 Found 7594 hits with Last Name = 'chu' and Initial = 's'
Found 7594 hits with Last Name = 'chu' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM533367
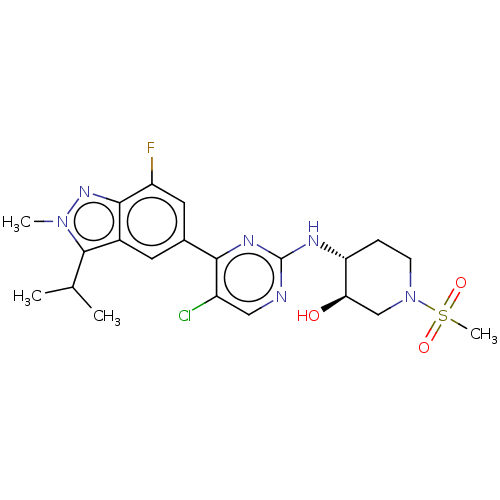
(US11220494, Example A25)Show SMILES CC(C)c1n(C)nc2c(F)cc(cc12)-c1nc(N[C@@H]2CCN(C[C@H]2O)S(C)(=O)=O)ncc1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The purpose CDK4/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitors by us... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XG9V9M |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM462197
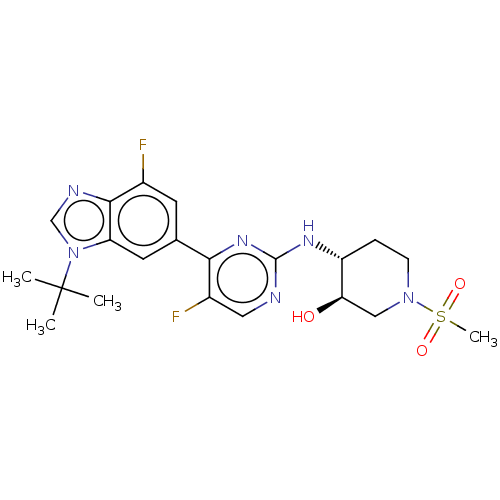
(US10766884, Example F15 | US11220494, Example F15)Show SMILES CC(C)(C)n1cnc2c(F)cc(cc12)-c1nc(N[C@@H]2CCN(C[C@H]2O)S(C)(=O)=O)ncc1F |r| Show InChI InChI=1S/C21H26F2N6O3S/c1-21(2,3)29-11-25-19-13(22)7-12(8-16(19)29)18-14(23)9-24-20(27-18)26-15-5-6-28(10-17(15)30)33(4,31)32/h7-9,11,15,17,30H,5-6,10H2,1-4H3,(H,24,26,27)/t15-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The purpose CDK4/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule inhibitors by us... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2XG9V9M |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50498230

(CHEMBL3577576)Show SMILES [H][C@]12OC[C@H](OC)[C@@]1([H])[C@H](CO2)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C29H40N2O9S/c1-19(2)15-31(41(34,35)22-12-10-21(36-3)11-13-22)16-24(32)23(14-20-8-6-5-7-9-20)30-29(33)40-26-18-39-28-27(26)25(37-4)17-38-28/h5-13,19,23-28,32H,14-18H2,1-4H3,(H,30,33)/t23-,24+,25-,26-,27-,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease |
J Med Chem 58: 5088-95 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00474
BindingDB Entry DOI: 10.7270/Q27D2Z42 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease |
J Med Chem 58: 5088-95 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00474
BindingDB Entry DOI: 10.7270/Q27D2Z42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50498229

