 Found 973 hits with Last Name = 'christensen' and Initial = 's'
Found 973 hits with Last Name = 'christensen' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118750
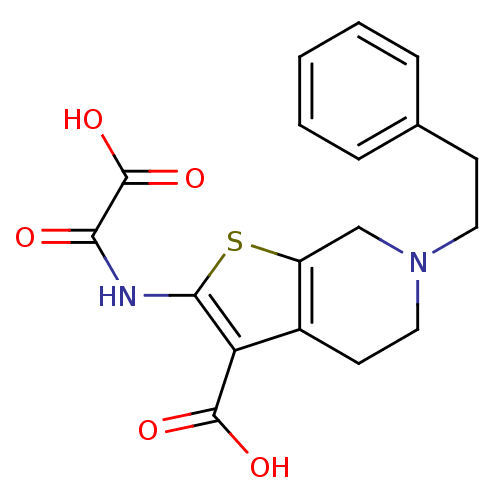
(2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C18H18N2O5S/c21-15(18(24)25)19-16-14(17(22)23)12-7-9-20(10-13(12)26-16)8-6-11-4-2-1-3-5-11/h1-5H,6-10H2,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118792
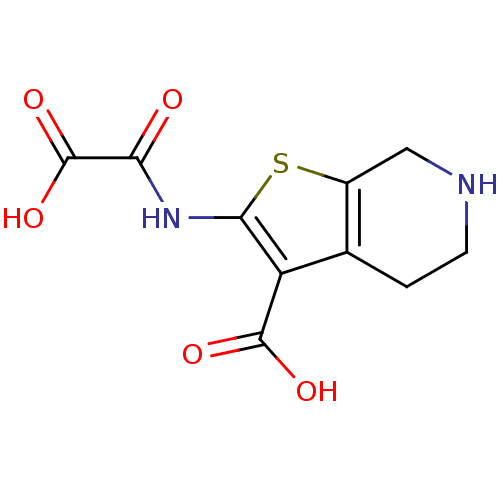
(2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...)Show InChI InChI=1S/C10H10N2O5S/c13-7(10(16)17)12-8-6(9(14)15)4-1-2-11-3-5(4)18-8/h11H,1-3H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118786
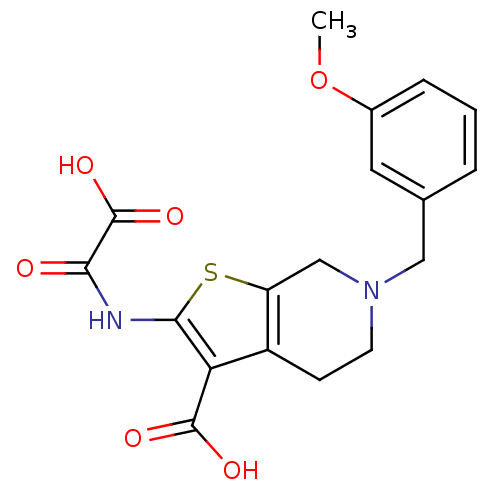
(6-(3-Methoxy-benzyl)-2-(oxalyl-amino)-4,5,6,7-tetr...)Show SMILES COc1cccc(CN2CCc3c(C2)sc(NC(=O)C(O)=O)c3C(O)=O)c1 Show InChI InChI=1S/C18H18N2O6S/c1-26-11-4-2-3-10(7-11)8-20-6-5-12-13(9-20)27-16(14(12)17(22)23)19-15(21)18(24)25/h2-4,7H,5-6,8-9H2,1H3,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118794
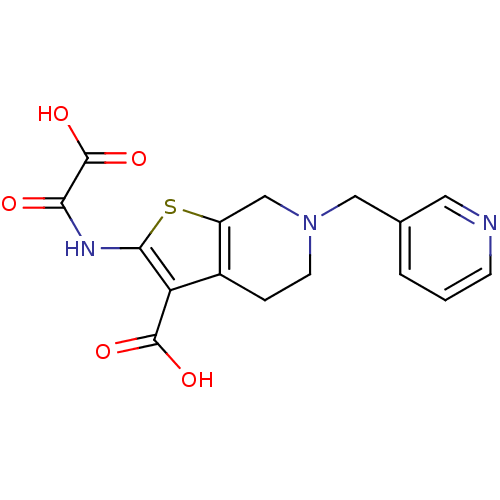
(2-(Oxalyl-amino)-6-pyridin-3-ylmethyl-4,5,6,7-tetr...)Show InChI InChI=1S/C16H15N3O5S/c20-13(16(23)24)18-14-12(15(21)22)10-3-5-19(8-11(10)25-14)7-9-2-1-4-17-6-9/h1-2,4,6H,3,5,7-8H2,(H,18,20)(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118742

