 Found 494 hits with Last Name = 'chuckowree' and Initial = 'i'
Found 494 hits with Last Name = 'chuckowree' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358203
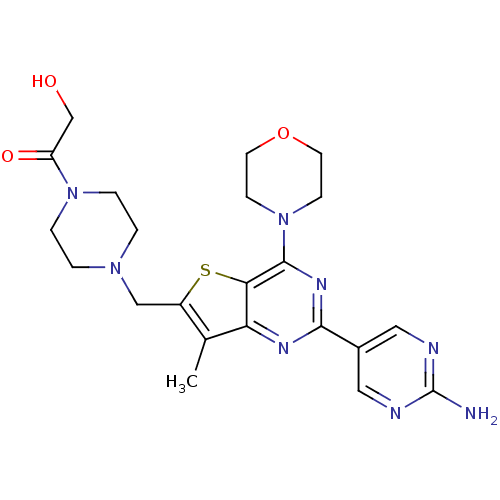
(CHEMBL1922093)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CO)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H28N8O3S/c1-14-16(12-28-2-4-29(5-3-28)17(32)13-31)34-19-18(14)26-20(15-10-24-22(23)25-11-15)27-21(19)30-6-8-33-9-7-30/h10-11,31H,2-9,12-13H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315915
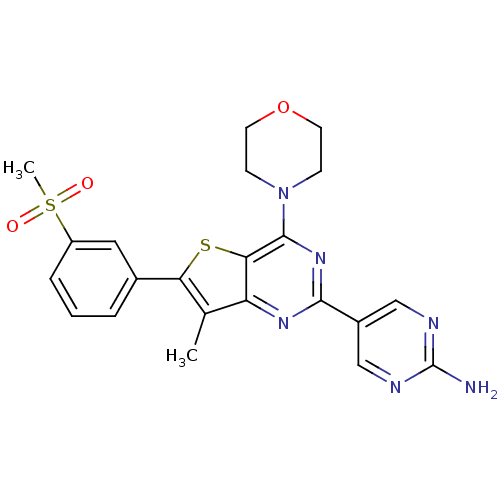
(5-(7-methyl-6-(3-(methylsulfonyl)phenyl)-4-morphol...)Show SMILES Cc1c(sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1)-c1cccc(c1)S(C)(=O)=O Show InChI InChI=1S/C22H22N6O3S2/c1-13-17-19(32-18(13)14-4-3-5-16(10-14)33(2,29)30)21(28-6-8-31-9-7-28)27-20(26-17)15-11-24-22(23)25-12-15/h3-5,10-12H,6-9H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315914

((3-(2-(2-aminopyrimidin-5-yl)-7-methyl-4-morpholin...)Show SMILES CN1CCN(CC1)C(=O)c1cccc(c1)-c1sc2c(nc(nc2c1C)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C27H30N8O2S/c1-17-21-23(25(34-10-12-37-13-11-34)32-24(31-21)20-15-29-27(28)30-16-20)38-22(17)18-4-3-5-19(14-18)26(36)35-8-6-33(2)7-9-35/h3-5,14-16H,6-13H2,1-2H3,(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315911
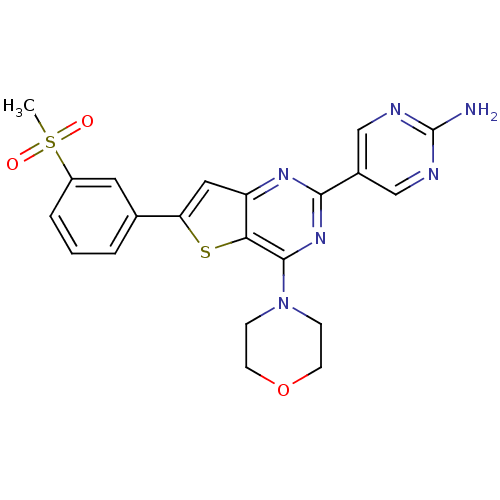
(5-(6-(3-(methylsulfonyl)phenyl)-4-morpholinothieno...)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1 Show InChI InChI=1S/C21H20N6O3S2/c1-32(28,29)15-4-2-3-13(9-15)17-10-16-18(31-17)20(27-5-7-30-8-6-27)26-19(25-16)14-11-23-21(22)24-12-14/h2-4,9-12H,5-8H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358206
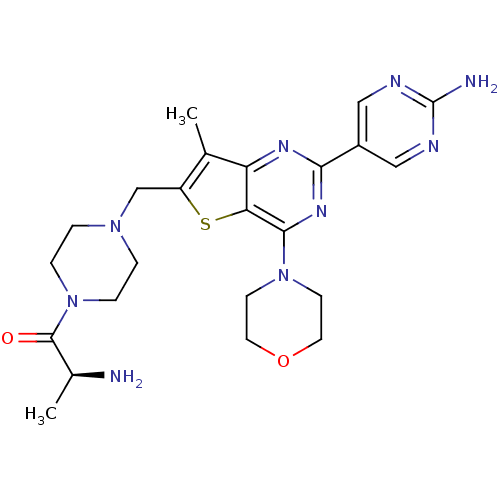
(CHEMBL1922091)Show SMILES C[C@H](N)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H31N9O2S/c1-14-17(13-30-3-5-32(6-4-30)22(33)15(2)24)35-19-18(14)28-20(16-11-26-23(25)27-12-16)29-21(19)31-7-9-34-10-8-31/h11-12,15H,3-10,13,24H2,1-2H3,(H2,25,26,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315913