(CHEMBL3577575)Show SMILES [H][C@@]12OC[C@H](OC(=O)N[C@@H](Cc3ccccc3)[C@H](O)CN(CC(C)C)S(=O)(=O)c3ccc(N)cc3)[C@]1([H])C(F)(F)CO2 |r| Show InChI InChI=1S/C27H35F2N3O7S/c1-17(2)13-32(40(35,36)20-10-8-19(30)9-11-20)14-22(33)21(12-18-6-4-3-5-7-18)31-26(34)39-23-15-37-25-24(23)27(28,29)16-38-25/h3-11,17,21-25,33H,12-16,30H2,1-2H3,(H,31,34)/t21-,22+,23-,24-,25-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease |
J Med Chem 58: 5088-95 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00474
BindingDB Entry DOI: 10.7270/Q27D2Z42 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110577
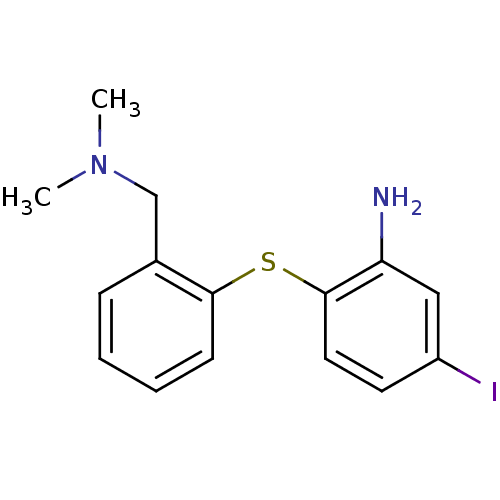
(2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...)Show InChI InChI=1S/C15H17IN2S/c1-18(2)10-11-5-3-4-6-14(11)19-15-8-7-12(16)9-13(15)17/h3-9H,10,17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease by fluorometric assay |
Bioorg Med Chem Lett 25: 4903-4909 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.052
BindingDB Entry DOI: 10.7270/Q2P84FW4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 55: 3387-97 (2012)
Article DOI: 10.1021/jm300072d
BindingDB Entry DOI: 10.7270/Q2T43WZW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50040079