(2-(Oxalyl-amino)-6-pyridin-2-ylmethyl-4,5,6,7-tetr...)Show InChI InChI=1S/C16H15N3O5S/c20-13(16(23)24)18-14-12(15(21)22)10-4-6-19(8-11(10)25-14)7-9-3-1-2-5-17-9/h1-3,5H,4,6-8H2,(H,18,20)(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118793

(6-Methyl-2-(oxalyl-amino)-4,5,6,7-tetrahydro-thien...)Show InChI InChI=1S/C11H12N2O5S/c1-13-3-2-5-6(4-13)19-9(7(5)10(15)16)12-8(14)11(17)18/h2-4H2,1H3,(H,12,14)(H,15,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118774
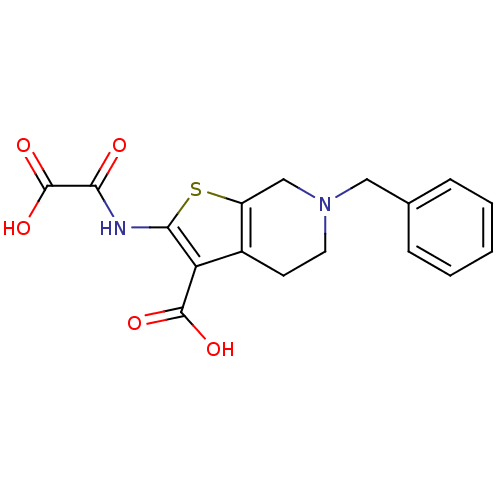
(6-Benzyl-2-(oxalyl-amino)-4,5,6,7-tetrahydro-thien...)Show InChI InChI=1S/C17H16N2O5S/c20-14(17(23)24)18-15-13(16(21)22)11-6-7-19(9-12(11)25-15)8-10-4-2-1-3-5-10/h1-5H,6-9H2,(H,18,20)(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118755
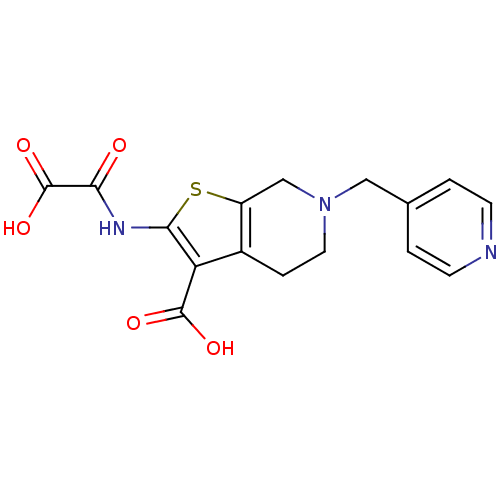
(2-(Oxalyl-amino)-6-pyridin-4-ylmethyl-4,5,6,7-tetr...)Show InChI InChI=1S/C16H15N3O5S/c20-13(16(23)24)18-14-12(15(21)22)10-3-6-19(8-11(10)25-14)7-9-1-4-17-5-2-9/h1-2,4-5H,3,6-8H2,(H,18,20)(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118772
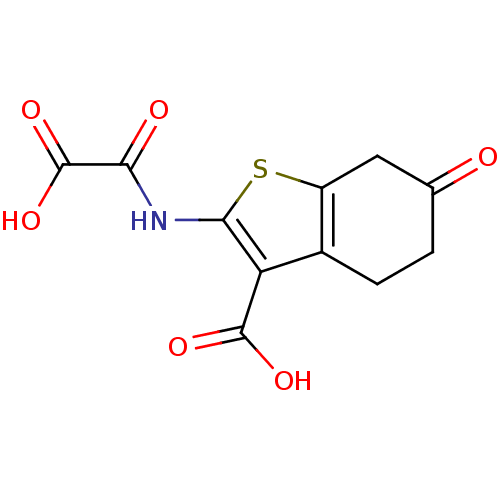
(2-(Oxalyl-amino)-6-oxo-4,5,6,7-tetrahydro-benzo[b]...)Show InChI InChI=1S/C11H9NO6S/c13-4-1-2-5-6(3-4)19-9(7(5)10(15)16)12-8(14)11(17)18/h1-3H2,(H,12,14)(H,15,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118767