((3-(2-(2-aminopyrimidin-5-yl)-4-morpholinothieno[3...)Show SMILES CN1CCN(CC1)C(=O)c1cccc(c1)-c1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1 Show InChI InChI=1S/C26H28N8O2S/c1-32-5-7-34(8-6-32)25(35)18-4-2-3-17(13-18)21-14-20-22(37-21)24(33-9-11-36-12-10-33)31-23(30-20)19-15-28-26(27)29-16-19/h2-4,13-16H,5-12H2,1H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315908

(CHEMBL1090598 | N-((2-(2-aminopyrimidin-5-yl)-4-mo...)Show SMILES CN(Cc1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C17H21N7O3S2/c1-23(29(2,25)26)10-12-7-13-14(28-12)16(24-3-5-27-6-4-24)22-15(21-13)11-8-19-17(18)20-9-11/h7-9H,3-6,10H2,1-2H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358205
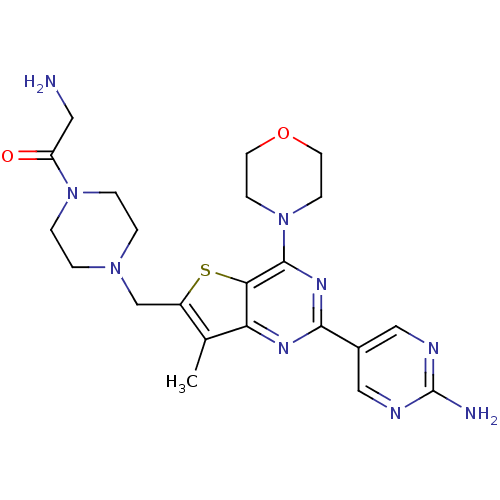
(CHEMBL1922090)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CN)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H29N9O2S/c1-14-16(13-29-2-4-30(5-3-29)17(32)10-23)34-19-18(14)27-20(15-11-25-22(24)26-12-15)28-21(19)31-6-8-33-9-7-31/h11-12H,2-10,13,23H2,1H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358209

(CHEMBL1922095)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)O)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H32N8O3S/c1-15-17(14-30-4-6-32(7-5-30)22(33)24(2,3)34)36-19-18(15)28-20(16-12-26-23(25)27-13-16)29-21(19)31-8-10-35-11-9-31/h12-13,34H,4-11,14H2,1-3H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358207