(CHEMBL308524 | N-(1-Ethyl-pyrrolidin-2-ylmethyl)-2...)Show InChI InChI=1S/C16H23IN2O4/c1-4-19-7-5-6-10(19)9-18-16(21)13-14(20)11(17)8-12(22-2)15(13)23-3/h8,10,20H,4-7,9H2,1-3H3,(H,18,21)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition constant against dopamine receptor D2 in rat |
J Med Chem 36: 221-8 (1993)
BindingDB Entry DOI: 10.7270/Q2TT4Q10 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008785
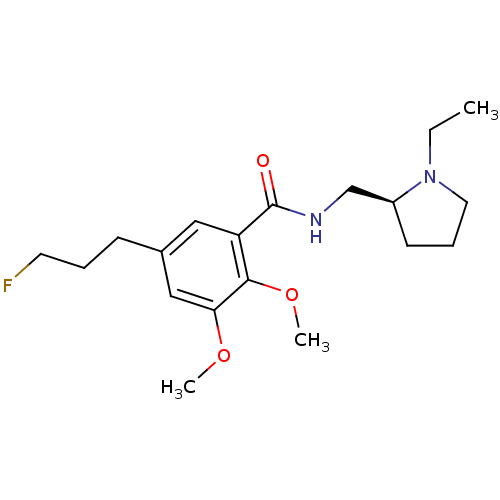
(CHEMBL42953 | N-(1-Ethyl-pyrrolidin-2-ylmethyl)-5-...)Show InChI InChI=1S/C19H29FN2O3/c1-4-22-10-6-8-15(22)13-21-19(23)16-11-14(7-5-9-20)12-17(24-2)18(16)25-3/h11-12,15H,4-10,13H2,1-3H3,(H,21,23)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition constant against dopamine receptor D2 in rat |
J Med Chem 36: 221-8 (1993)
BindingDB Entry DOI: 10.7270/Q2TT4Q10 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008782
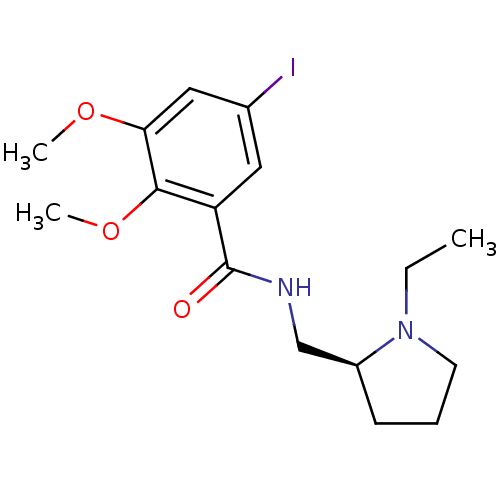
(CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...)Show InChI InChI=1S/C16H23IN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition constant against dopamine receptor D2 in rat |
J Med Chem 36: 221-8 (1993)
BindingDB Entry DOI: 10.7270/Q2TT4Q10 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50341508
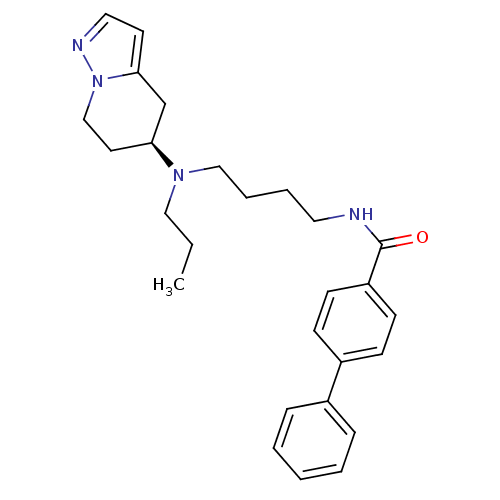
((S)-N-(4-(4-Phenylbenzoylamino)butyl)-N-propyl-5-a...)Show SMILES CCCN(CCCCNC(=O)c1ccc(cc1)-c1ccccc1)[C@H]1CCn2nccc2C1 |r| Show InChI InChI=1S/C27H34N4O/c1-2-18-30(25-15-20-31-26(21-25)14-17-29-31)19-7-6-16-28-27(32)24-12-10-23(11-13-24)22-8-4-3-5-9-22/h3-5,8-14,17,25H,2,6-7,15-16,18-21H2,1H3,(H,28,32)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University
Curated by ChEMBL
| Assay Description
Displacement of [3H]7-OH-DPAT from human dopamine D3 receptor expressed in CHO cells after 60 mins |
J Med Chem 54: 2477-91 (2011)
Article DOI: 10.1021/jm101639t
BindingDB Entry DOI: 10.7270/Q2V1253W |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110578
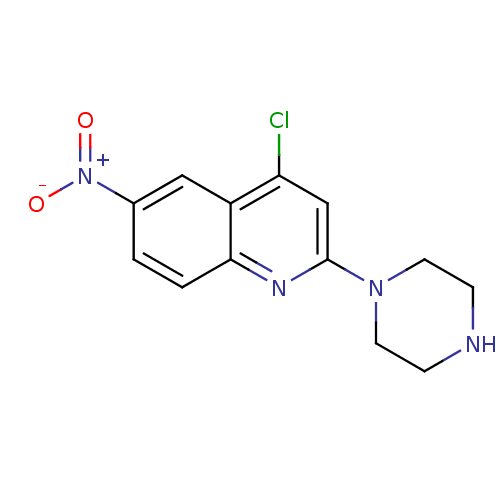
(4-Chloro-6-nitro-2-piperazin-1-yl-quinoline | CHEM...)Show InChI InChI=1S/C13H13ClN4O2/c14-11-8-13(17-5-3-15-4-6-17)16-12-2-1-9(18(19)20)7-10(11)12/h1-2,7-8,15H,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31822
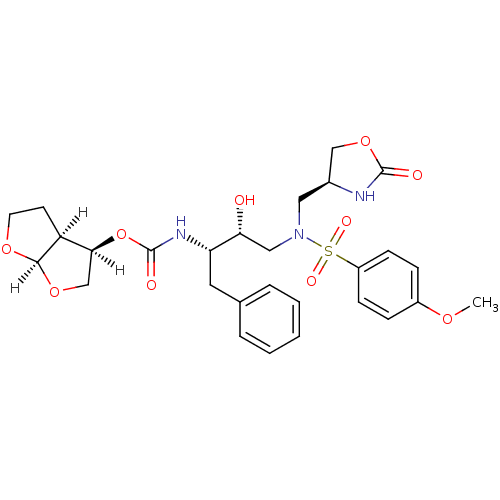
(oxazolidinone, 31)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1COC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C28H35N3O10S/c1-37-20-7-9-21(10-8-20)42(35,36)31(14-19-16-40-27(33)29-19)15-24(32)23(13-18-5-3-2-4-6-18)30-28(34)41-25-17-39-26-22(25)11-12-38-26/h2-10,19,22-26,32H,11-17H2,1H3,(H,29,33)(H,30,34)/t19-,22-,23-,24+,25-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0350 | -59.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50366620