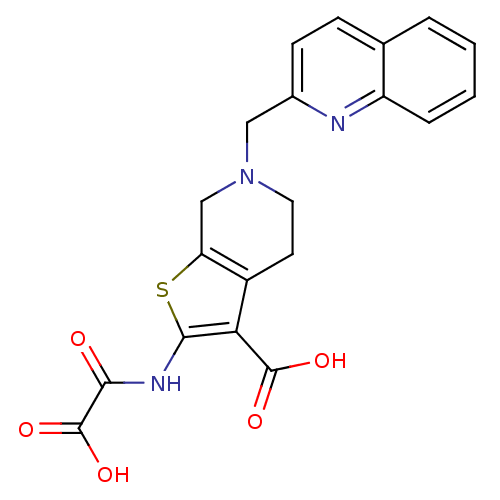
(2-(Oxalyl-amino)-6-quinolin-2-ylmethyl-4,5,6,7-tet...)Show SMILES OC(=O)C(=O)Nc1sc2CN(Cc3ccc4ccccc4n3)CCc2c1C(O)=O Show InChI InChI=1S/C20H17N3O5S/c24-17(20(27)28)22-18-16(19(25)26)13-7-8-23(10-15(13)29-18)9-12-6-5-11-3-1-2-4-14(11)21-12/h1-6H,7-10H2,(H,22,24)(H,25,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118771
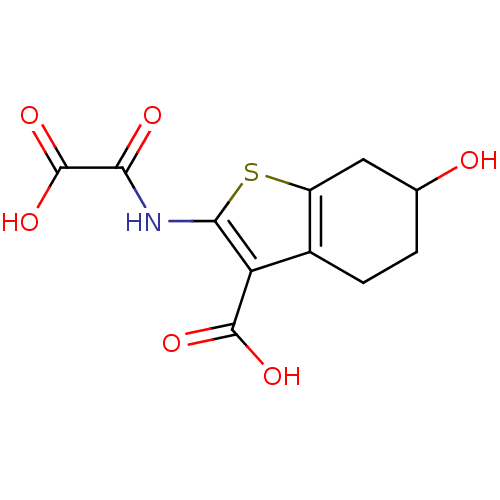
(2-(carboxyformamido)-6-hydroxy-4,5,6,7-tetrahydrob...)Show InChI InChI=1S/C11H11NO6S/c13-4-1-2-5-6(3-4)19-9(7(5)10(15)16)12-8(14)11(17)18/h4,13H,1-3H2,(H,12,14)(H,15,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118741
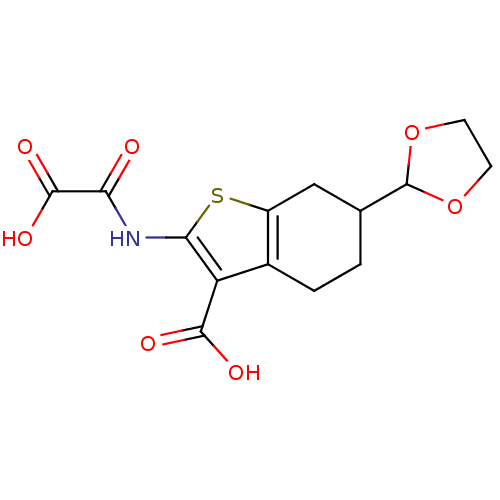
(6-[1,3]Dioxolan-2-yl-2-(oxalyl-amino)-4,5,6,7-tetr...)Show InChI InChI=1S/C14H15NO7S/c16-10(13(19)20)15-11-9(12(17)18)7-2-1-6(5-8(7)23-11)14-21-3-4-22-14/h6,14H,1-5H2,(H,15,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118765
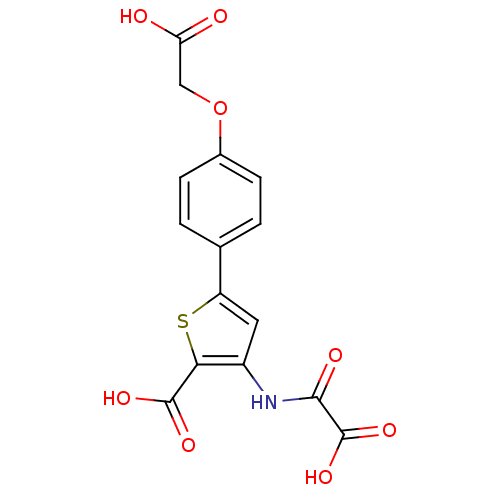
(3-(carboxyformamido)-5-(4-(carboxymethoxy)phenyl)t...)Show SMILES OC(=O)COc1ccc(cc1)-c1cc(NC(=O)C(O)=O)c(s1)C(O)=O Show InChI InChI=1S/C15H11NO8S/c17-11(18)6-24-8-3-1-7(2-4-8)10-5-9(12(25-10)14(20)21)16-13(19)15(22)23/h1-5H,6H2,(H,16,19)(H,17,18)(H,20,21)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118750

