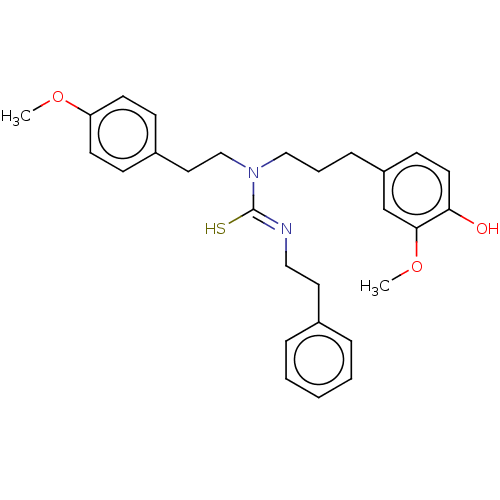 (CHEMBL267205) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344779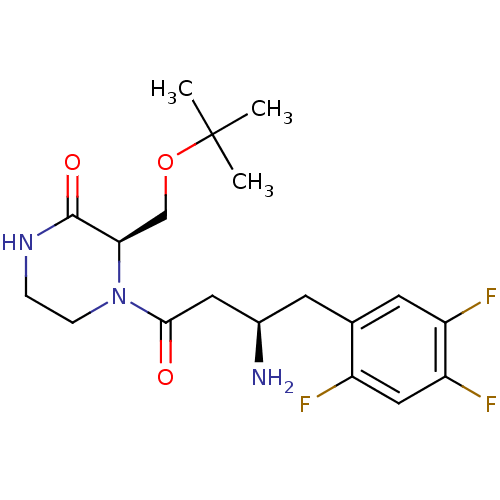 ((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222649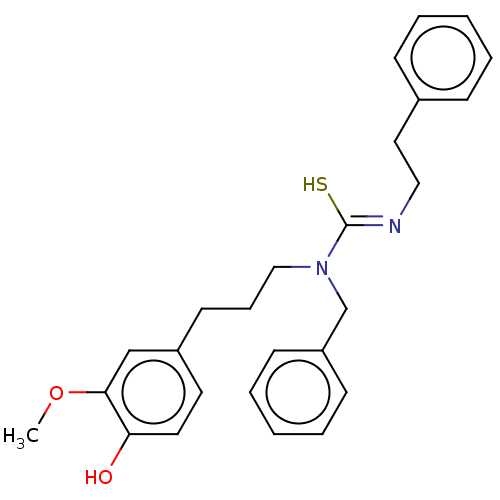 (CHEMBL155730) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222647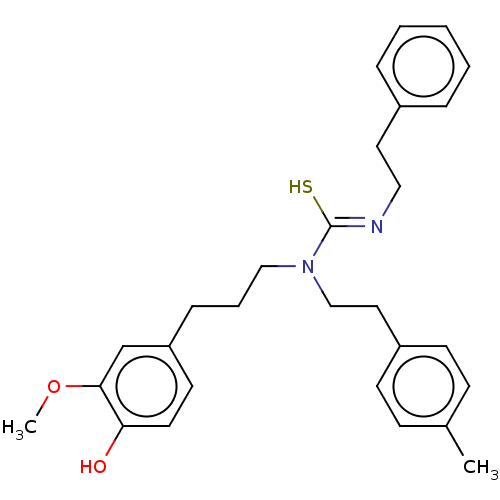 (CHEMBL154990) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222641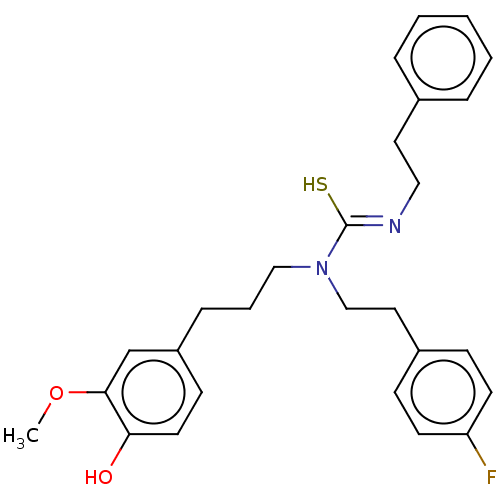 (CHEMBL157962) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344783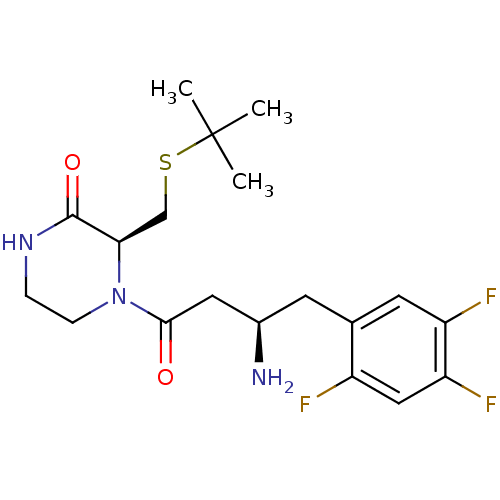 ((S)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344777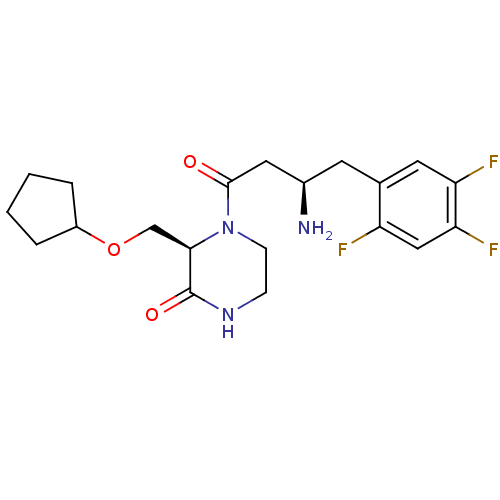 ((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344778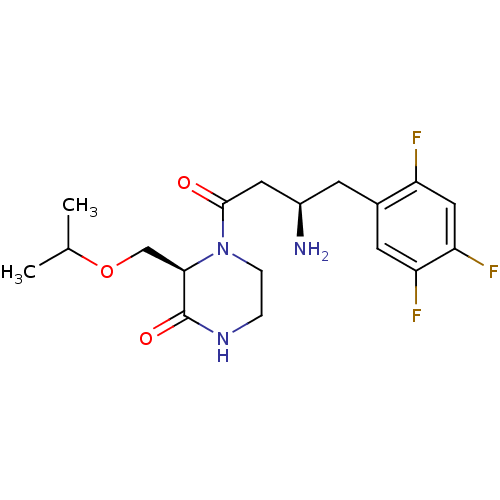 ((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222638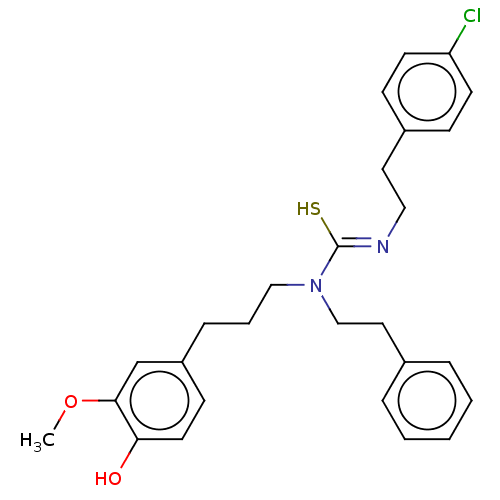 (CHEMBL422702) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222644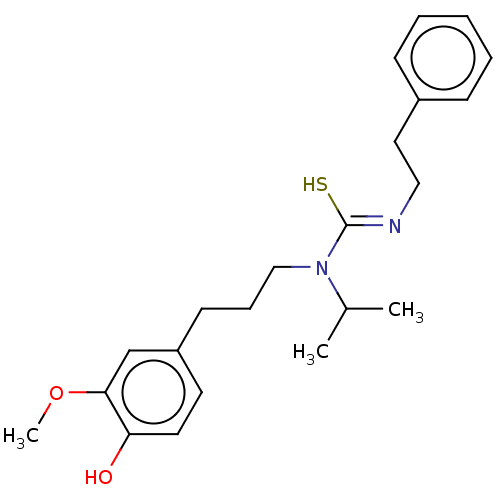 (CHEMBL434295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222648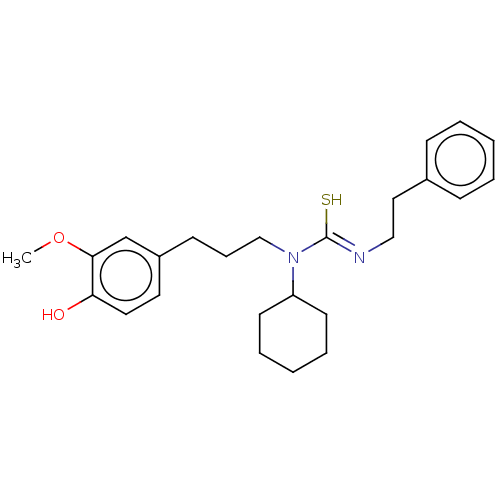 (CHEMBL156693) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222639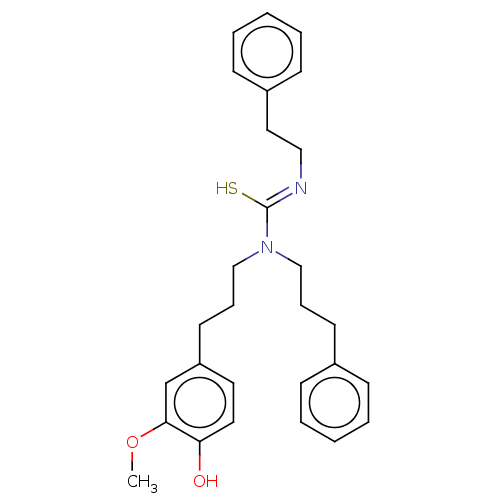 (CHEMBL356682) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344776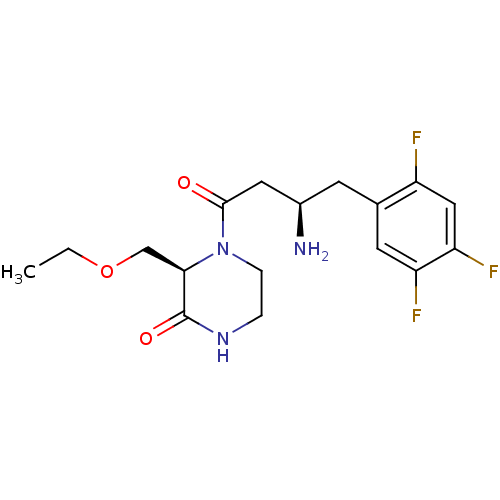 ((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222646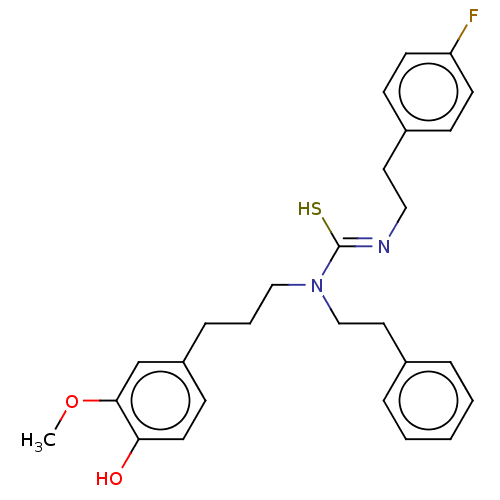 (CHEMBL157917) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344782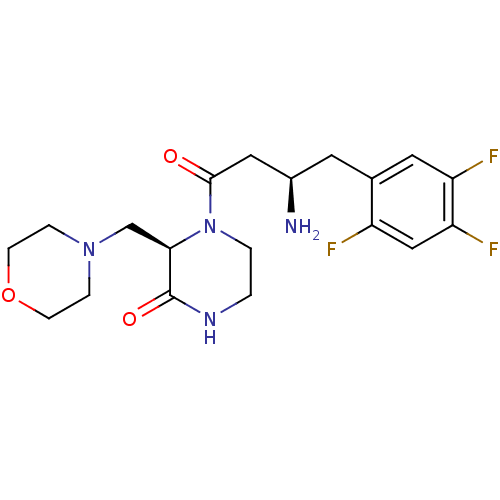 ((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222694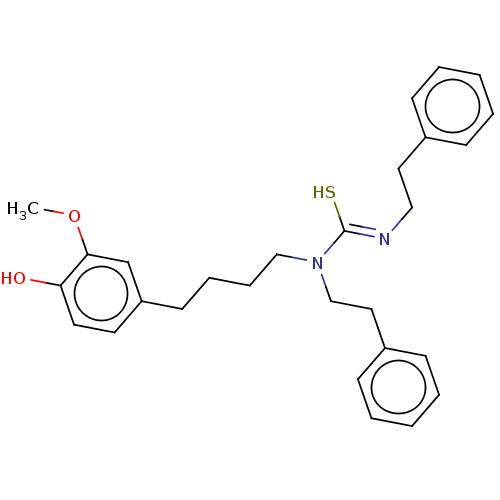 (CHEMBL158189) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344788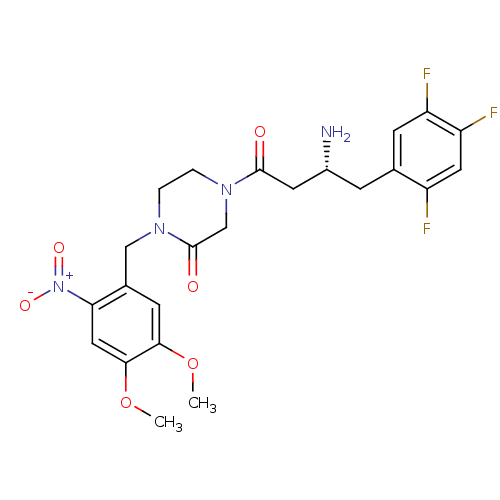 ((R)-4-(3-amino-4-(2,4,5-trifluorophenyl)butanoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344775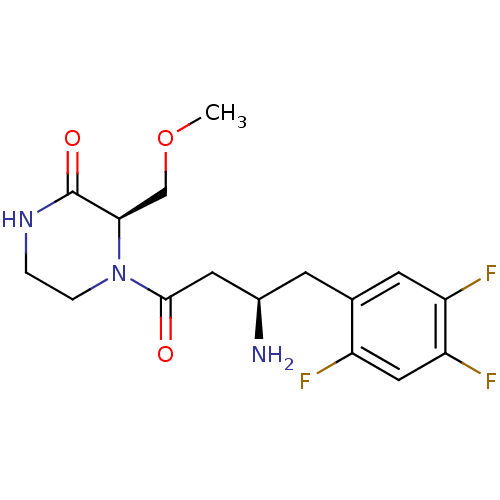 ((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222691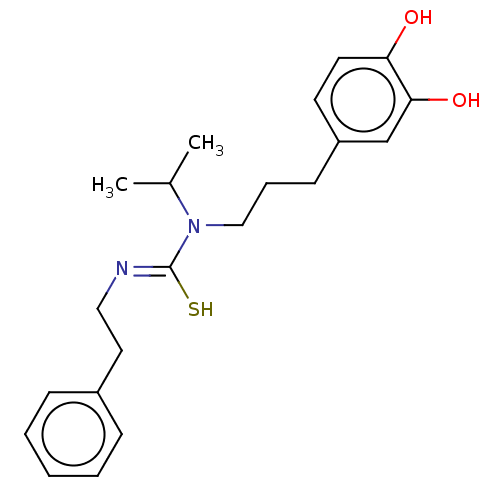 (CHEMBL156927) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222690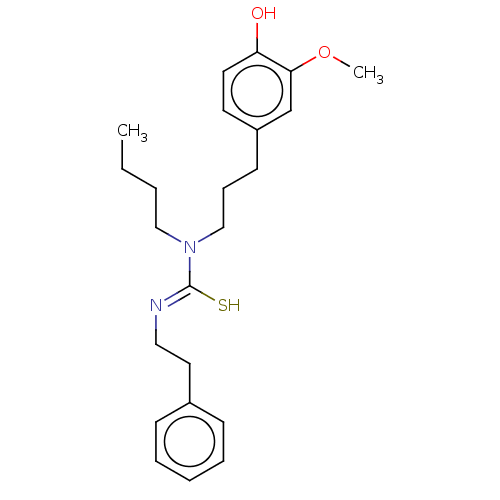 (CHEMBL345157) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344781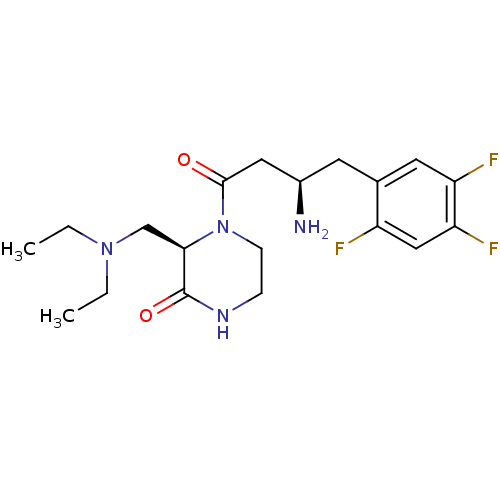 ((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222645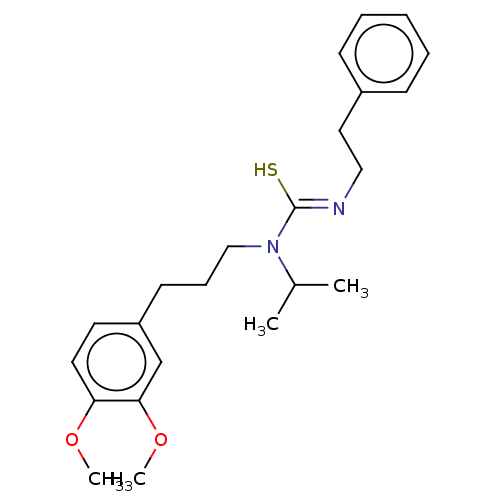 (CHEMBL157918) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50222693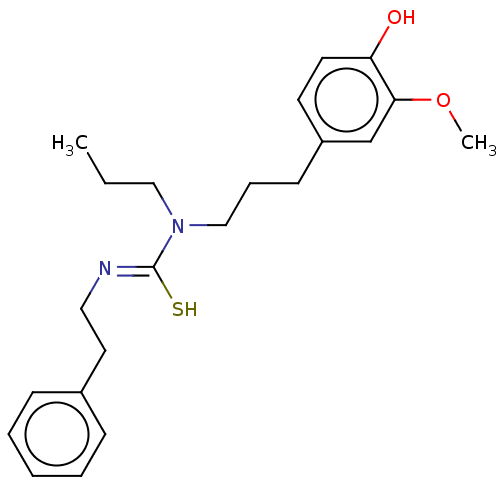 (CHEMBL157579) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonistic activity against vanilloid receptor as concentration to reduce the response to 0.5 mM capsaicin by 50% from neonatal rat culture spinal ... | Bioorg Med Chem Lett 13: 601-4 (2003) BindingDB Entry DOI: 10.7270/Q2XP7747 