

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50089636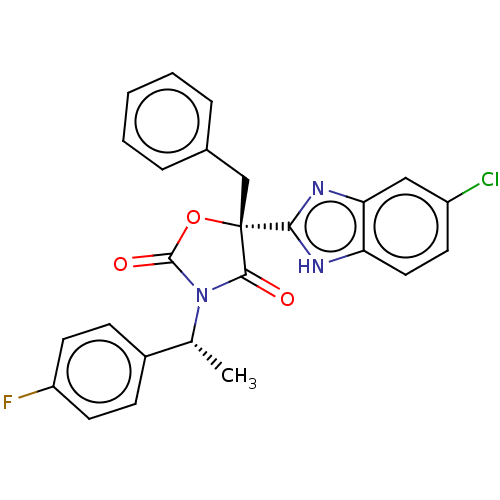 (CHEMBL3578271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to mineralocorticoid receptor (unknown origin) | ACS Med Chem Lett 6: 461-5 (2015) Article DOI: 10.1021/acsmedchemlett.5b00010 BindingDB Entry DOI: 10.7270/Q21J9CH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476445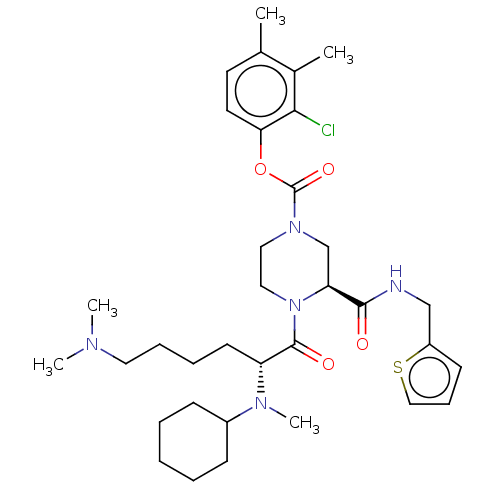 (2-chloro-3,4-dimethylphenyl (3S)-4- (N2-cyclohexyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170939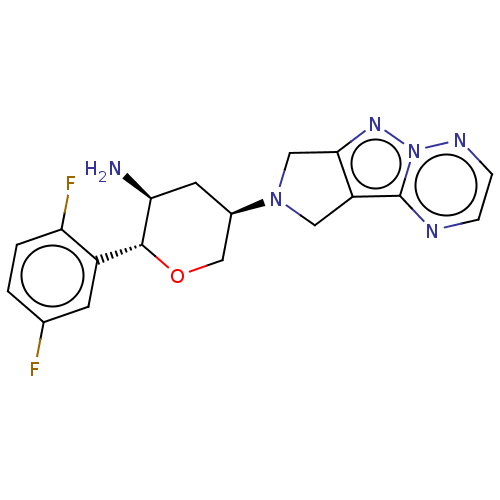 (CHEMBL3806052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170919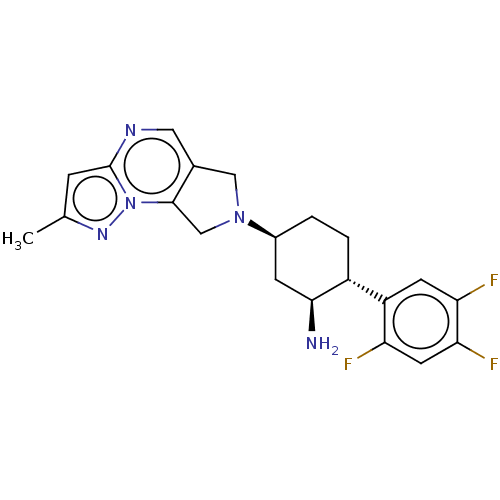 (CHEMBL3805966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476448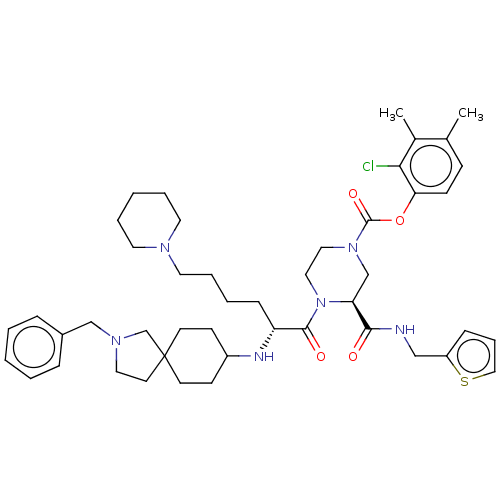 (2-chloro-3,4-dimethylphenyl (3S)-4- [N-(2-benzyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170922 (CHEMBL3805629) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170923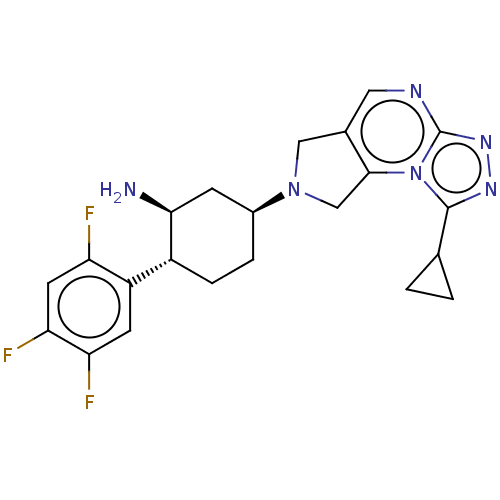 (CHEMBL3806041) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476454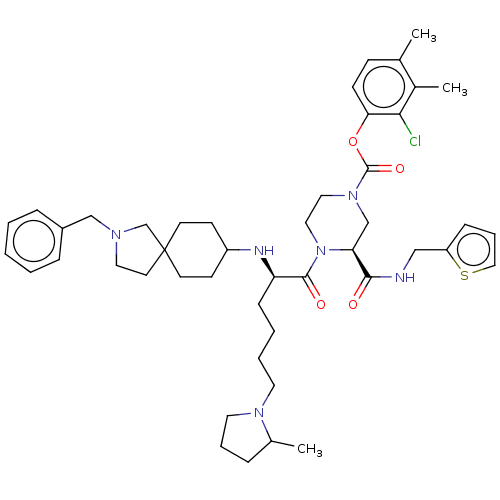 (2-chloro-3,4-dimethylphenyl (3S)-4- [N-(2-benzyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476452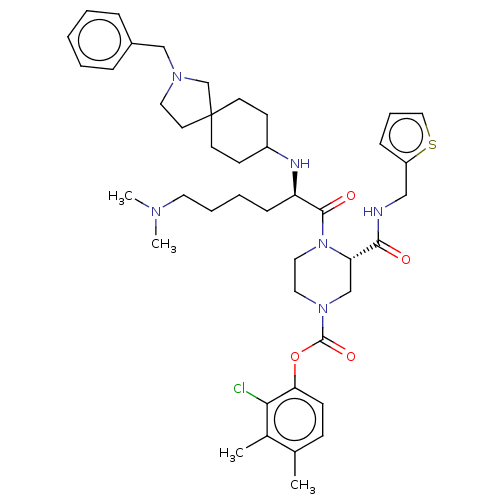 (2-chloro-3,4-dimethylphenyl (3S)-4- [N2-(2-benzyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM120806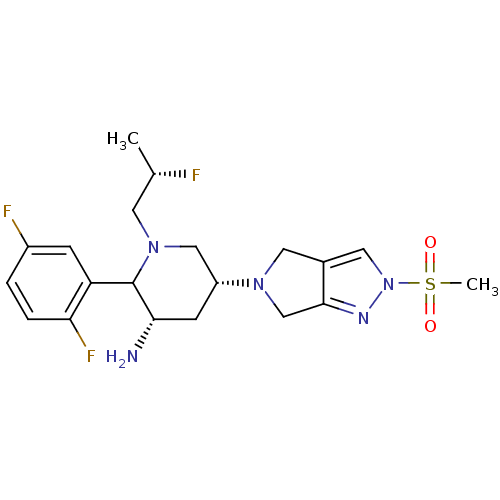 (US8716482, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8716482 (2014) BindingDB Entry DOI: 10.7270/Q2G73CC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476486 ((2S)-1-[N2-(2-benzyl-2- azaspiro[4.5]dec-8-yl)-N6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476515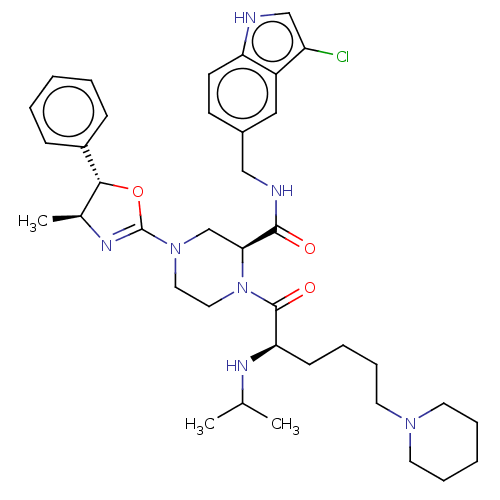 ((2S)-N-[(3-chloro-1H-indol-5- yl)methyl]-1-[N-(1-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170953 (CHEMBL3805154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170929 (CHEMBL3805751) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476444 (2-chloro-3,4-dimethylphenyl (3S)-4- [N2-(2-benzyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476449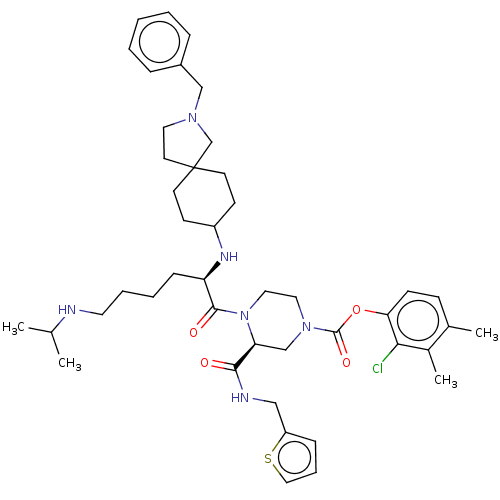 (2-chloro-3,4-dimethylphenyl (3S)-4- [N2-(2-benzyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170921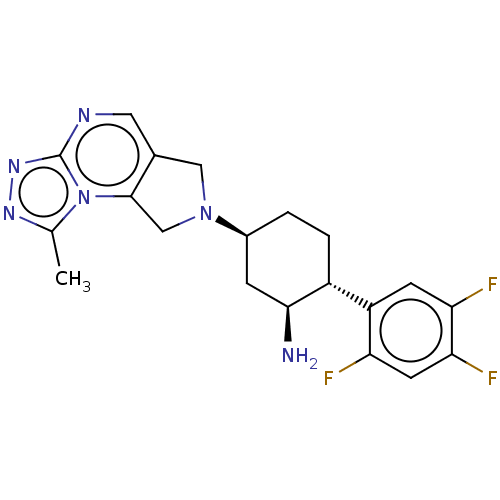 (CHEMBL3806023) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170952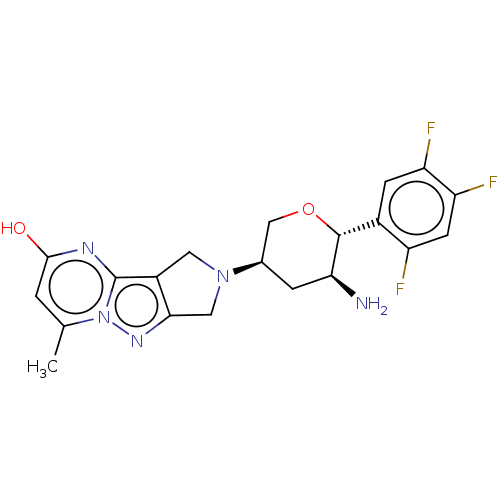 (CHEMBL3806310) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476516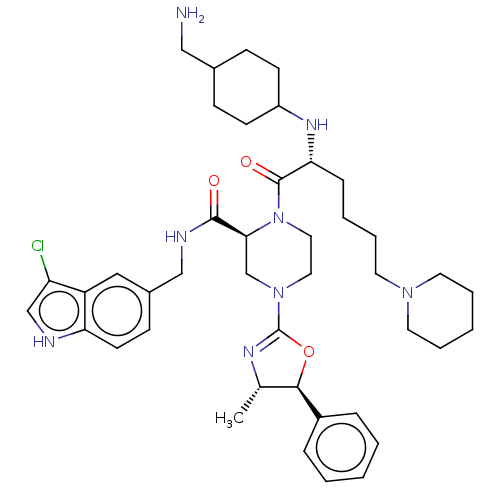 ((2S)-1-{N-[4- (aminomethyl)cyclohexyl]-6- piperidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170957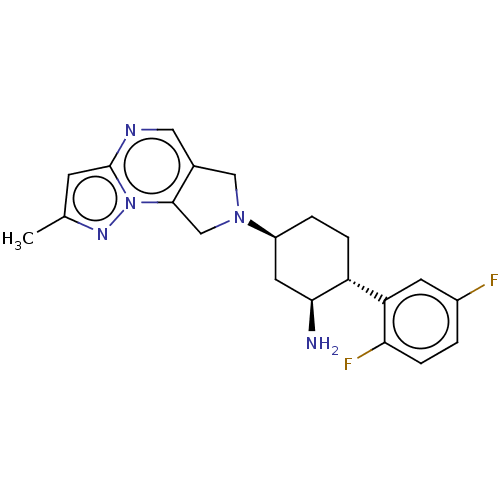 (CHEMBL3806157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170934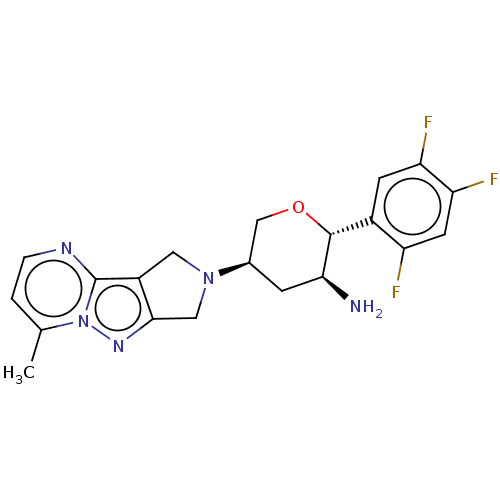 (CHEMBL3806021) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170927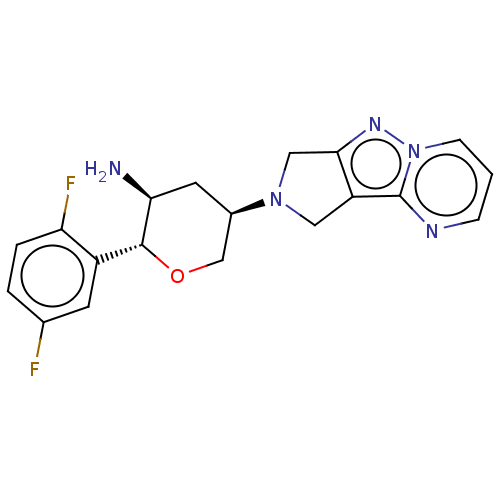 (CHEMBL3806216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476434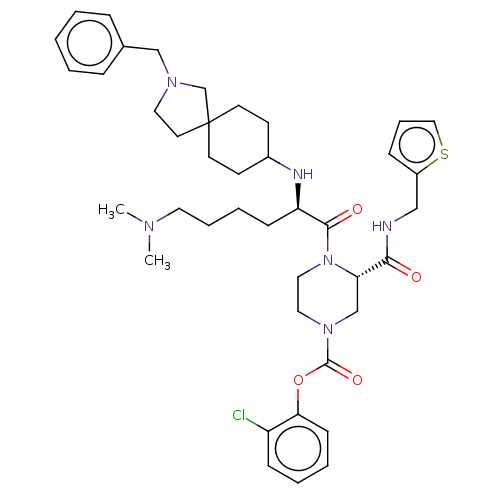 (2-chlorophenyl (3S)-4-[N2-(2-benzyl- 2-azaspiro[4....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM120799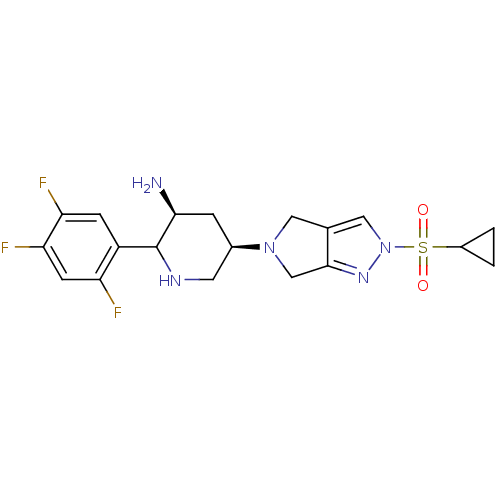 (US8716482, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8716482 (2014) BindingDB Entry DOI: 10.7270/Q2G73CC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170956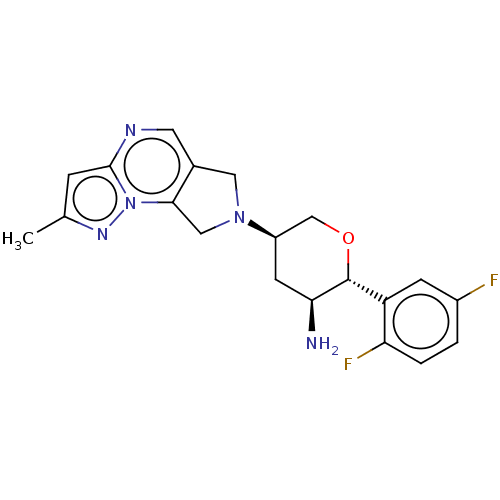 (CHEMBL3805294) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170920 (CHEMBL3806029) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476446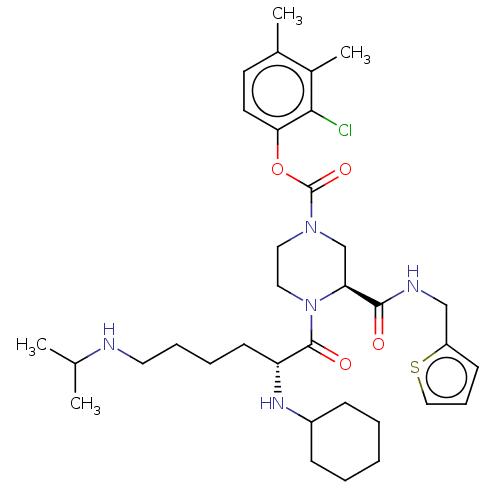 (2-chloro-3,4-dimethylphenyl (3S)-4- [N2-cyclohexyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476441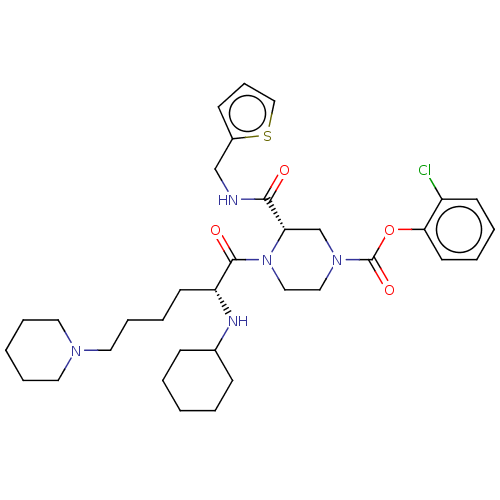 (2-chlorophenyl (3S)-4-(N-cyclohexyl- 6-piperidin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476479 ((2S)-1-[N2-(2-benzyl-2- azaspiro[4.5]dec-8-yl)-N6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM120802 (US8716482, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8716482 (2014) BindingDB Entry DOI: 10.7270/Q2G73CC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476422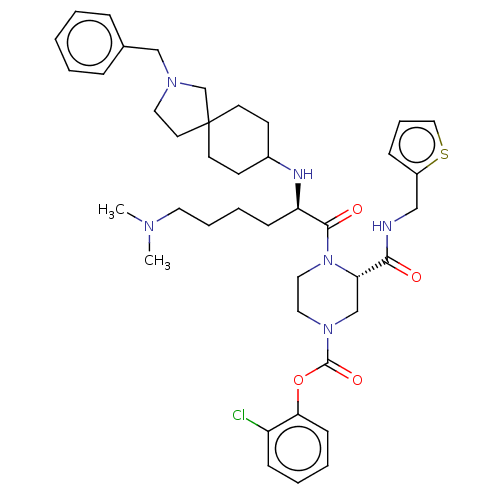 (2-chlorophenyl (3S)-4-[N2-(2-benzyl- 2-azaspiro[4....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476432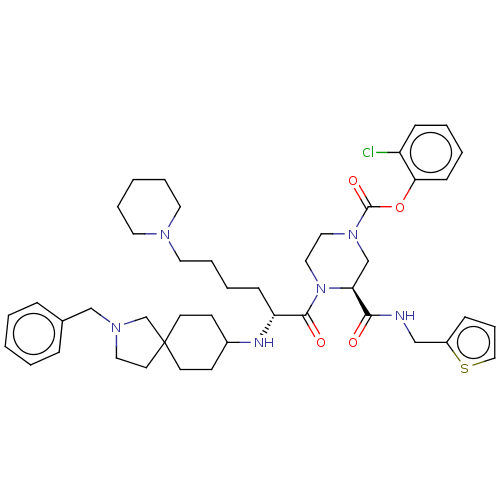 (2-chlorophenyl (3S)-4-[N-(2-benzyl- 2-azaspiro[4.5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476453 (2-chloro-3,4-dimethylphenyl (3S)-4- [N-(2-benzyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476431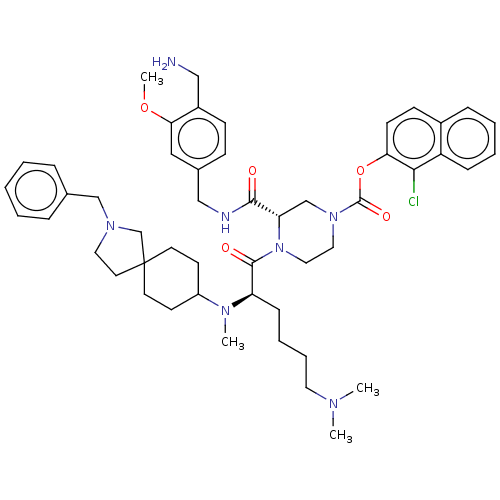 (1-chloronaphthalen-2-yl (3S)-3-{[4- (aminomethyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM120801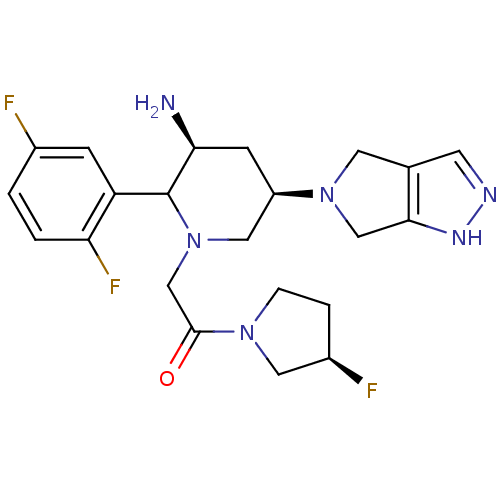 (US8716482, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description A continuous fluorometric assay is employed with the substrate Gly-Pro-AMC, which is cleaved by DPP-4 to release the fluorescent AMC leaving group. T... | US Patent US8716482 (2014) BindingDB Entry DOI: 10.7270/Q2G73CC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170959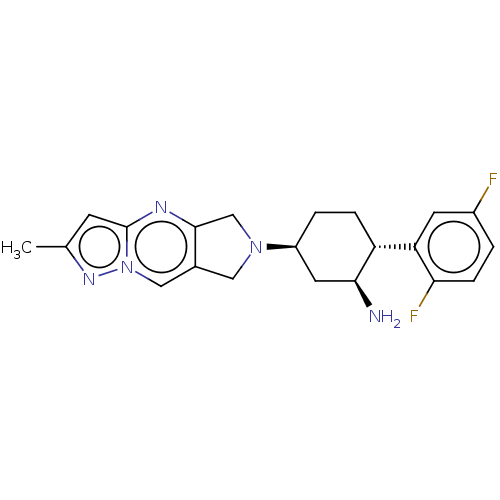 (CHEMBL3805226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170931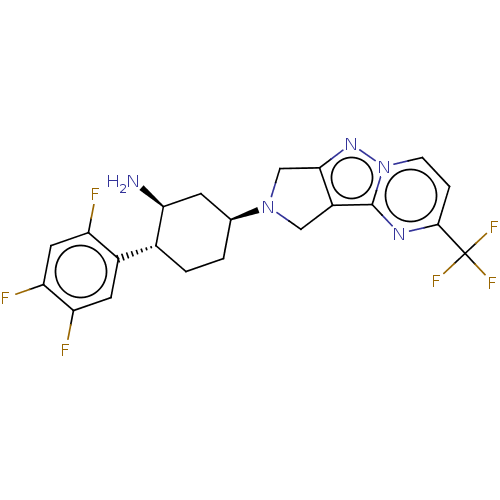 (CHEMBL3805871) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476399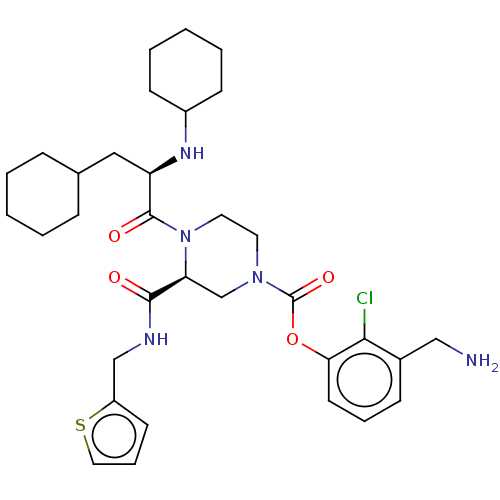 (3-(aminomethyl)-2-chlorophenyl (3S)- 4-(N,3-dicycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170936 (CHEMBL3805179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476417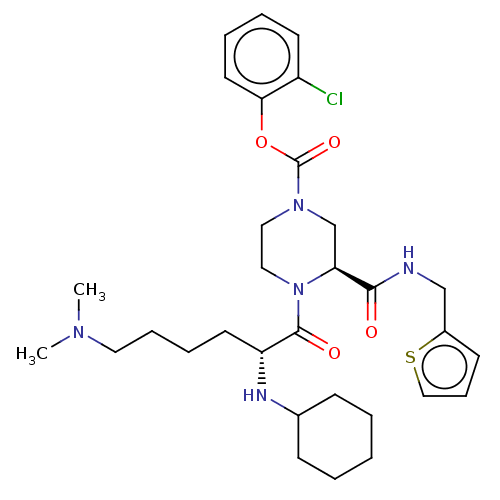 (2-chlorophenyl (3S)-4-(N2- cyclohexyl-N6,N6-dimeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476423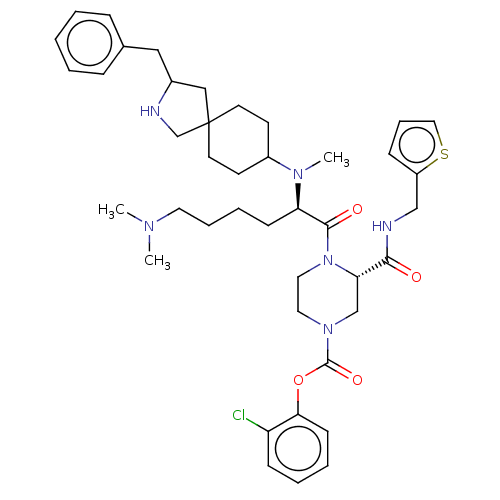 (2-chlorophenyl (3S)-4-[N2-(2-benzyl- 2-azaspiro[4....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476474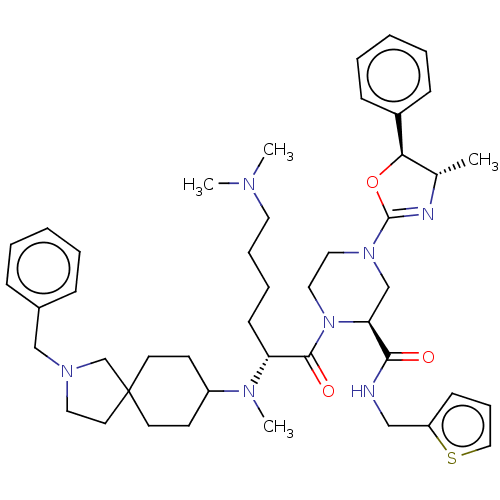 ((2S)-1-[N2-(2-benzyl-2- azaspiro[4.5]dec-8-yl)-N6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170955 (CHEMBL3805937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476447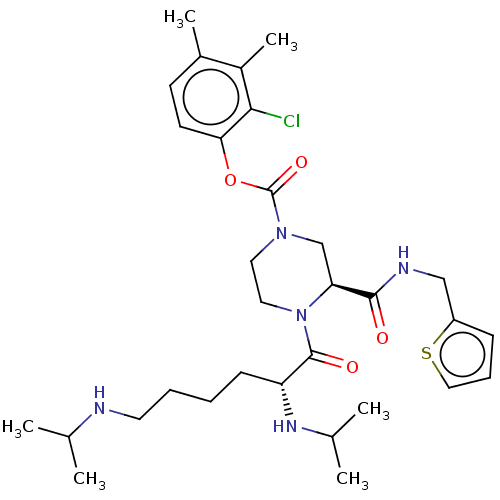 (2-chloro-3,4-dimethylphenyl (3S)-4- [N2,N6-bis(1-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476482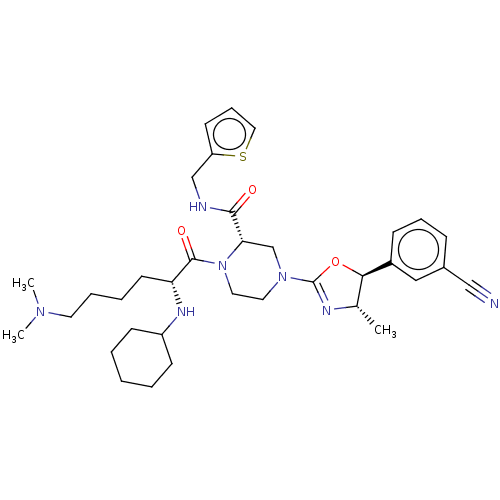 ((2S)-4[(4S,5S)-5-(3-cyanophenyl)-4- methyl-4,5-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM498923 (N-{(3S,4R)-1-[N~2~- (2-benzyl-2- azaspiro[4.5]dec-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIIa activity