(CHEMBL1922092)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)N)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H33N9O2S/c1-15-17(14-31-4-6-33(7-5-31)22(34)24(2,3)26)36-19-18(15)29-20(16-12-27-23(25)28-13-16)30-21(19)32-8-10-35-11-9-32/h12-13H,4-11,14,26H2,1-3H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358204
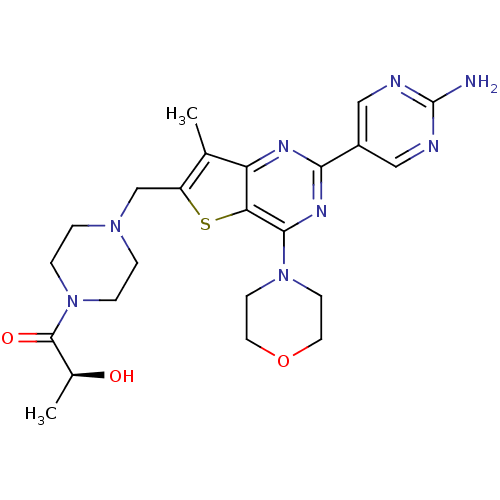
(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315907
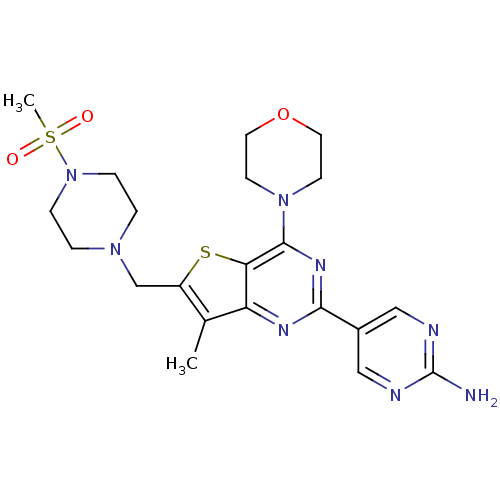
(5-(7-methyl-6-((4-(methylsulfonyl)piperazin-1-yl)m...)Show SMILES Cc1c(CN2CCN(CC2)S(C)(=O)=O)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C21H28N8O3S2/c1-14-16(13-27-3-5-29(6-4-27)34(2,30)31)33-18-17(14)25-19(15-11-23-21(22)24-12-15)26-20(18)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315907

(5-(7-methyl-6-((4-(methylsulfonyl)piperazin-1-yl)m...)Show SMILES Cc1c(CN2CCN(CC2)S(C)(=O)=O)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C21H28N8O3S2/c1-14-16(13-27-3-5-29(6-4-27)34(2,30)31)33-18-17(14)25-19(15-11-23-21(22)24-12-15)26-20(18)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50312606
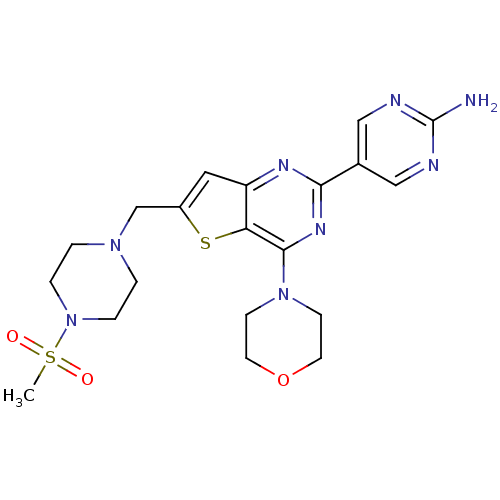
(5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-17(32-15)19(27-6-8-31-9-7-27)25-18(24-16)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50312606

(5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-17(32-15)19(27-6-8-31-9-7-27)25-18(24-16)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315912
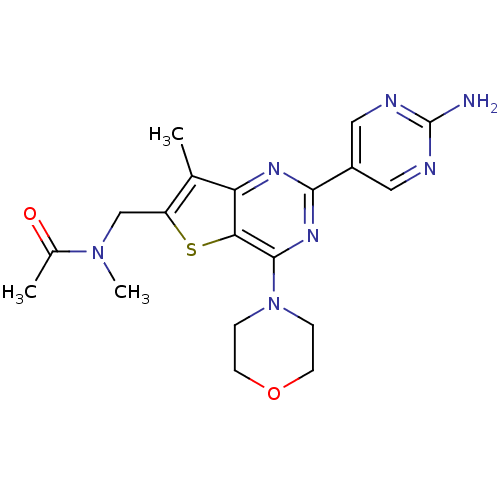
(CHEMBL1091979 | N-((2-(2-aminopyrimidin-5-yl)-7-me...)Show SMILES CN(Cc1sc2c(nc(nc2c1C)-c1cnc(N)nc1)N1CCOCC1)C(C)=O Show InChI InChI=1S/C19H23N7O2S/c1-11-14(10-25(3)12(2)27)29-16-15(11)23-17(13-8-21-19(20)22-9-13)24-18(16)26-4-6-28-7-5-26/h8-9H,4-7,10H2,1-3H3,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50304689