(2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C18H18N2O5S/c21-15(18(24)25)19-16-14(17(22)23)12-7-9-20(10-13(12)26-16)8-6-11-4-2-1-3-5-11/h1-5H,6-10H2,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118750

(2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C18H18N2O5S/c21-15(18(24)25)19-16-14(17(22)23)12-7-9-20(10-13(12)26-16)8-6-11-4-2-1-3-5-11/h1-5H,6-10H2,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118782
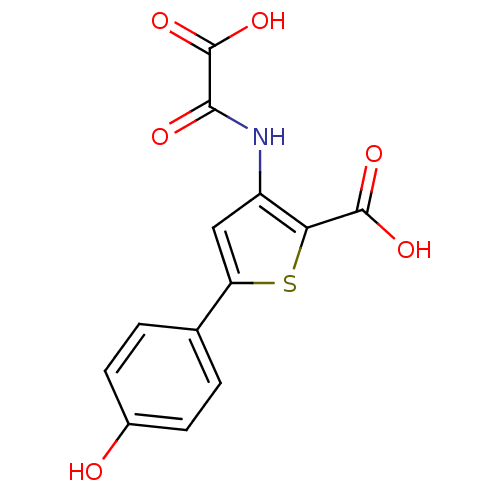
(3-(carboxyformamido)-5-(4-hydroxyphenyl)thiophene-...)Show InChI InChI=1S/C13H9NO6S/c15-7-3-1-6(2-4-7)9-5-8(10(21-9)12(17)18)14-11(16)13(19)20/h1-5,15H,(H,14,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118780
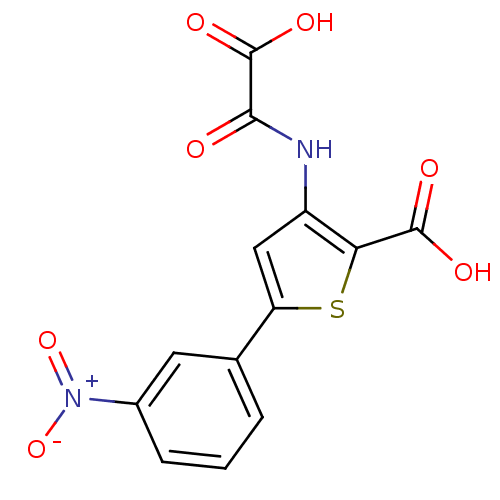
(3-(carboxyformamido)-5-(3-nitrophenyl)thiophene-2-...)Show SMILES OC(=O)C(=O)Nc1cc(sc1C(O)=O)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C13H8N2O7S/c16-11(13(19)20)14-8-5-9(23-10(8)12(17)18)6-2-1-3-7(4-6)15(21)22/h1-5H,(H,14,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118792

(2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...)Show InChI InChI=1S/C10H10N2O5S/c13-7(10(16)17)12-8-6(9(14)15)4-1-2-11-3-5(4)18-8/h11H,1-3H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118783

(2-(Oxalyl-amino)-6-oxo-4,5,6,7-tetrahydro-6lambda*...)Show InChI InChI=1S/C10H9NO6S2/c12-7(10(15)16)11-8-6(9(13)14)4-1-2-19(17)3-5(4)18-8/h1-3H2,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50118752

(6-(3,3-Diphenyl-propyl)-2-(oxalyl-amino)-4,5,6,7-t...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCC(c3ccccc3)c3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C25H24N2O5S/c28-22(25(31)32)26-23-21(24(29)30)19-12-14-27(15-20(19)33-23)13-11-18(16-7-3-1-4-8-16)17-9-5-2-6-10-17/h1-10,18H,11-15H2,(H,26,28)(H,29,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against T cell protein tyrosine phosphatase (TC-PTP) using p-nitrophenyl phosphate as substrate at pH 7.0 |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118752

(6-(3,3-Diphenyl-propyl)-2-(oxalyl-amino)-4,5,6,7-t...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCC(c3ccccc3)c3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C25H24N2O5S/c28-22(25(31)32)26-23-21(24(29)30)19-12-14-27(15-20(19)33-23)13-11-18(16-7-3-1-4-8-16)17-9-5-2-6-10-17/h1-10,18H,11-15H2,(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118758
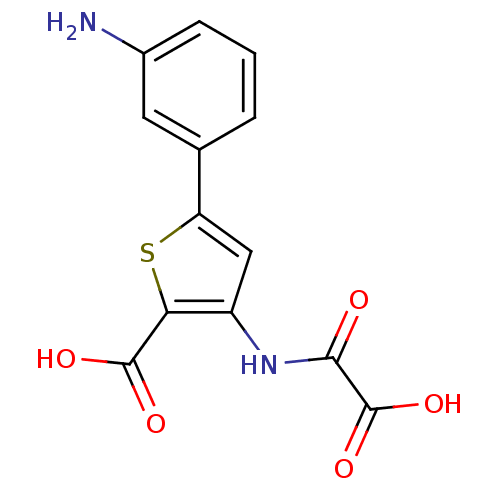
(5-(3-Amino-phenyl)-3-(oxalyl-amino)-thiophene-2-ca...)Show InChI InChI=1S/C13H10N2O5S/c14-7-3-1-2-6(4-7)9-5-8(10(21-9)12(17)18)15-11(16)13(19)20/h1-5H,14H2,(H,15,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118763

(2-(Oxalyl-amino)-6,6-dioxo-4,5,6,7-tetrahydro-6lam...)Show InChI InChI=1S/C10H9NO7S2/c12-7(10(15)16)11-8-6(9(13)14)4-1-2-20(17,18)3-5(4)19-8/h1-3H2,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118790