(RESINIFERATOXIN)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606126
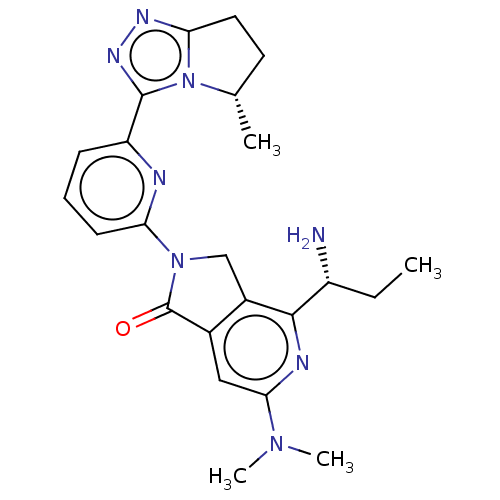
(4-[(1R)-1- aminopropyl]- 6-(dimethyl- amino)-2-{6-...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606125

(4-[(1$#958;)-1- aminoethyl]-2- {6-[(5$#958;)-5- me...)Show SMILES C[C@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](C)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606205

(2-{6-[(5S,7S)- 5,7-dimethyl-6,7- dihydro-5H- pyrro...)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2[C@@H](C)C[C@H](C)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606129

(4-[(1R)-1- aminopropyl]- 2-{6-[(5R)-5- (hydroxy- m...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606115

(4-(2- aminopropan- 2-yl)-2-{6- [(5S)-5-methyl- 6,7...)Show SMILES C[C@@H]1CCCN1c1cc2C(=O)N(Cc2c(n1)C(C)(C)N)c1cccc(n1)-c1nnc2CC[C@H](C)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606114

(4-[(1S)-1- aminopropyl]- 2-{6-[(5$#958;)-5- ethyl-...)Show SMILES CC[C@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](CC)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606118

(4-[(1R)-1- aminopropyl]- 2-{6-[(5R)-5- (hydroxy- m...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606123

(4-[(1$#958;)-1- aminoethyl]-2- {6-[(5$#958;)-5- me...)Show SMILES C[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](C)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606098
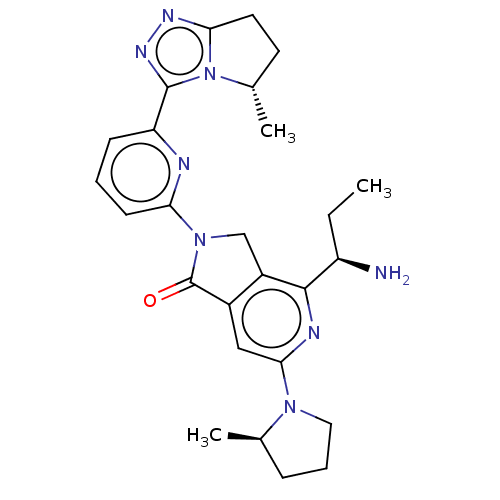
(US11684616, Example 1)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606099