(5-(4-morpholinothieno[3,2-d]pyrimidin-2-yl)pyrimid...)Show InChI InChI=1S/C14H14N6OS/c15-14-16-7-9(8-17-14)12-18-10-1-6-22-11(10)13(19-12)20-2-4-21-5-3-20/h1,6-8H,2-5H2,(H2,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315909
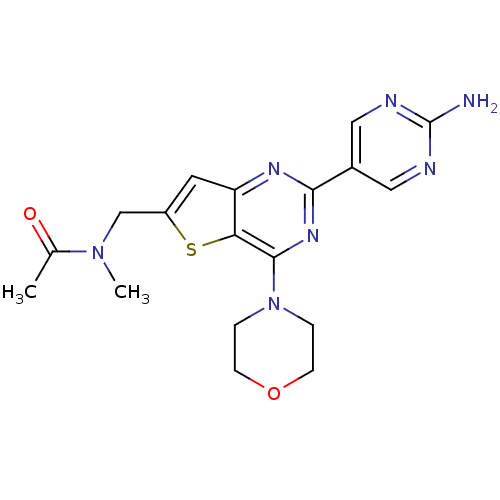
(CHEMBL1090599 | N-((2-(2-aminopyrimidin-5-yl)-4-mo...)Show SMILES CN(Cc1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1)C(C)=O Show InChI InChI=1S/C18H21N7O2S/c1-11(26)24(2)10-13-7-14-15(28-13)17(25-3-5-27-6-4-25)23-16(22-14)12-8-20-18(19)21-9-12/h7-9H,3-6,10H2,1-2H3,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315910
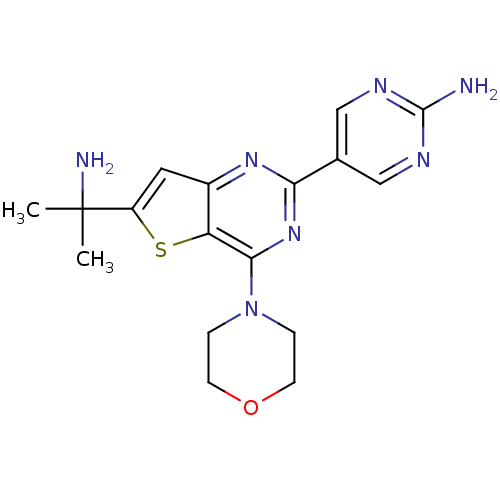
(5-(6-(2-aminopropan-2-yl)-4-morpholinothieno[3,2-d...)Show SMILES CC(C)(N)c1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1 Show InChI InChI=1S/C17H21N7OS/c1-17(2,19)12-7-11-13(26-12)15(24-3-5-25-6-4-24)23-14(22-11)10-8-20-16(18)21-9-10/h7-9H,3-6,19H2,1-2H3,(H2,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR assessed as reduction in GFP-4EBP1 phosphorylation after 30 mins by FRET assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 measured after 30 mins by fluo... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 580 | -35.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Piramed Pharma
| Assay Description
Mammalian target of rapamycin (mTOR) was assayed by monitoring phosphorylation of GFP-4EBP using a homogeneous time-resolved fluorescence resonance e... |
J Med Chem 51: 5522-32 (2008)
Article DOI: 10.1021/jm800295d
BindingDB Entry DOI: 10.7270/Q2222S23 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50328085
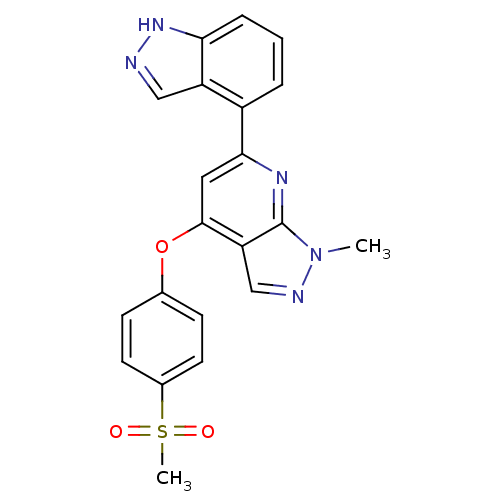
(6-(1H-indazol-4-yl)-1-methyl-4-(4-(methylsulfonyl)...)Show SMILES Cn1ncc2c(Oc3ccc(cc3)S(C)(=O)=O)cc(nc12)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C21H17N5O3S/c1-26-21-17(12-23-26)20(29-13-6-8-14(9-7-13)30(2,27)28)10-19(24-21)15-4-3-5-18-16(15)11-22-25-18/h3-12H,1-2H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6048-51 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.067
BindingDB Entry DOI: 10.7270/Q2ST7Q28 |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572440