(2-(Oxalyl-amino)-6-(3-phenyl-propyl)-4,5,6,7-tetra...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCCc3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C19H20N2O5S/c22-16(19(25)26)20-17-15(18(23)24)13-8-10-21(11-14(13)27-17)9-4-7-12-5-2-1-3-6-12/h1-3,5-6H,4,7-11H2,(H,20,22)(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118754
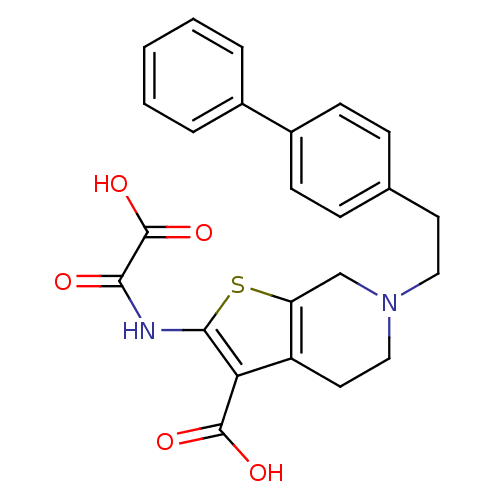
(6-(2-Biphenyl-4-yl-ethyl)-2-(oxalyl-amino)-4,5,6,7...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccc(cc3)-c3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C24H22N2O5S/c27-21(24(30)31)25-22-20(23(28)29)18-11-13-26(14-19(18)32-22)12-10-15-6-8-17(9-7-15)16-4-2-1-3-5-16/h1-9H,10-14H2,(H,25,27)(H,28,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118740

(3-(carboxyformamido)-5-(4-fluorophenyl)thiophene-2...)Show InChI InChI=1S/C13H8FNO5S/c14-7-3-1-6(2-4-7)9-5-8(10(21-9)12(17)18)15-11(16)13(19)20/h1-5H,(H,15,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118796

(6-(OXALYL-AMINO)-1H-INDOLE-5-CARBOXYLIC ACID | 6-(...)Show InChI InChI=1S/C11H8N2O5/c14-9(11(17)18)13-8-4-7-5(1-2-12-7)3-6(8)10(15)16/h1-4,12H,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118756

(3-(carboxyformamido)-5-(3,5-dimethoxyphenyl)thioph...)Show SMILES COc1cc(OC)cc(c1)-c1cc(NC(=O)C(O)=O)c(s1)C(O)=O Show InChI InChI=1S/C15H13NO7S/c1-22-8-3-7(4-9(5-8)23-2)11-6-10(12(24-11)14(18)19)16-13(17)15(20)21/h3-6H,1-2H3,(H,16,17)(H,18,19)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118762
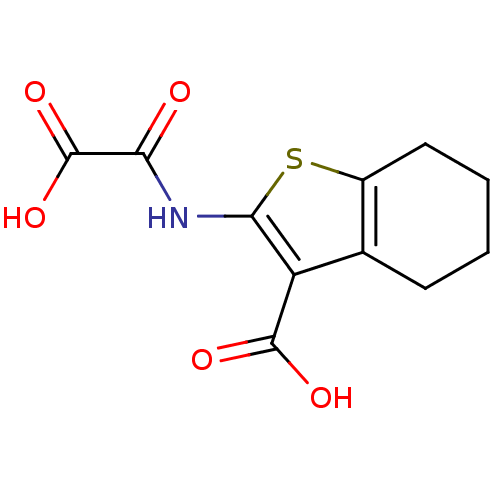
(2-(Oxalyl-amino)-4,5,6,7-tetrahydro-benzo[b]thioph...)Show InChI InChI=1S/C11H11NO5S/c13-8(11(16)17)12-9-7(10(14)15)5-3-1-2-4-6(5)18-9/h1-4H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118787
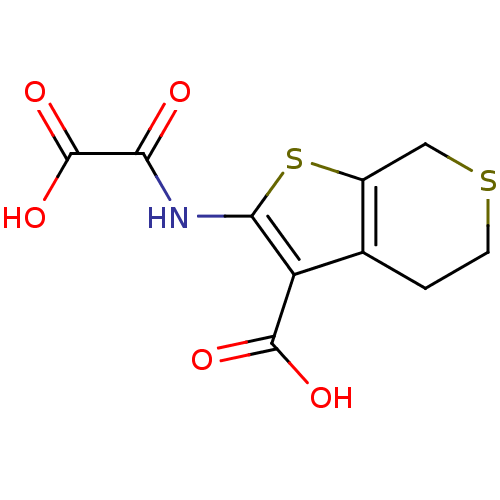
(2-(Oxalyl-amino)-4,7-dihydro-5H-thieno[2,3-c]thiop...)Show InChI InChI=1S/C10H9NO5S2/c12-7(10(15)16)11-8-6(9(13)14)4-1-2-17-3-5(4)18-8/h1-3H2,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118786