(US11684616, Example 2)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4[C@@H](C)N)N4CCC[C@H]4C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606100
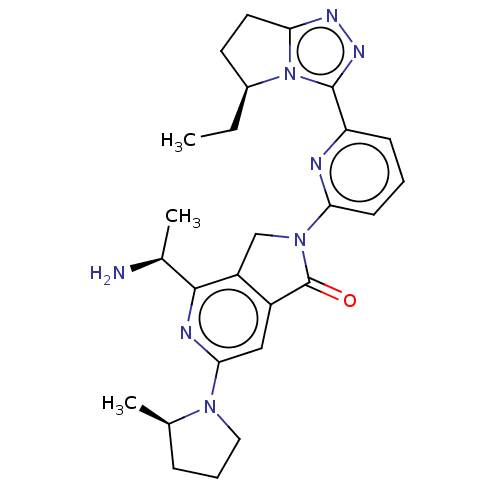
(US11684616, Example 3)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4[C@H](C)N)N4CCC[C@H]4C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606101

(US11684616, Example 4)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4[C@@H](C)N)N(C)C(C)C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606102

(US11684616, Example 5)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606135
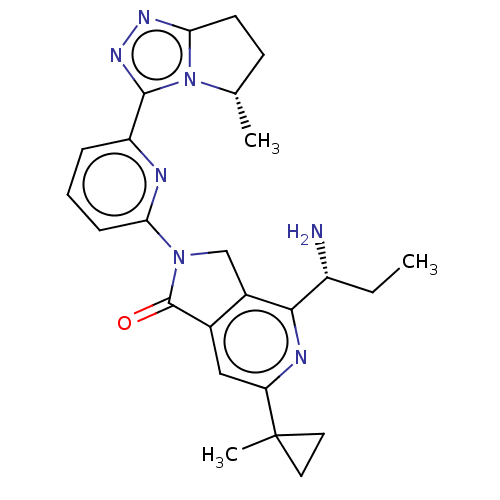
(4-[(1$#958;)-1- aminopropyl]- 6-(1- methylcyclo- p...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)C1(C)CC1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606137
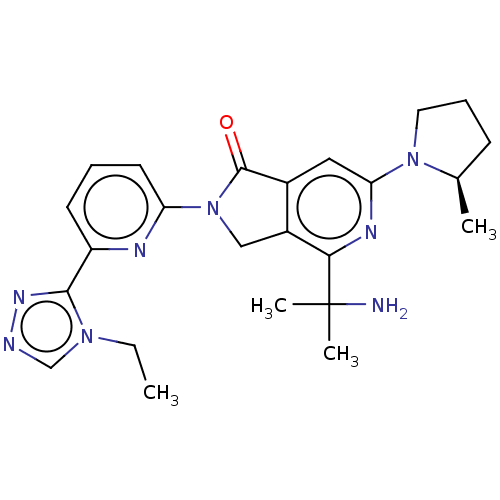
(US11684616, Example 100)Show SMILES CCn1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2C(C)(C)N)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606194

(US11684616, Example 200)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4CNC)N4CCC[C@H]4C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606195

(US11684616, Example 201)Show SMILES CC[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4CNC)N(C)C(C)C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606199

(2-{6-[(5R)-5- (fluoromethyl)- 6,7-dihydro-5H- pyrr...)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CF)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606204