(CHEMBL4469438)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3ccccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572444
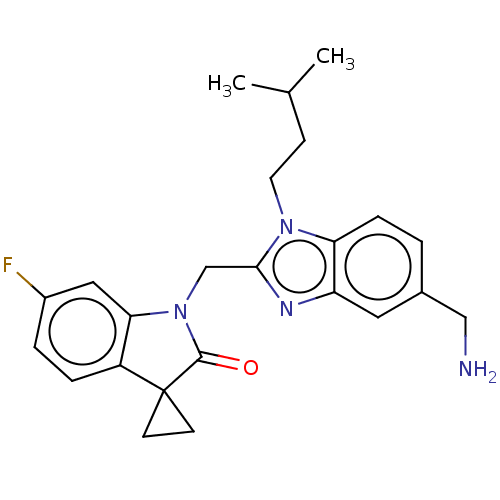
(CHEMBL4878302)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3ccc(F)cc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572466

(CHEMBL4876200)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CCOCC3)c3ccccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus fusion protein |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50312614
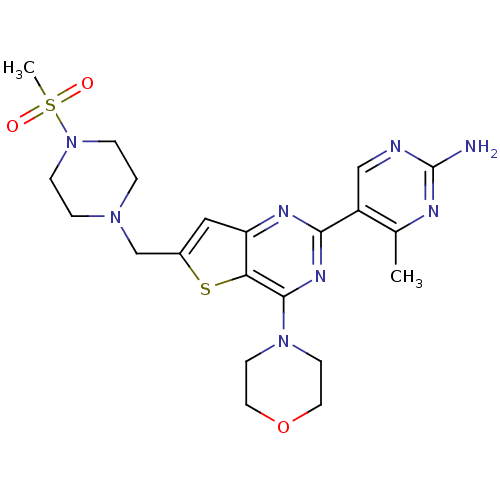
(4-methyl-5-(6-{[4-(methylsulfonyl)piperazin-1-yl]m...)Show SMILES Cc1nc(N)ncc1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)S(C)(=O)=O)cc2n1 Show InChI InChI=1S/C21H28N8O3S2/c1-14-16(12-23-21(22)24-14)19-25-17-11-15(13-27-3-5-29(6-4-27)34(2,30)31)33-18(17)20(26-19)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358205

(CHEMBL1922090)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CN)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H29N9O2S/c1-14-16(13-29-2-4-30(5-3-29)17(32)10-23)34-19-18(14)27-20(15-11-25-22(24)26-12-15)28-21(19)31-6-8-33-9-7-31/h11-12H,2-10,13,23H2,1H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572442

(CHEMBL4872095)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3cc(F)ccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50312612
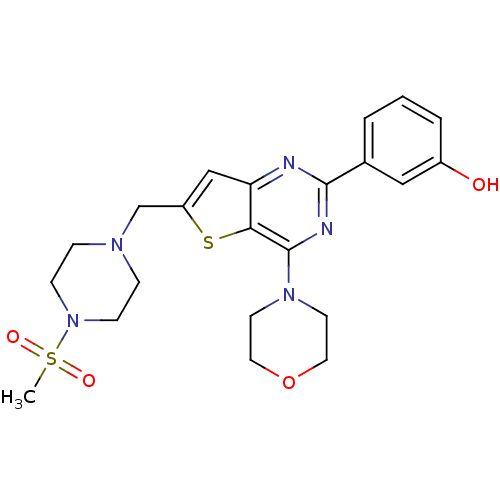
(3-[6-(4-Methanesulfonyl-piperazin-1-ylmethyl)-4-mo...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(O)c2)CC1 Show InChI InChI=1S/C22H27N5O4S2/c1-33(29,30)27-7-5-25(6-8-27)15-18-14-19-20(32-18)22(26-9-11-31-12-10-26)24-21(23-19)16-3-2-4-17(28)13-16/h2-4,13-14,28H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572464