(6-(3-Methoxy-benzyl)-2-(oxalyl-amino)-4,5,6,7-tetr...)Show SMILES COc1cccc(CN2CCc3c(C2)sc(NC(=O)C(O)=O)c3C(O)=O)c1 Show InChI InChI=1S/C18H18N2O6S/c1-26-11-4-2-3-10(7-11)8-20-6-5-12-13(9-20)27-16(14(12)17(22)23)19-15(21)18(24)25/h2-4,7H,5-6,8-9H2,1H3,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118748
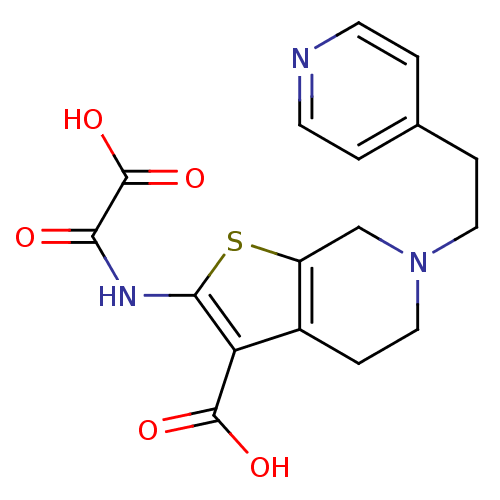
(2-(Oxalyl-amino)-6-(2-pyridin-4-yl-ethyl)-4,5,6,7-...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccncc3)CCc2c1C(O)=O Show InChI InChI=1S/C17H17N3O5S/c21-14(17(24)25)19-15-13(16(22)23)11-4-8-20(9-12(11)26-15)7-3-10-1-5-18-6-2-10/h1-2,5-6H,3-4,7-9H2,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118770

(2-(Oxalyl-amino)-6-(2-thiophen-3-yl-ethyl)-4,5,6,7...)Show InChI InChI=1S/C16H16N2O5S2/c19-13(16(22)23)17-14-12(15(20)21)10-2-5-18(7-11(10)25-14)4-1-9-3-6-24-8-9/h3,6,8H,1-2,4-5,7H2,(H,17,19)(H,20,21)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118759
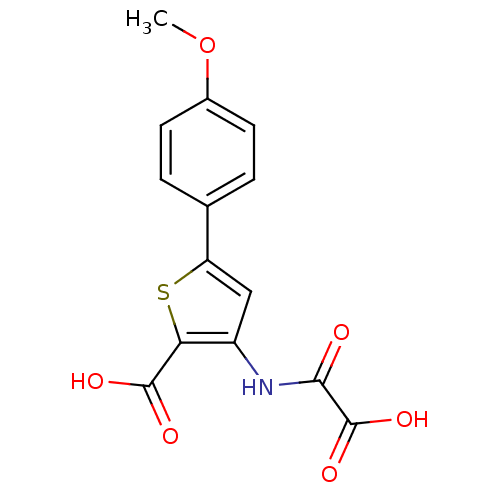
(3-(carboxyformamido)-5-(4-methoxyphenyl)thiophene-...)Show InChI InChI=1S/C14H11NO6S/c1-21-8-4-2-7(3-5-8)10-6-9(11(22-10)13(17)18)15-12(16)14(19)20/h2-6H,1H3,(H,15,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118792

(2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...)Show InChI InChI=1S/C10H10N2O5S/c13-7(10(16)17)12-8-6(9(14)15)4-1-2-11-3-5(4)18-8/h11H,1-3H2,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0 |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118784

(6-[2-(4-Benzyloxy-phenyl)-ethyl]-2-(oxalyl-amino)-...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccc(OCc4ccccc4)cc3)CCc2c1C(O)=O Show InChI InChI=1S/C25H24N2O6S/c28-22(25(31)32)26-23-21(24(29)30)19-11-13-27(14-20(19)34-23)12-10-16-6-8-18(9-7-16)33-15-17-4-2-1-3-5-17/h1-9H,10-15H2,(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0 |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118760
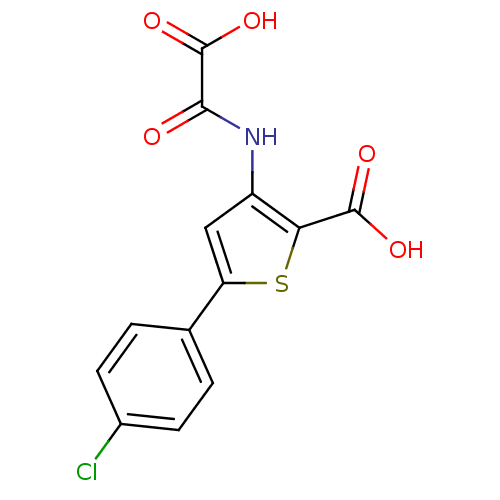
(3-(carboxyformamido)-5-(4-chlorophenyl)thiophene-2...)Show InChI InChI=1S/C13H8ClNO5S/c14-7-3-1-6(2-4-7)9-5-8(10(21-9)12(17)18)15-11(16)13(19)20/h1-5H,(H,15,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118793