(4-[(methyl- amino)methyl]- 2-{6-[(5S)- 5-methyl-6,...)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](C)n12)N(C)C(C)C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606128
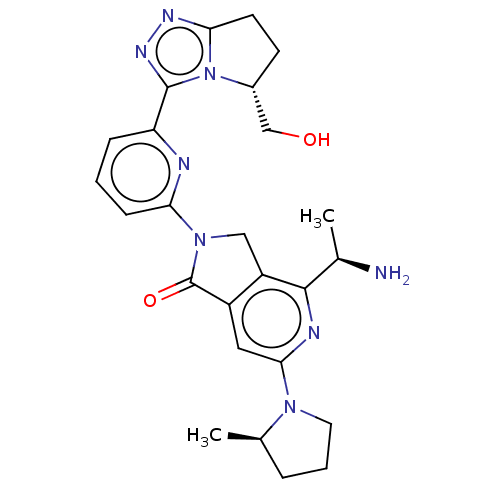
(4-[(1R)-1- aminoethyl]-2- {6-[(5R)-5- (hydroxy- me...)Show SMILES C[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606130
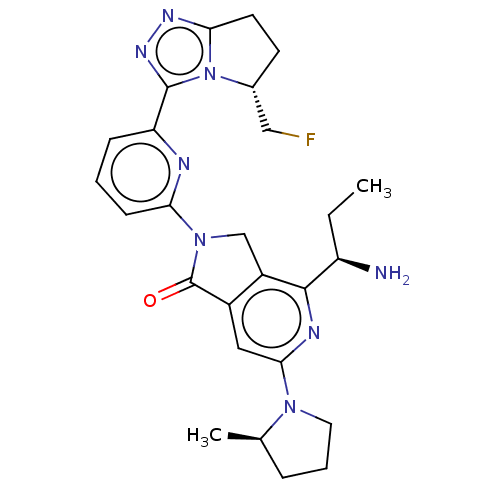
(4-[(1R)-1- aminopropyl]- 2-{6-[(5R)-5- (fluorometh...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CF)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606196

(US11684616, Example 202)Show SMILES CNCc1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CF)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606119
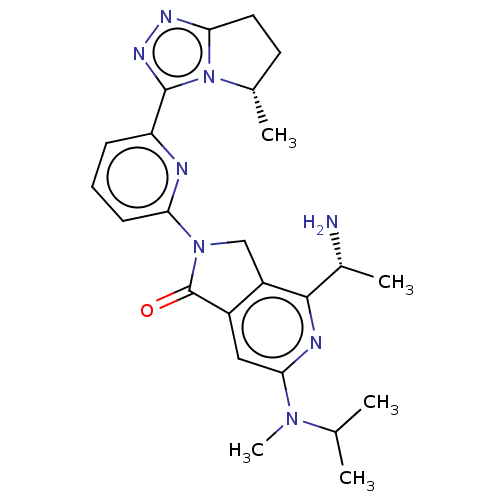
(4-[(1R)-1- aminoethyl]-2- {6-[(5S)-5- methyl-6,7- ...)Show SMILES CC(C)N(C)c1cc2C(=O)N(Cc2c(n1)[C@@H](C)N)c1cccc(n1)-c1nnc2CC[C@H](C)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606178

(4-[(1R)-1- aminopropyl]- 2-[6-(4-ethyl- 4H-1,2,4- ...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nncn1CC)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606111

(4-[(1R)-1- aminopropyl]- 2-{6-[(5$#958;)-5- ethyl-...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](CC)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50247744
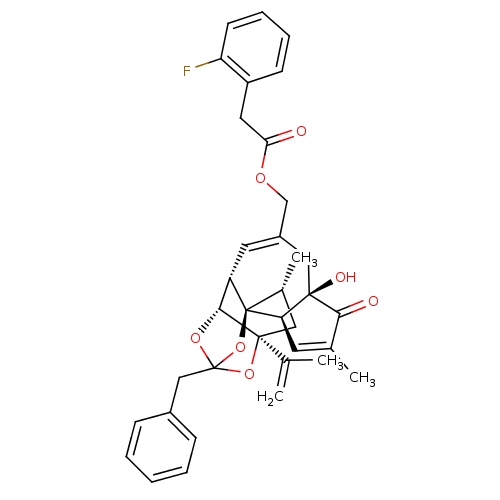
(CHEMBL504725 | [(1R,2R,6R,10S,11R,15S,17R)-13-benz...)Show SMILES C[C@@H]1C[C@]2(OC3(Cc4ccccc4)O[C@@H]2[C@@H]2C=C(COC(=O)Cc4ccccc4F)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]12O3)C(C)=C |r,t:18,37,TLB:6:5:15:3.2.1,THB:4:5:15:3.2.1| Show InChI InChI=1S/C36H37FO7/c1-21(2)34-17-23(4)36-27(32(34)42-35(43-34,44-36)19-24-10-6-5-7-11-24)15-25(18-33(40)29(36)14-22(3)31(33)39)20-41-30(38)16-26-12-8-9-13-28(26)37/h5-15,23,27,29,32,40H,1,16-20H2,2-4H3/t23-,27+,29-,32-,33-,34+,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell system |
Bioorg Med Chem 17: 690-8 (2009)
Article DOI: 10.1016/j.bmc.2008.11.085
BindingDB Entry DOI: 10.7270/Q2CJ8FCW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606134