(CHEMBL4858040)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3ccncc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus fusion protein |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572450
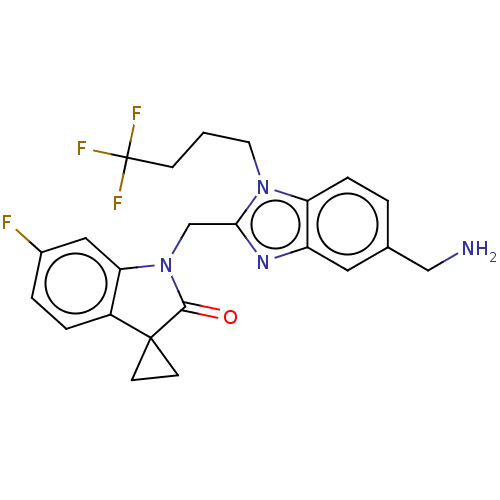
(Rv-521 | Rv521 | Sisunatovir)Show SMILES NCc1ccc2n(CCCC(F)(F)F)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572463

(CHEMBL4871101)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3cccnc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus fusion protein |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358207

(CHEMBL1922092)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)N)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H33N9O2S/c1-15-17(14-31-4-6-33(7-5-31)22(34)24(2,3)26)36-19-18(15)29-20(16-12-27-23(25)28-13-16)30-21(19)32-8-10-35-11-9-32/h12-13H,4-11,14,26H2,1-3H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572451
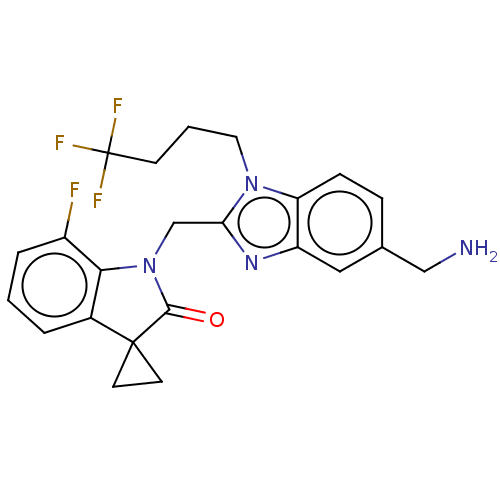
(CHEMBL4867108)Show SMILES NCc1ccc2n(CCCC(F)(F)F)c(CN3C(=O)C4(CC4)c4cccc(F)c34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315913

((3-(2-(2-aminopyrimidin-5-yl)-4-morpholinothieno[3...)Show SMILES CN1CCN(CC1)C(=O)c1cccc(c1)-c1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1 Show InChI InChI=1S/C26H28N8O2S/c1-32-5-7-34(8-6-32)25(35)18-4-2-3-17(13-18)21-14-20-22(37-21)24(33-9-11-36-12-10-33)31-23(30-20)19-15-28-26(27)29-16-19/h2-4,13-16H,5-12H2,1H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha assessed as reduction in 3,4,5-inositoltriphosphate accumulation after 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315908

(CHEMBL1090598 | N-((2-(2-aminopyrimidin-5-yl)-4-mo...)Show SMILES CN(Cc1cc2nc(nc(N3CCOCC3)c2s1)-c1cnc(N)nc1)S(C)(=O)=O Show InChI InChI=1S/C17H21N7O3S2/c1-23(29(2,25)26)10-12-7-13-14(28-12)16(24-3-5-27-6-4-24)22-15(21-13)11-8-19-17(18)20-9-11/h7-9H,3-6,10H2,1-2H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha assessed as reduction in 3,4,5-inositoltriphosphate accumulation after 30 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2408-11 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.046
BindingDB Entry DOI: 10.7270/Q2GM87FX |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572465
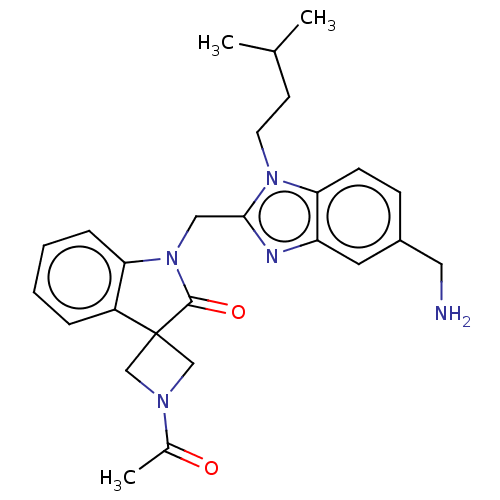
(CHEMBL4858949)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CN(C3)C(C)=O)c3ccccc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus fusion protein |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312614