(6-Methyl-2-(oxalyl-amino)-4,5,6,7-tetrahydro-thien...)Show InChI InChI=1S/C11H12N2O5S/c1-13-3-2-5-6(4-13)19-9(7(5)10(15)16)12-8(14)11(17)18/h2-4H2,1H3,(H,12,14)(H,15,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118761
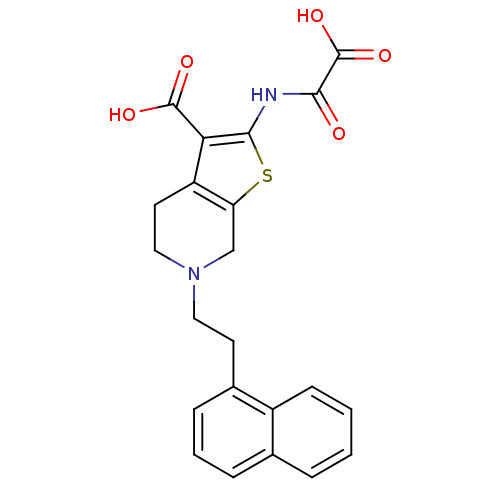
(6-(2-Naphthalen-1-yl-ethyl)-2-(oxalyl-amino)-4,5,6...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3cccc4ccccc34)CCc2c1C(O)=O Show InChI InChI=1S/C22H20N2O5S/c25-19(22(28)29)23-20-18(21(26)27)16-9-11-24(12-17(16)30-20)10-8-14-6-3-5-13-4-1-2-7-15(13)14/h1-7H,8-12H2,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118761

(6-(2-Naphthalen-1-yl-ethyl)-2-(oxalyl-amino)-4,5,6...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3cccc4ccccc34)CCc2c1C(O)=O Show InChI InChI=1S/C22H20N2O5S/c25-19(22(28)29)23-20-18(21(26)27)16-9-11-24(12-17(16)30-20)10-8-14-6-3-5-13-4-1-2-7-15(13)14/h1-7H,8-12H2,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0 |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118764
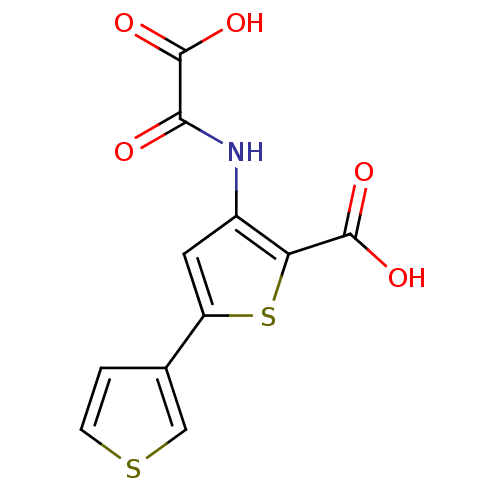
(3-(carboxyformamido)-5-(thiophen-3-yl)thiophene-2-...)Show InChI InChI=1S/C11H7NO5S2/c13-9(11(16)17)12-6-3-7(5-1-2-18-4-5)19-8(6)10(14)15/h1-4H,(H,12,13)(H,14,15)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118770

(2-(Oxalyl-amino)-6-(2-thiophen-3-yl-ethyl)-4,5,6,7...)Show InChI InChI=1S/C16H16N2O5S2/c19-13(16(22)23)17-14-12(15(20)21)10-2-5-18(7-11(10)25-14)4-1-9-3-6-24-8-9/h3,6,8H,1-2,4-5,7H2,(H,17,19)(H,20,21)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0 |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118788
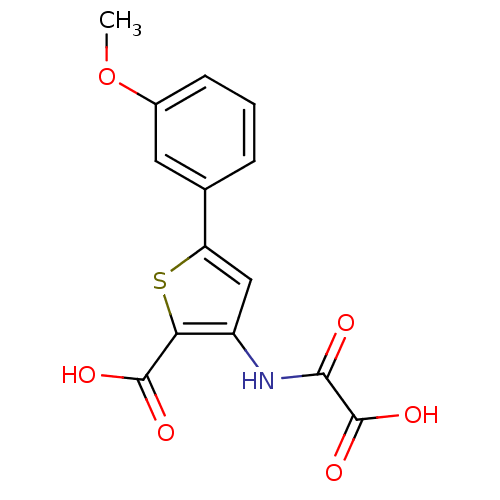
(3-(carboxyformamido)-5-(3-methoxyphenyl)thiophene-...)Show InChI InChI=1S/C14H11NO6S/c1-21-8-4-2-3-7(5-8)10-6-9(11(22-10)13(17)18)15-12(16)14(19)20/h2-6H,1H3,(H,15,16)(H,17,18)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118791