(4-[(1$#958;)-1- aminopropyl]- 2-{6-[(5R)-5- (hydro...)Show SMILES CC[C@@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@H](CO)n12)C1(C)CC1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50341508

((S)-N-(4-(4-Phenylbenzoylamino)butyl)-N-propyl-5-a...)Show SMILES CCCN(CCCCNC(=O)c1ccc(cc1)-c1ccccc1)[C@H]1CCn2nccc2C1 |r| Show InChI InChI=1S/C27H34N4O/c1-2-18-30(25-15-20-31-26(21-25)14-17-29-31)19-7-6-16-28-27(32)24-12-10-23(11-13-24)22-8-4-3-5-9-22/h3-5,8-14,17,25H,2,6-7,15-16,18-21H2,1H3,(H,28,32)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from wild type human dopamine D3 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 54: 2477-91 (2011)
Article DOI: 10.1021/jm101639t
BindingDB Entry DOI: 10.7270/Q2V1253W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606120
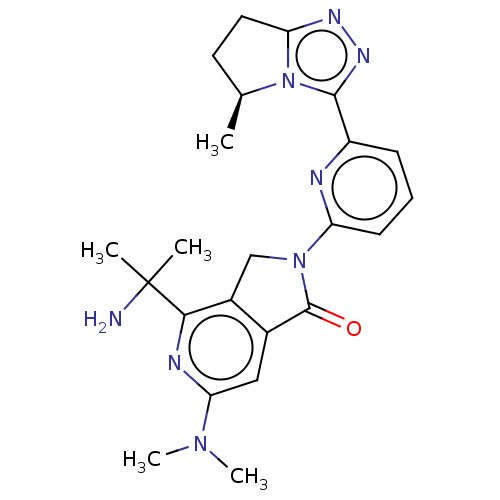
(4-(2- aminopropan- 2-yl)-6- (dimethyl- amino)-2-{6...)Show SMILES C[C@H]1CCc2nnc(-c3cccc(n3)N3Cc4c(cc(nc4C(C)(C)N)N(C)C)C3=O)n12 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition constant against dopamine receptor D2 in rat |
J Med Chem 36: 221-8 (1993)
BindingDB Entry DOI: 10.7270/Q2TT4Q10 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606147
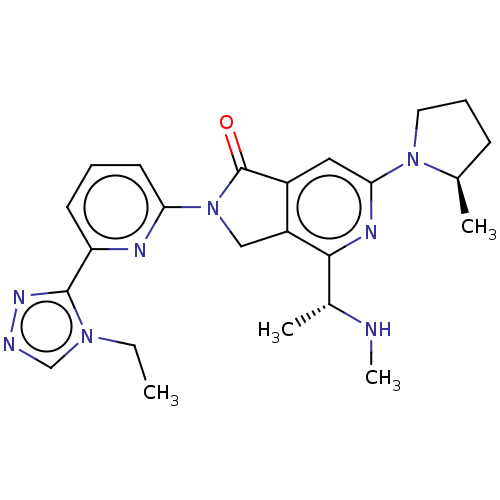
(2-[6-(4-ethyl- 4H-1,2,4- triazol-3- yl)pyridin-2- ...)Show SMILES CCn1cnnc1-c1cccc(n1)N1Cc2c(cc(nc2[C@@H](C)NC)N2CCC[C@H]2C)C1=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM606114