(4-methyl-5-(6-{[4-(methylsulfonyl)piperazin-1-yl]m...)Show SMILES Cc1nc(N)ncc1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)S(C)(=O)=O)cc2n1 Show InChI InChI=1S/C21H28N8O3S2/c1-14-16(12-23-21(22)24-14)19-25-17-11-15(13-27-3-5-29(6-4-27)34(2,30)31)33-18(17)20(26-19)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50328080
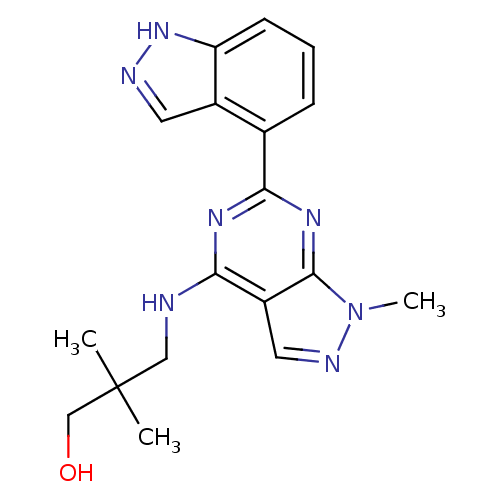
(3-(6-(1H-indazol-4-yl)-1-methyl-1H-pyrazolo[3,4-d]...)Show SMILES Cn1ncc2c(NCC(C)(C)CO)nc(nc12)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C18H21N7O/c1-18(2,10-26)9-19-15-13-8-21-25(3)17(13)23-16(22-15)11-5-4-6-14-12(11)7-20-24-14/h4-8,26H,9-10H2,1-3H3,(H,20,24)(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6048-51 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.067
BindingDB Entry DOI: 10.7270/Q2ST7Q28 |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572453

(CHEMBL4874709)Show SMILES NCc1ccc2n(C3CCOCC3)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572445

(CHEMBL4865906)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3ccc(Cl)cc23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572447
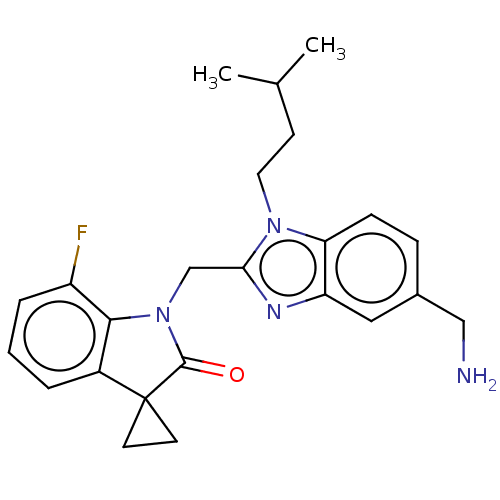
(CHEMBL4860271)Show SMILES CC(C)CCn1c(CN2C(=O)C3(CC3)c3cccc(F)c23)nc2cc(CN)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50328082

(6-(1H-indazol-4-yl)-1-methyl-N-(4-(methylsulfonyl)...)Show SMILES Cn1ncc2c(Nc3ccc(cc3)S(C)(=O)=O)nc(nc12)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C20H17N7O2S/c1-27-20-16(11-22-27)19(23-12-6-8-13(9-7-12)30(2,28)29)24-18(25-20)14-4-3-5-17-15(14)10-21-26-17/h3-11H,1-2H3,(H,21,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6048-51 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.067
BindingDB Entry DOI: 10.7270/Q2ST7Q28 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50328079
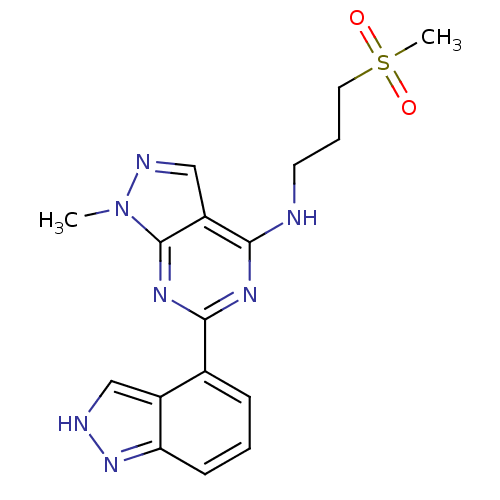
(6-(1H-indazol-4-yl)-1-methyl-N-(3-(methylsulfonyl)...)Show SMILES Cn1ncc2c(NCCCS(C)(=O)=O)nc(nc12)-c1cccc2n[nH]cc12 Show InChI InChI=1S/C17H19N7O2S/c1-24-17-13(10-20-24)15(18-7-4-8-27(2,25)26)21-16(22-17)11-5-3-6-14-12(11)9-19-23-14/h3,5-6,9-10H,4,7-8H2,1-2H3,(H,19,23)(H,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6048-51 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.067
BindingDB Entry DOI: 10.7270/Q2ST7Q28 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50328076