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344774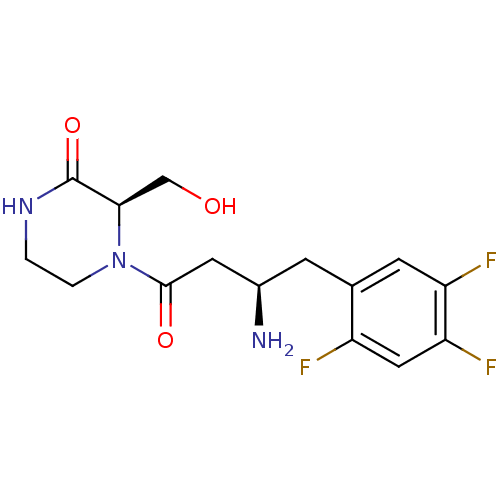 ((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344769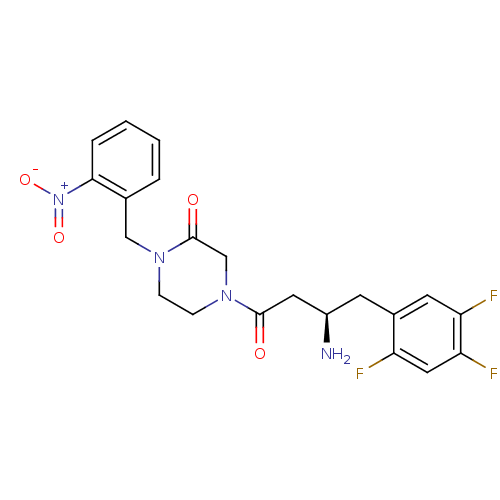 ((R)-4-(3-amino-4-(2,4,5-trifluorophenyl)butanoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50224228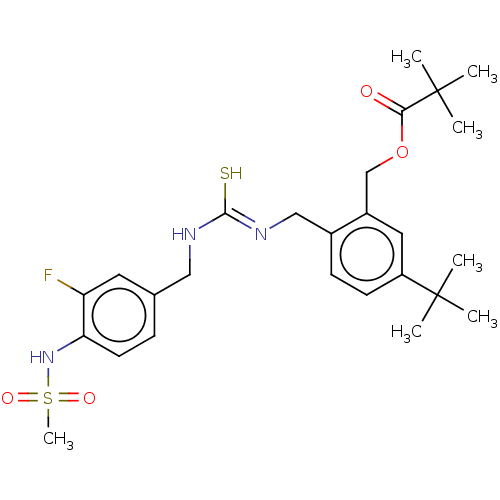 (CHEMBL46233) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro inhibitory concentration was determined against [45Ca2+]- influx in rat DRG neurons | Bioorg Med Chem Lett 14: 1693-6 (2004) BindingDB Entry DOI: 10.7270/Q23B6294 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344780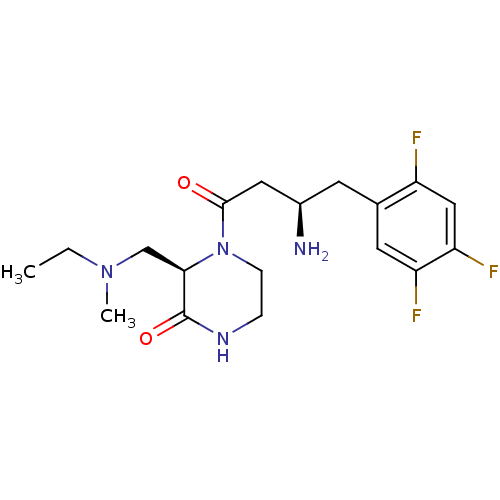 ((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1/2/4 (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50224175 (CHEMBL48061) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro inhibitory concentration was determined against [45Ca2+]- influx in rat DRG neurons | Bioorg Med Chem Lett 14: 1693-6 (2004) BindingDB Entry DOI: 10.7270/Q23B6294 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50344786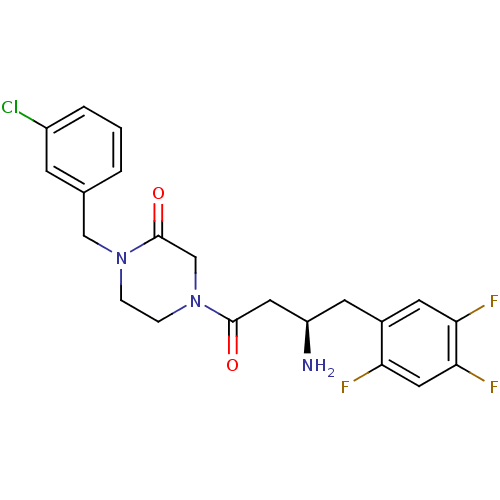 ((R)-4-(3-amino-4-(2,4,5-trifluorophenyl)butanoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay | Bioorg Med Chem Lett 21: 3809-12 (2011) Article DOI: 10.1016/j.bmcl.2011.04.029 BindingDB Entry DOI: 10.7270/Q2XS5VQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 203 total ) | Next | Last >> |