(4-[(1S)-1- aminopropyl]- 2-{6-[(5$#958;)-5- ethyl-...)Show SMILES CC[C@H](N)c1nc(cc2C(=O)N(Cc12)c1cccc(n1)-c1nnc2CC[C@@H](CC)n12)N1CCC[C@H]1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SF317X |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM31817

(GRL-02031 | methyl-2-pyrrolidinone, 19b)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(C[C@H]1CCC(=O)N1)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C30H39N3O8S/c1-39-23-8-10-25(11-9-23)42(37,38)33(18-22-7-12-29(35)31-22)19-27(34)26(15-20-5-3-2-4-6-20)32-30(36)41-24-16-21-13-14-40-28(21)17-24/h2-6,8-11,21-22,24,26-28,34H,7,12-19H2,1H3,(H,31,35)(H,32,36)/t21-,22+,24+,26-,27+,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0990 | -57.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 52: 3902-14 (2009)
Article DOI: 10.1021/jm900303m
BindingDB Entry DOI: 10.7270/Q20G3HHH |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50100983
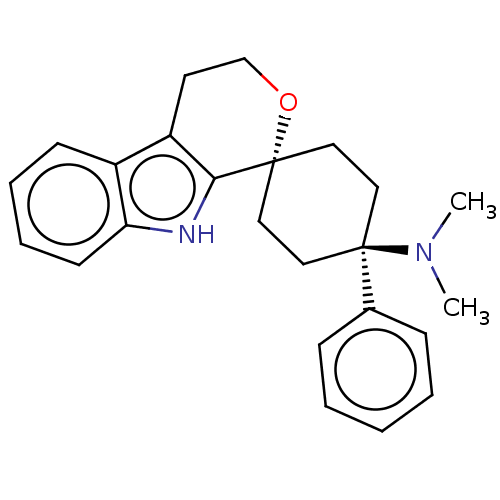
(CHEMBL3326224)Show SMILES Cl.CN(C)[C@]1(CC[C@@]2(CC1)OCCc1c2[nH]c2ccccc12)c1ccccc1 |r,wU:4.2,wD:7.9,(22.71,-7.97,;18.3,-7.93,;16.96,-7.16,;16.96,-5.62,;15.63,-7.93,;14.86,-9.26,;13.32,-9.27,;12.55,-7.94,;13.32,-6.61,;14.86,-6.6,;11.79,-9.28,;10.25,-9.27,;9.47,-7.94,;10.25,-6.6,;11.79,-6.61,;12.26,-5.14,;11.03,-4.24,;10.87,-2.7,;9.47,-2.07,;8.21,-2.98,;8.37,-4.51,;9.77,-5.14,;16.43,-9.25,;15.68,-10.6,;16.48,-11.91,;18.02,-11.88,;18.76,-10.52,;17.96,-9.21,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay |
ACS Med Chem Lett 5: 857-62 (2014)
Article DOI: 10.1021/ml500117c
BindingDB Entry DOI: 10.7270/Q25140ZK |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM462100

(US10766884, Example A17 | US11220494, Example A17)Show SMILES CCC1COCc2nc3c(F)cc(cc3n12)-c1nc(N[C@@H]2CCN(C[C@H]2O)S(C)(=O)=O)ncc1F |r| Show InChI InChI=1S/C22H26F2N6O4S/c1-3-13-10-34-11-19-27-21-14(23)6-12(7-17(21)30(13)19)20-15(24)8-25-22(28-20)26-16-4-5-29(9-18(16)31)35(2,32)33/h6-8,13,16,18,31H,3-5,9-11H2,1-2H3,(H,25,26,28)/t13?,16-,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
CDK4/Cyclin D1: The purpose CDK4/Cyclin D1 assay is to evaluate the inhibition (% inhibition, Kiapp and Ki values) in the presence of small molecule ... |
US Patent US10766884 (2020)
BindingDB Entry DOI: 10.7270/Q23F4SQG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data