(6-(1H-indazol-4-yl)-N-(3-methoxyphenyl)-1-methyl-1...)Show SMILES COc1cccc(Nc2nc(nc3n(C)ncc23)-c2cccc3[nH]ncc23)c1 Show InChI InChI=1S/C20H17N7O/c1-27-20-16(11-22-27)19(23-12-5-3-6-13(9-12)28-2)24-18(25-20)14-7-4-8-17-15(14)10-21-26-17/h3-11H,1-2H3,(H,21,26)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 6048-51 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.067
BindingDB Entry DOI: 10.7270/Q2ST7Q28 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50312609
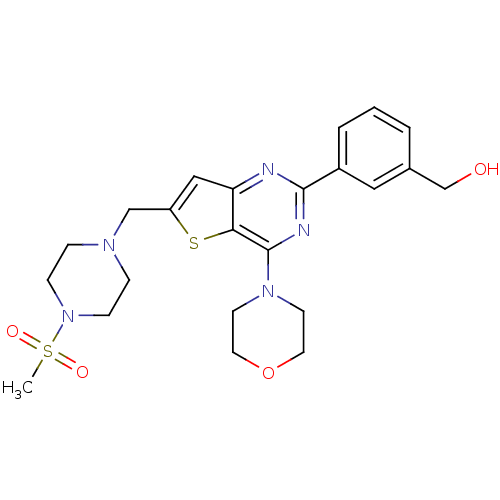
((3-(6-((4-(Methylsulfonyl)piperazin-1-yl)methyl)-4...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(CO)c2)CC1 Show InChI InChI=1S/C23H29N5O4S2/c1-34(30,31)28-7-5-26(6-8-28)15-19-14-20-21(33-19)23(27-9-11-32-12-10-27)25-22(24-20)18-4-2-3-17(13-18)16-29/h2-4,13-14,29H,5-12,15-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kdelta assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50358207

(CHEMBL1922092)Show SMILES Cc1c(CN2CCN(CC2)C(=O)C(C)(C)N)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C24H33N9O2S/c1-15-17(14-31-4-6-33(7-5-31)22(34)24(2,3)26)36-19-18(15)29-20(16-12-27-23(25)28-13-16)30-21(19)32-8-10-35-11-9-32/h12-13H,4-11,14,26H2,1-3H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110delta assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Fusion glycoprotein F0
(Human respiratory syncytial virus A (strain A2)) | BDBM50572449

(CHEMBL4853602)Show SMILES NCc1ccc2n(CCCCO)c(CN3C(=O)C4(CC4)c4ccc(F)cc34)nc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Respiratory syncytial virus A2 fusion protein expressed in HEK293T cells harboring GAL4-NF-kappaB assessed as reduction in cell-cell fu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01882
BindingDB Entry DOI: 10.7270/Q2QF8XNB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50312612

(3-[6-(4-Methanesulfonyl-piperazin-1-ylmethyl)-4-mo...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc(O)c2)CC1 Show InChI InChI=1S/C22H27N5O4S2/c1-33(29,30)27-7-5-25(6-8-27)15-18-14-19-20(32-18)22(26-9-11-31-12-10-26)24-21(23-19)16-3-2-4-17(28)13-16/h2-4,13-14,28H,5-12,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as PIP3 product formation by fluorescence polarization assay |
J Med Chem 53: 1086-97 (2010)
Article DOI: 10.1021/jm901284w
BindingDB Entry DOI: 10.7270/Q2FJ2GXT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data