determinations were made in 50 mM HEPES buffer containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US11014920 (2021) BindingDB Entry DOI: 10.7270/Q2QF8X0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476463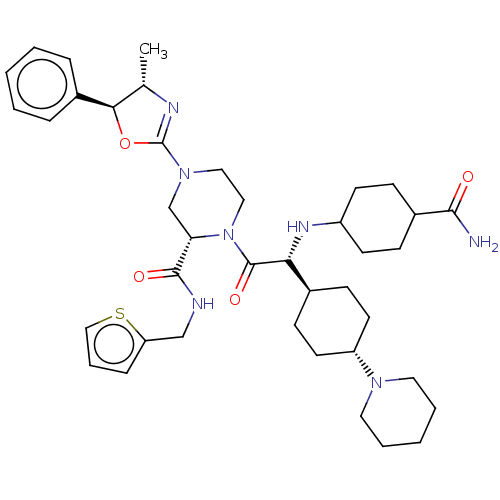 ((2S)-1-[(2R)-2-[(4- carbamoylcyclohexyl)amino]-2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170930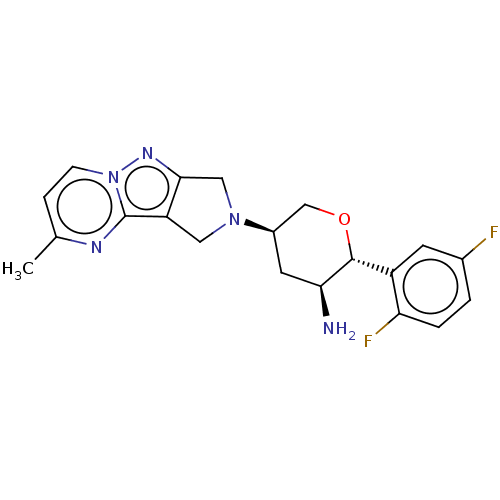 (CHEMBL3805400) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50170932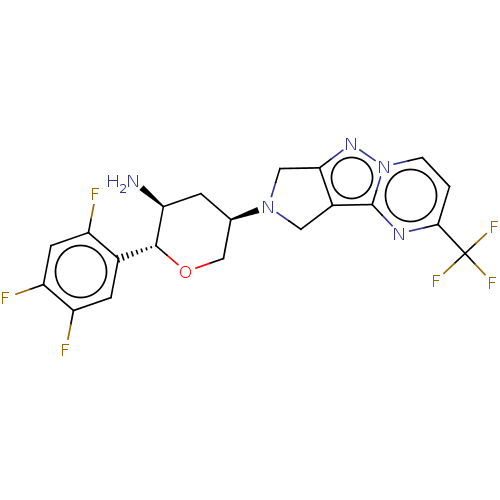 (CHEMBL3805502) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 26: 2622-6 (2016) Article DOI: 10.1016/j.bmcl.2016.04.020 BindingDB Entry DOI: 10.7270/Q2Z321JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476368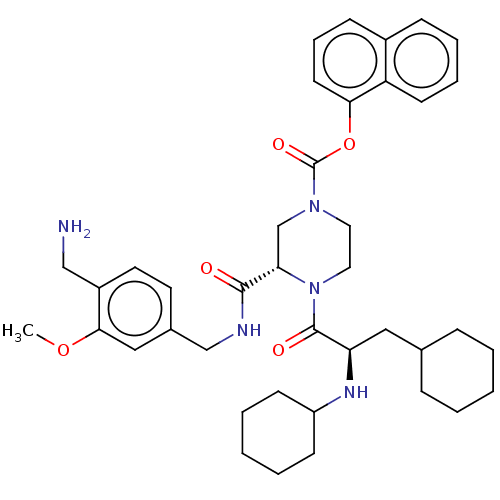 (US10875851, Example 24 | naphthalen-1-yl (3S)-3-{[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 825 total ) | Next | Last >> |