(2-(Oxalyl-amino)-6-(2-pyridin-2-yl-ethyl)-4,5,6,7-...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccccn3)CCc2c1C(O)=O Show InChI InChI=1S/C17H17N3O5S/c21-14(17(24)25)19-15-13(16(22)23)11-5-8-20(9-12(11)26-15)7-4-10-3-1-2-6-18-10/h1-3,6H,4-5,7-9H2,(H,19,21)(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118785
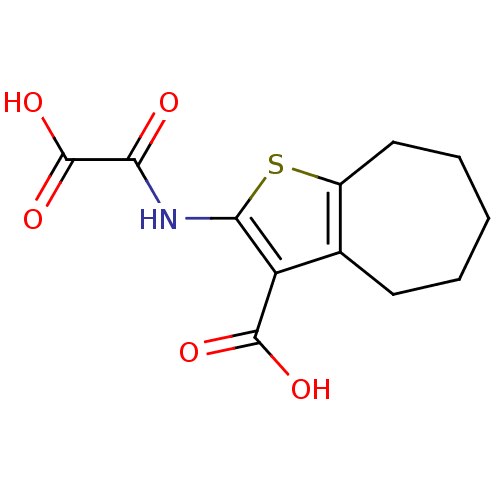
(2-(Oxalyl-amino)-5,6,7,8-tetrahydro-4H-cyclohepta[...)Show InChI InChI=1S/C12H13NO5S/c14-9(12(17)18)13-10-8(11(15)16)6-4-2-1-3-5-7(6)19-10/h1-5H2,(H,13,14)(H,15,16)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118754

(6-(2-Biphenyl-4-yl-ethyl)-2-(oxalyl-amino)-4,5,6,7...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCc3ccc(cc3)-c3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C24H22N2O5S/c27-21(24(30)31)25-22-20(23(28)29)18-11-13-26(14-19(18)32-22)12-10-15-6-8-17(9-7-15)16-4-2-1-3-5-16/h1-9H,10-14H2,(H,25,27)(H,28,29)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0 |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118790

(2-(Oxalyl-amino)-6-(3-phenyl-propyl)-4,5,6,7-tetra...)Show SMILES OC(=O)C(=O)Nc1sc2CN(CCCc3ccccc3)CCc2c1C(O)=O Show InChI InChI=1S/C19H20N2O5S/c22-16(19(25)26)20-17-15(18(23)24)13-8-10-21(11-14(13)27-17)9-4-7-12-5-2-1-3-6-12/h1-3,5-6H,4,7-11H2,(H,20,22)(H,23,24)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of T cell protein tyrosine phosphatase (TC-PTP) at pH 7.0 |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118794

(2-(Oxalyl-amino)-6-pyridin-3-ylmethyl-4,5,6,7-tetr...)Show InChI InChI=1S/C16H15N3O5S/c20-13(16(23)24)18-14-12(15(21)22)10-3-5-19(8-11(10)25-14)7-9-2-1-4-17-6-9/h1-2,4,6H,3,5,7-8H2,(H,18,20)(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118753
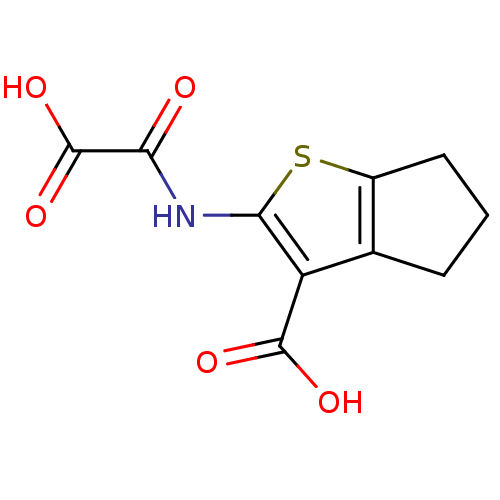
(2-(Oxalyl-amino)-5,6-dihydro-4H-cyclopenta[b]thiop...)Show InChI InChI=1S/C10H9NO5S/c12-7(10(15)16)11-8-6(9(13)14)4-2-1-3-5(4)17-8/h1-3H2,(H,11,12)(H,13,14)(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50118755

(2-(Oxalyl-amino)-6-pyridin-4-ylmethyl-4,5,6,7-tetr...)Show InChI InChI=1S/C16H15N3O5S/c20-13(16(23)24)18-14-12(15(21)22)10-3-6-19(8-11(10)25-14)7-9-1-4-17-5-2-9/h1-2,4-5H,3,6-8H2,(H,18,20)(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Protein-tyrosine phosphatase 1B. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data